Desirudin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
SPINAL/EPIDURAL HEMATOMAS:
|
Overview
Desirudin is a direct thrombin inhibitor that is FDA approved for the {{{indicationType}}} of deep vein thrombosis in patients undergoing elective hip replacement surgery. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hemorrhage, injection site mass, wound secretion, anemia, deep thrombophlebitis, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Deep Vein Thrombosis
- Dosing Information
- All patients should be evaluated for bleeding disorder risk before prophylactic administration of Iprivask.
- Initial Dosage
- In patients undergoing hip replacement surgery, the recommended dose of Iprivask is 15 mg every 12 hours administered by subcutaneous injection with the initial dose given up to 5 to 15 minutes prior to surgery, but after induction of regional block anesthesia, if used. Up to 12 days administration (average duration 9 to 12 days) of Iprivask has been well tolerated in controlled clinical trials.
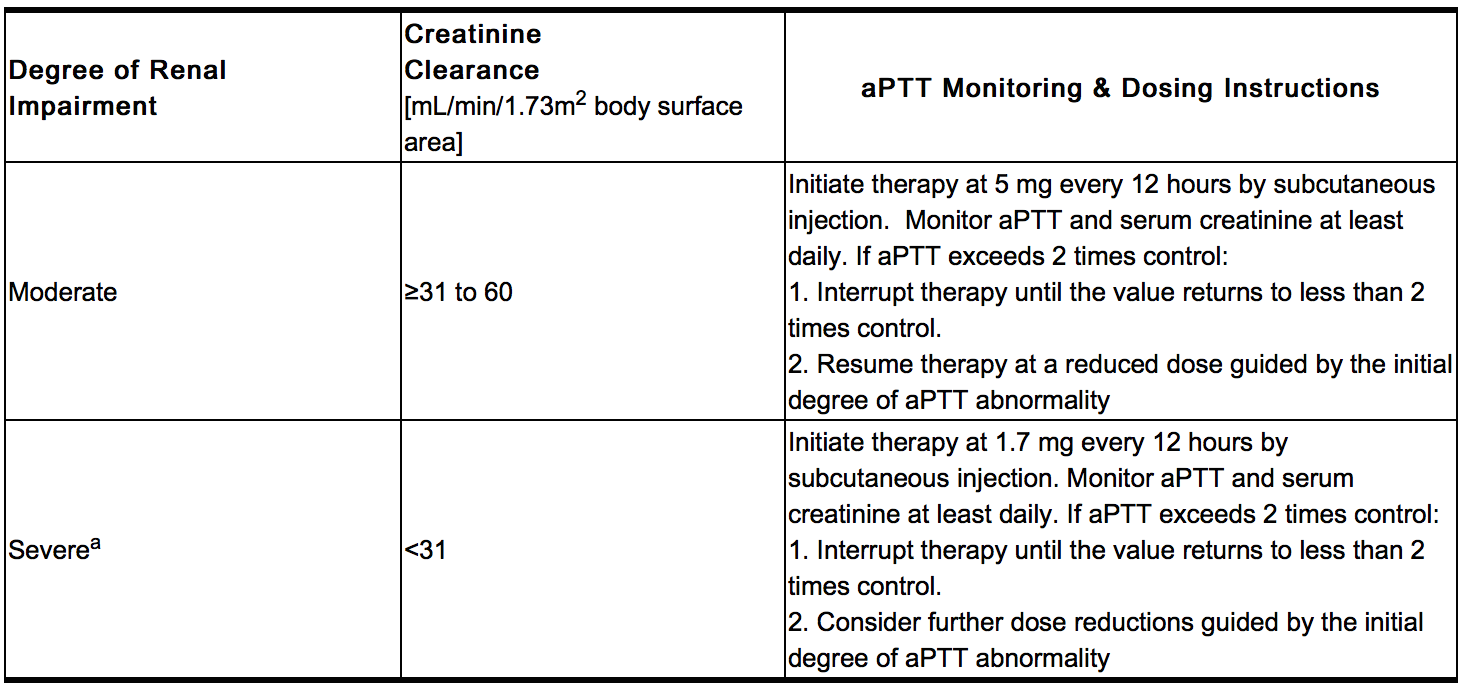
- Dose in Renal Impairment
- Dose in Hepatic Impairment
- In the absence of clinical studies in this population, dosing recommendations cannot be made at this time.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desirudin in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Coronary Thrombolysis in Acute Coronary Syndrome
- Equivalent or slightly better efficacy compared to heparin for thrombolysis in patients with acute coronary syndrome; desirudin administration is also associated with a lower early reinfarction rate.[1][2][3][4]
Percutaneous Transluminal Coronary Angioplasty
- Intravenous desirudin (20 mgbolus followed by an infusion of 0.16 mg/kg/hr for 24 hours) is associated with a reduced early cardiac events in patients undergoing percutaneous transluminal coronary angioplasty.[5][6]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desirudin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desirudin in pediatric patients.
