Denileukin diftitox: Difference between revisions
No edit summary |
m (Protected "Denileukin diftitox": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (22 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=[[antineoplastic agent]] | |drugClass=[[antineoplastic agent]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=patients with persistent or | |indication=patients with persistent or [[cutaneous T-cell lymphoma|recurrent cutaneous T-cell lymphoma]] whose malignant cells express the CD25 component of the [[IL-2 receptor]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=[[peripheral edema]], [[pruritus]], [[rash]], [[diarrhea]], [[loss of appetite]], [[nausea]], [[Vomiting]],Increased liver enzymes, [[arthralgia]], [[backache]], [[myalgia]], [[asthenia]], [[headache]], [[cough]], [[dyspnea]],[[fatigue]], [[fever]], [[pain]], [[rigor]]. | ||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=WARNING | |blackBoxWarningTitle=WARNING | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">SERIOUS INFUSION REACTIONS, CAPILLARY LEAK SYNDROME AND LOSS OF VISUAL ACUITY. </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">SERIOUS INFUSION REACTIONS, CAPILLARY LEAK SYNDROME AND LOSS OF VISUAL ACUITY. </span></i> | ||
* The following adverse reactions have been reported: | * The following adverse reactions have been reported: | ||
:*Serious and fatal infusion reactions. Administer | :*Serious and fatal infusion reactions. Administer Denileukin in a facility equipped and staffed for cardiopulmonary resuscitation. Immediately stop and permanently discontinue Denileukin for serious infusion reactions . | ||
:*Capillary leak syndrome resulting in death. Monitor weight, edema, blood pressure and serum albumin levels prior to and during | :*Capillary leak syndrome resulting in death. Monitor weight, edema, blood pressure and serum albumin levels prior to and during Denileukin treatment. | ||
**Loss of visual acuity and color vision | **Loss of visual acuity and color vision | ||
| Line 19: | Line 20: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Cutaneous T-cell lymphoma===== | |fdaLIADAdult= | ||
* | |||
=====Cutaneous T-cell lymphoma===== | |||
*Denileukin ® is indicated for the treatment of patients with persistent or [[cutaneous T-cell lymphoma|recurrent cutaneous T-cell lymphoma]] whose malignant cells express the [[CD25]] component of the [[IL-2 receptor]] | |||
=====Dosing Schedule and Administration===== | =====Dosing Schedule and Administration===== | ||
*Premedicate with an antihistamine and acetaminophen prior to each | *Premedicate with an [[antihistamine]] and [[acetaminophen]] prior to each Denileukin infusion. | ||
*Administer at 9 or 18 mcg/kg/day by intravenous infusion over 30-60 minutes for 5 consecutive days every 21 days for 8 cycles. | *Administer at 9 or 18 mcg/kg/day by intravenous infusion over 30-60 minutes for 5 consecutive days every 21 days for 8 cycles. | ||
*Do not administer as a bolus injection. | *Do not administer as a bolus injection. | ||
*Withhold administration of | *Withhold administration of Denileukin if [[serum albumin]] levels are less than 3.0 g/dL. | ||
*Discontinue for adverse infusion reactions. | *Discontinue for adverse [[infusion reactions]]. | ||
=====Preparation and Administration===== | =====Preparation and Administration===== | ||
*Thaw vials in the refrigerator at 2 to 8°C (36 to 46°F) for not more than 24 hours or at room temperature for 1 to 2 hours. | *Thaw vials in the refrigerator at 2 to 8°C (36 to 46°F) for not more than 24 hours or at room temperature for 1 to 2 hours. | ||
*Bring | *Bring Denileukin to room temperature, before preparing the dose. | ||
*Mix the solution in the vial by gentle swirling; do not shake. | *Mix the solution in the vial by gentle swirling; do not shake. | ||
*Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Use only if the solution is clear, colorless and without visible particulate matter. After thawing, a haze may be visible which should clear when the solution is at room temperature. | *Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Use only if the solution is clear, colorless and without visible particulate matter. After thawing, a haze may be visible which should clear when the solution is at room temperature. | ||
*Do not refreeze | *Do not refreeze Denileukin after thawing. | ||
*Prepare and hold diluted | *Prepare and hold diluted Denileukin in plastic syringes or soft plastic IV bags. Do not use glass containers. | ||
*Maintain concentration of | *Maintain concentration of Denileukin at 15 mcg/mL or higher. | ||