Contraindications
- Iprivask is contraindicated in patients with known hypersensitivity to natural or recombinant hirudins, and in patients with active bleeding and/or irreversible coagulation disorders.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
SPINAL/EPIDURAL HEMATOMAS:
|
- Spinal/Epidural Anesthesia
- As with other anticoagulants, there is a risk of neuraxial hematoma formation with the concurrent use of desirudin and spinal/epidural anesthesia, which has the potential to result in long term or permanent paralysis. The risk may be greater with the use of post-operative indwelling catheters or the concomitant use of additional drugs affecting hemostasis such as NSAIDs (Non-Steroidal Anti-Inflammatory Drugs), platelet inhibitors or other anticoagulants. The risk may also be increased by traumatic or repeated neuraxial puncture.
- To reduce the potential risk of bleeding associated with the concurrent use of desirudin and epidural or spinal anesthesia/analgesia, the pharmacokinetic profile of the drug should be considered when scheduling or using epidural or spinal anesthesia in proximity to desirudin administration. The physician should consider placement of the catheter prior to initiating desirudin and removal of the catheter when the anticoagulant effect of desirudin is low.
- Should the physician decide to administer anticoagulation in the context of epidural/spinal anesthesia, extreme vigilance and frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. Patients should be instructed to inform their physician immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, urgent diagnosis and treatment including spinal cord decompression should be initiated.
- The physician should consider the potential benefit versus risk before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis.
- Iprivask cannot be used interchangeably with other hirudins as they differ in manufacturing process and specific biological activity (ATUs). Each of these medicines has its own instructions for use.
- Iprivask must be used with caution in patients with renal impairment, particularly in those with moderate and severe renal impairment (creatinine clearance ≤60 mL/min/1.73 m2 body surface area). Dose reductions by factors of three and nine are recommended for patients with moderate and severe renal impairment respectively. In addition, daily aPTT and serum creatinine monitoring are recommended for patients with moderate or severe renal impairment.
- Hemorrhagic Events
- Iprivask is not intended for intramuscular injection as local hematoma formation may result.
- Iprivask, like other anticoagulants, should be used with caution in patients with increased risks of hemorrhage such as those with recent major surgery, organ biopsy or puncture of a non-compressible vessel within the last month; a history of hemorrhagic stroke, intracranial or intraocular bleeding including diabetic retinopathy; recent ischemic stroke, severe uncontrolled hypertension, bacterial endocarditis, a known hemostatic disorder (congenital or acquired, e.g. hemophilia, liver disease) or a history of gastrointestinal or pulmonary bleeding within the past 3 months.
- Bleeding can occur at any site during therapy with Iprivask. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site.
- Antibodies/Re-exposure
- Antibodies have been reported in patients treated with hirudins. Potential for cross-sensitivity to hirudin products cannot be excluded. Irritative skin reactions were observed in 9/322 volunteers exposed to Iprivask by subcutaneous injection or IV bolus or infusion in single or multiple administrations of the drug. Allergic events were reported in <2% of patients who were administered desirudin in Phase III clinical trials. Allergic events were reported in 1% of patients receiving unfractionated heparin and 1% of patients receiving enoxaparin. Hirudin-specific IgE evaluations may not be indicative of sensitivity to Iprivask as this test was not always positive in the presence of symptoms. Very rarely, anti-hirudin antibodies have been detected upon re-exposure to desirudin. Fatal anaphylactoid reactions have been reported during hirudin therapy.
- Hepatic Impairment/Liver Injury
- No information is available about the use of desirudin in patients with hepatic impairment/liver injury. Although Iprivask is not significantly metabolized by the liver, hepatic impairment or serious liver injury (e.g., liver cirrhosis) may alter the anticoagulant effect of Iprivask due to coagulation defects secondary to reduced generation of vitamin K-dependent coagulation factors. Iprivask should be used with caution in these patients.
- Laboratory Tests
- Activated partial thromboplastin time (aPTT) should be monitored daily in patients with increased risk of bleeding and/or renal impairment. Serum creatinine should be monitored daily in patients with renal impairment. Peak aPTT should not exceed two times control. Should peak aPTT exceed this level, dose reduction is advised based on the degree of aPTT abnormality. If necessary, therapy with desirudin should be interrupted until aPTT falls to less than two times control, at which time treatment with desirudin can be resumed at a reduced dose. Thrombin time (TT) is not a suitable test for routine monitoring of Iprivask therapy. Dose adjustments based on serum creatinine may be necessary.
Adverse Reactions
Clinical Trials Experience
- In the Phase II and III clinical studies, desirudin was administered to 2159 patients undergoing elective hip replacement surgery to determine the safety and efficacy of Iprivask in preventing VTE in this population. Below is the safety profile of the Iprivask 15 mg (q12h) regimen from these 5 multicenter clinical trials.
- Hemorrhagic Events: The following rates of hemorrhagic events have been reported during clinical trials.
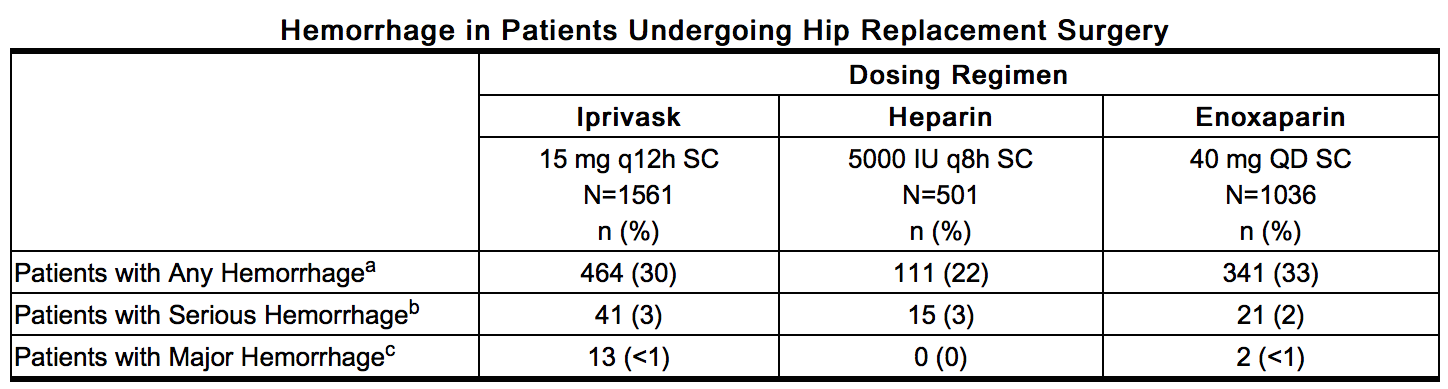
This image is provided by the National Library of Medicine. - a – Includes hematomas which occurred at an incidence of 6% in the Iprivask and enoxaparin treatment groups and 5% in the heparin treatment group.