*during all steps in the preparation of the solution for IV infusion. | *during all steps in the preparation of the solution for IV infusion. | ||
*Withdraw the calculated dose from the vial(s) and inject it into an empty IV infusion bag. Do not add more than 9 mL of sterile saline without preservative to the IV bag for each 1 mL of | *Withdraw the calculated dose from the vial(s) and inject it into an empty IV infusion bag. Do not add more than 9 mL of sterile saline without preservative to the IV bag for each 1 mL of Denileukin . | ||
*Do not mix | *Do not mix Denileukin with other drugs. | ||
*Do not administer | *Do not administer Denileukin through an in-line filter. | ||
*Administer prepared solutions of | *Administer prepared solutions of Denileukin within 6 hours, using a syringe pump or IV infusion bag. | ||
*Discard unused portions of | *Discard unused portions of Denileukin immediately. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Denileukin diftitox in adult patients. | ||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |||
|offLabelAdultNoGuideSupport= | |||
=====Chronic lymphoid leukemia===== | |||
*18 micrograms/kilogram/day intravenously infused over 60 minutes for 5 days, every 21 days, for a maximum of 8 cycles <ref name="pmid16586495">{{cite journal| author=Frankel AE, Surendranathan A, Black JH, White A, Ganjoo K, Cripe LD| title=Phase II clinical studies of denileukin diftitox diphtheria toxin fusion protein in patients with previously treated chronic lymphocytic leukemia. | journal=Cancer | year= 2006 | volume= 106 | issue= 10 | pages= 2158-64 | pmid=16586495 | doi=10.1002/cncr.21851 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16586495 }} </ref> | |||
|fdaLIADPed=Safety and effectiveness in pediatric patients have not been established. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=Safety and effectiveness in pediatric patients have not been established. | ||
|offLabelPedNoGuideSupport=Safety and effectiveness in pediatric patients have not been established. | |||
|offLabelPedNoGuideSupport= | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=None. | |contraindications=None. | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings======Infusion Reactions===== | |warnings= | ||
*Infusion reactions, defined as symptoms occurring within 24 hours of infusion and resolving within 48 hours of the last infusion in that course, were reported in 70.5% (165/234) of | |||
*For patients completing at least 4 courses of | =====Infusion Reactions===== | ||
*Resuscitative equipment should be available during | *[[Infusion reactions]], defined as symptoms occurring within 24 hours of infusion and resolving within 48 hours of the last infusion in that course, were reported in 70.5% (165/234) of Denileukin -treated patients across 3 clinical studies utilizing the approved doses and schedule. [[Infusion reaction|Serious infusion reactions]] were reported in 8.1% (19/234) of Denileukin -treated patients. There have been post-marketing reports of infusion reactions resulting in death. | ||
*For patients completing at least 4 courses of Denileukin treatment in Study 1 , the incidence of infusion reactions was lower in the 3rd and 4th cycles as compared to the 1st and 2nd cycles of Denileukin . | |||
*Resuscitative equipment should be available during Denileukin administration. Immediately stop and permanently discontinue Denileukin for serious infusion reactions. | |||
=====Capillary Leak Syndrome===== | =====Capillary Leak Syndrome===== | ||
*Capillary leak syndrome was defined as the occurrence of at least 2 of the following 3 symptoms (hypotension, edema, serum albumin <3.0 g/dL) at any time during | *[[Capillary leak syndrome]] was defined as the occurrence of at least 2 of the following 3 symptoms ([[hypotension]], [[edema]], [[serum albumin]] <3.0 g/dL) at any time during Denileukin therapy. These symptoms were not required to occur simultaneously to be characterized as [[capillary leak syndrome]]. As defined, [[capillary leak syndrome]] was reported in 32.5% (76/234) of Denileukin -treated patients. Among these 76 patients with [[capillary leak syndrome]], one-third required hospitalization or medical intervention to prevent hospitalization. There have been post-marketing reports of [[capillary leak syndrome]] resulting in death. | ||
*The onset of symptoms in patients with capillary leak syndrome may be delayed, occurring up to 2 weeks following infusion. Symptoms may persist or worsen after the cessation of | *The onset of symptoms in patients with [[capillary leak syndrome]] may be delayed, occurring up to 2 weeks following infusion. Symptoms may persist or worsen after the cessation of Denileukin . | ||