- b – Bleeding complications were considered serious if preoperative transfusion requirements exceeded 5 units of whole blood or packed red cells, or if total transfusion requirements up to postoperative Day 6 inclusive exceeded 7 units of whole blood or packed red cells, or total blood loss up to postoperative Day 6 inclusive exceeded 3500 mL.
- c – Bleeding complications were considered major if the hemorrhage was: (1) overt and it produced a fall in hemoglobin of ≥2g/dL or if it lead to a transfusion of 2 or more units of whole or packed cells outside the perioperative period (the time from start of surgery until up to 12 hours after); (2) Retroperitoneal, intracranial, intraocular, intraspinal, or occurred in a major prosthetic joint.
- Non-hemorrhagic Events
- Non-hemorrhagic adverse events occurring at ≥2% incidence in patients treated with Iprivask 15 mg (q 12h) during elective hip replacement surgery and considered to be remotely, possibly, or probably related to desirudin are provided below.
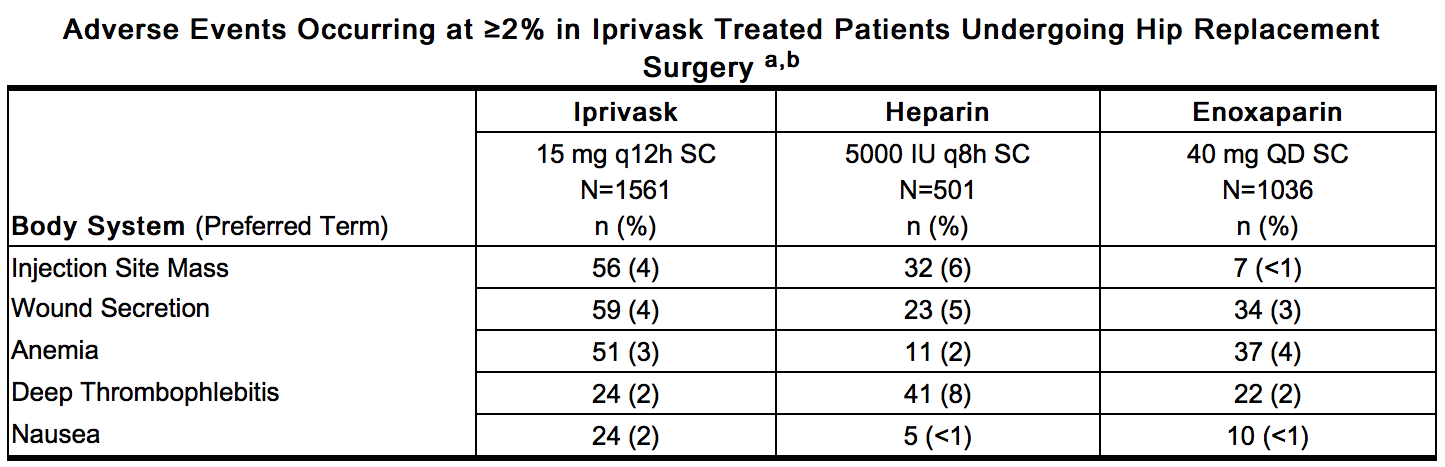
This image is provided by the National Library of Medicine. - a – Represents events reported while on treatment, excluding unrelated adverse events
- b – All hemorrhages that occurred are included in Hemorrhagic Events.
- Related Adverse Events with a Frequency of <2% and >0.2% (in decreasing order of frequency): thrombosis, hypotension, leg edema, fever, decreased hemoglobin, hematuria, dizziness, epistaxis, vomiting, impaired healing, cerebrovascular disorder, leg pain, hematemesis.
- Allergic Reactions
- In clinical studies, allergic events were reported <2% overall and in 2% of patients who were administered 15 mg desirudin.
Postmarketing Experience
- In addition to adverse events reported from clinical trials the following adverse events have been identified during post approval use of Iprivask. These events were reported voluntarily from a population of unknown size and the frequency of occurrence cannot be determined precisely: rare reports of major hemorrhages, some of which were fatal, and anaphylactic/anaphylactoid reactions.
Drug Interactions
- Any agent which may enhance the risk of hemorrhage should be discontinued prior to initiation of desirudin therapy. These agents include medications such as Dextran 40, systemic glucocorticoids, thrombolytics, and anticoagulants. If co-administration cannot be avoided, close clinical and laboratory monitoring should be conducted. During prophylaxis of venous thromboembolism, concomitant treatment with heparins (unfractionated and low-molecular weight heparins) or dextrans is not recommended. The effects of desirudin and unfractionated heparins on prolongation of aPTT are additive.
- As with other anticoagulants, desirudin should be used with caution in conjunction with drugs which affect platelet function. These medications include systemic salicylates, NSAIDs including ketorolac, acetylsalicylic acid, ticlopidine, dipyridamole, sulfinpyrazone, clopidogrel, abciximab and other glycoprotein IIb/IIIa antagonists.
- Use in patients switching from oral anticoagulants to Iprivask or from Iprivask to oral anticoagulants. The concomitant administration of warfarin did not significantly affect the pharmacokinetic effects of desirudin. When warfarin and desirudin were co-administered, greater inhibition of hemostasis measured by activated partial thromboplastin time (aPTT), prothrombin time (PT), and international normalized ratio (INR) was observed. If a patient is switched from oral anticoagulants to Iprivask therapy or from Iprivask to oral anticoagulants, the anticoagulant activity should continue to be closely monitored with appropriate methods. That activity should be taken into account in the evaluation of the overall coagulation status of the patient during the switch.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well controlled studies in pregnant women. Iprivask should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Animal Data
- Teratology studies have been performed in rats at subcutaneous doses in a range of 1 to 15 mg/kg/day (about 0.3 to 4 times the recommended human dose based on body surface area) and in rabbits at IV doses in a range of 0.6 to 6 mg/kg/day (about 0.3 to 3 times the recommended human dose based on body surface area) and have revealed desirudin to be teratogenic. Observed teratogenic findings were: omphalocele, asymmetric and fused sternebrae, edema, shortened hind limbs, etc. in rats; and spina bifida, malrotated hind limb, hydrocephaly, gastroschisis, etc. in rabbits.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desirudin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desirudin during labor and delivery.
Nursing Mothers
- It is not known whether desirudin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when desirudin is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- In three clinical studies of Iprivask, the percentage of patients greater than 65 years of age treated with 15 mg of Iprivask subcutaneously every 12 hours was 58.5%, while 20.8% were 75 years of age or older. Elderly patients treated with Iprivask had a reduction in the incidence of VTE similar to that observed in the younger patients, and a slightly lower incidence of VTE compared to those patients treated with heparin or enoxaparin.
- Regarding safety, in the clinical studies the incidence of hemorrhage (major or otherwise) in patients 65 years of age or older was similar to that in patients less than 65 years of age. In addition, the elderly had a similar incidence of total, treatment-related, or serious adverse events compared to those patients less than 65 years of age. Serious adverse events occurred more frequently in patients 75 years of age or older as compared to those less than 65 years of age. In general, 15 mg desirudin every 12 hours can be used safely in the geriatric population as in the population of patients less than 65 years of age so long as renal function is adequate.
Gender
There is no FDA guidance on the use of Desirudin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desirudin with respect to specific racial populations.
Renal Impairment
- Iprivask must be used with caution in patients with renal impairment, particularly in those with moderate and severe renal impairment (creatinine clearance ≤60 mL/min/1.73 m2 body surface area). Dose reductions by factors of three and nine are recommended for patients with moderate and severe renal impairment respectively. In addition, daily aPTT and serum creatinine monitoring are recommended for patients with moderate or severe renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desirudin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desirudin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desirudin in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
Epidural or Spinal Anesthesia
- Should the physician decide to administer anticoagulation in the context of epidural/spinal anesthesia, extreme vigilance and frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. Patients should be instructed to inform their physician immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, urgent diagnosis and treatment including spinal cord decompression should be initiated.
Renal Insufficiency
- Daily aPTT and serum creatinine monitoring are recommended for patients with moderate or severe renal impairment.
Patients with Increased Risk of Bleeding
- Activated partial thromboplastin time (aPTT) should be monitored daily in patients with increased risk of bleeding.
- Peak aPTT should not exceed two times control.
- Should peak aPTT exceed this level, dose reduction is advised based on the degree of aPTT abnormality. If necessary, therapy with desirudin should be interrupted until aPTT falls to less than two times control, at which time treatment with desirudin can be resumed at a reduced dose.
- Thrombin time (TT) is not a suitable test for routine monitoring of Iprivask therapy.
- Dose adjustments based on serum creatinine may be necessary.
Co-Administration of Agents which Increase the Risk of Bleeding
- Any agent which may enhance the risk of hemorrhage should be discontinued prior to initiation of desirudin therapy.
- These agents include medications such as Dextran 40, systemic glucocorticoids, thrombolytics, and anticoagulants.
- If co-administration cannot be avoided, close clinical and laboratory monitoring should be conducted.
Switching to or from Oral Anticoagulants
- The concomitant administration of warfarin did not significantly affect the pharmacokinetic effects of desirudin.
- When warfarin and desirudin were co-administered, greater inhibition of hemostasis measured by activated partial thromboplastin time (aPTT), prothrombin time (PT), and international normalized ratio (INR) was observed. If a patient is switched from oral anticoagulants to Iprivask therapy or from Iprivask to oral anticoagulants, the anticoagulant activity should continue to be closely monitored with appropriate methods.
IV Compatibility
There is limited information regarding IV Compatibility of Desirudin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- In an open, pilot, dose-ascending study to assess safety, the highest dose of desirudin (40 mg q12h) caused excessive hemorrhage.
- In case of overdose, most likely reflected in hemorrhagic complications or suggested by excessively high aPTT values, Iprivask therapy should be discontinued.
- Emergency procedures should be instituted as appropriate (for example, determination of aPTT and other coagulation levels, hemoglobin, the use of blood transfusion or plasma expanders).
Management
- No specific antidote for Iprivask is available; however, the anticoagulant effect of desirudin is partially reversible using thrombin-rich plasma concentrates while aPTT levels can be reduced by the IV administration of 0.3 µg/kg DDAVP (desmopressin). The clinical effectiveness of DDAVP in treating bleeding due to desirudin overdose has not been studied.
Chronic Overdose
There is limited information regarding Chronic Overdose of Desirudin in the drug label.
Pharmacology
There is limited information regarding Desirudin Pharmacology in the drug label.
Mechanism of Action
- Desirudin is a selective inhibitor of free circulating and clot-bound thrombin. The anticoagulant properties of desirudin are demonstrated by its ability to prolong the clotting time of human plasma. One molecule of desirudin binds to one molecule of thrombin and thereby blocks the thrombogenic activity of thrombin. As a result, all thrombin-dependent coagulation assays are affected. Activated partial thromboplastin time (aPTT) is a measure of the anticoagulant activity of desirudin and increases in a dose-dependent fashion.
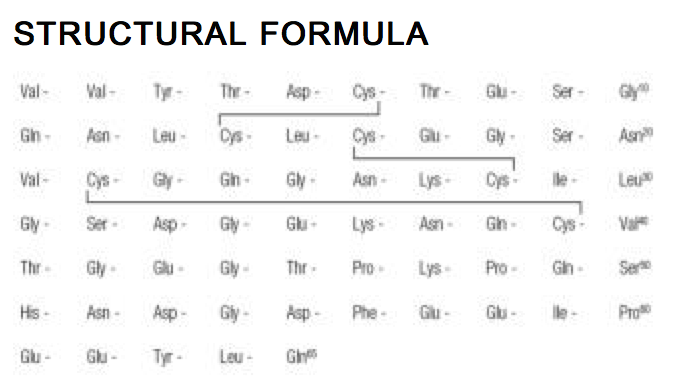
Structure
- Iprivask® (desirudin for injection) is a specific inhibitor of human thrombin. It has a protein structure that is similar to that of hirudin, the naturally occurring anticoagulant present in the peripharyngeal glands in the medicinal leech,Hirudo medicinalis. Hirudin is a single polypeptide chain of 65 amino acids residues and contains three disulfide bridges. Desirudin has a chemical formula of C287H440N80O110S6 with a molecular weight of 6963.52. Desirudin, which is expressed in yeast (Saccharomyces cerevisiae, strain TR 1456) by recombinant DNA technology differs from the natural hirudin by lack of a sulfate group on Tyr-63. The biological activity of desirudin is determined through a chromogenic assay which measures the ability of desirudin to inhibit the hydrolysis of a chromogenic peptidic substrate by thrombin in comparison to a desirudin standard. One vial of desirudin contains 15.75 mg desirudin corresponding to approximately 315,000 antithrombin units (ATU) or 20,000 ATU per milligram of desirudin with reference to the WHO International Standard (prepared 1991) for alphathrombin.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Desirudin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Desirudin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Desirudin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Desirudin in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Desirudin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Desirudin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desirudin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Desirudin in the drug label.
Precautions with Alcohol
- Alcohol-Desirudin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Iprivask®[7]
Look-Alike Drug Names
- N/A[8]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb investigators". N Engl J Med. 335 (11): 775–82. 1996. doi:10.1056/NEJM199609123351103. PMID 8778585.
- ↑ Bahit MC, Topol EJ, Califf RM, Armstrong PW, Criger DA, Hasselblad V; et al. (2001). "Reactivation of ischemic events in acute coronary syndromes: results from GUSTO-IIb. Gobal Use of Strategies To Open occluded arteries in acute coronary syndromes". J Am Coll Cardiol. 37 (4): 1001–7. PMID 11263599.
- ↑ Metz BK, White HD, Granger CB, Simes RJ, Armstrong PW, Hirsh J; et al. (1998). "Randomized comparison of direct thrombin inhibition versus heparin in conjunction with fibrinolytic therapy for acute myocardial infarction: results from the GUSTO-IIb Trial. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO-IIb) Investigators". J Am Coll Cardiol. 31 (7): 1493–8. PMID 9626825.
- ↑ McGuire DK, Emanuelsson H, Granger CB, Magnus Ohman E, Moliterno DJ, White HD; et al. (2000). "Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO-IIb study. GUSTO IIb Investigators". Eur Heart J. 21 (21): 1750–8. doi:10.1053/euhj.2000.2317. PMID 11052839.
- ↑ van den Bos AA, Deckers JW, Heyndrickx GR, Laarman GJ, Suryapranata H, Zijlstra F; et al. (1993). "Safety and efficacy of recombinant hirudin (CGP 39 393) versus heparin in patients with stable angina undergoing coronary angioplasty". Circulation. 88 (5 Pt 1): 2058–66. PMID 8222099.
- ↑ Serruys PW, Herrman JP, Simon R, Rutsch W, Bode C, Laarman GJ; et al. (1995). "A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. Helvetica Investigators". N Engl J Med. 333 (12): 757–63. doi:10.1056/NEJM199509213331203. PMID 7643882.
- ↑ "IPRIVASK (desirudin) kit".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Desirudin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Desirudin |Label Name=Desirudin11.png
}}
{{#subobject:
|Label Page=Desirudin |Label Name=Desirudin11.png
}}