*Regularly assess patients for weight gain, new onset or worsening edema, hypotension (including orthostatic changes) and monitor serum albumin levels prior to the initiation of each course of therapy and more often as clinically indicated. Withhold | *Regularly assess patients for [[weight gain]], new onset or worsening [[edema]], [[hypotension]] (including orthostatic changes) and monitor [[serum albumin]] levels prior to the initiation of each course of therapy and more often as clinically indicated. Withhold Denileukin for serum albumin levels of less than 3.0 g/dL . | ||
=====Visual Loss===== | =====Visual Loss===== | ||
*Loss of visual acuity, usually with loss of color vision, with or without retinal pigment mottling has been reported following administration of | *[[visual acuity|Loss of visual acuity]], usually with [[color blindness|loss of color vision]], with or without [[retinal pigmentation|retinal pigment mottling]] has been reported following administration of Denileukin . Recovery was reported in some of the affected patients; however, most patients reported persistent [[visual impairment]]. | ||
=====CD25 Tumor Expression and Evaluation===== | =====CD25 Tumor Expression and Evaluation===== | ||
*Confirm that the patient's malignant cells express CD25 prior to administration of | *Confirm that the patient's malignant cells express [[CD25]] prior to administration of Denileukin . A testing service for the assay of [[CD25]] expression in tumor biopsy samples is available. For information on this service call 877-873-4724. | ||
=====Laboratory Monitoring/Hypoalbuminemia===== | =====Laboratory Monitoring/[[Hypoalbuminemia]]===== | ||
*Monitor serum albumin levels prior to the initiation of each treatment course. Withhold administration of | *Monitor serum albumin levels prior to the initiation of each treatment course. Withhold administration of Denileukin if [[serum albumin]] levels are less than 3.0 g/dL | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 141: | Line 84: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*The following adverse reactions are discussed in greater detail in other sections of the label: | |clinicalTrials=*The following adverse reactions are discussed in greater detail in other sections of the label: | ||
:*Infusion Reactions | :*[[Infusion Reactions]] | ||
:*Capillary Leak Syndrome | :*[[Capillary Leak Syndrome]] | ||
:*Visual Loss | :*[[Visual Loss]] | ||
=====Clinical Studies Experience===== | =====Clinical Studies Experience===== | ||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | *Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
*Safety data are available for 3 clinical studies in which 234 patients received | *Safety data are available for 3 clinical studies in which 234 patients received Denileukin at 9 mcg/kg (n=80) or 18 mcg/kg (n=154) at the recommended schedule. Of these studies, 1 was placebo-controlled and dose-ranging (Study 1, 100 Denileukin -treated patients), one was a dose-comparison of 9 and 18 mcg/kg (Study 2, n=71), and the third was a single-arm study using 18 mcg/kg (n=63); all studies were limited to adult patients with [[cutaneous T-cell lymphoma|CTCL]]. The median age of patients across the clinical studies was 60 years (range 23-91 years) and 36% (n=85) were 65 years of age or older; 55% were men and 85% were Caucasian. | ||
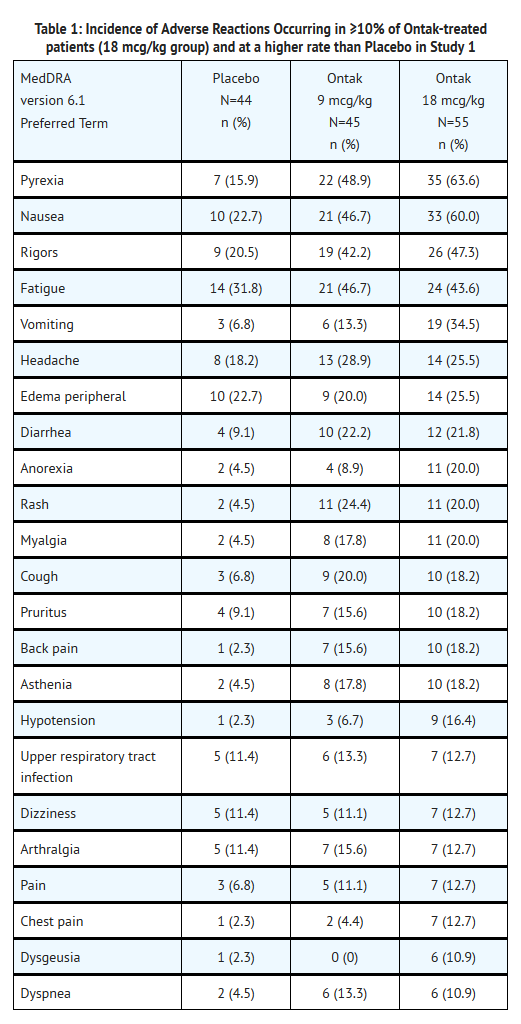
*Across all 3 studies, the most common adverse reactions in | *Across all 3 studies, the most common adverse reactions in Denileukin -treated patients (≥20%) were [[pyrexia]], [[nausea]], [[fatigue]], [[rigors]], [[vomiting]], [[diarrhea]], [[headache]], [[peripheral edema]], [[cough]], [[dyspnea]] and [[pruritus]]. The most common serious adverse reactions were [[capillary leak syndrome]] (11.1%), [[infusion reactions]] (8.1%), and visual changes including [[loss of visual acuity]] (4%). Denileukin was discontinued in 28.2% (66/234) of patients due to adverse reactions. | ||
*The data described in Table 1 reflect exposure to | *The data described in Table 1 reflect exposure to Denileukin in 100 patients administered as a single agent at the recommended dosing schedule in the randomized placebo-controlled trial (Study 1). The median number of Denileukin cycles was 7 (range 1-10) for the 9 mcg/kg cohort and 6 (range 1-11) for the 18 mcg/kg cohort. The median age of patients was 59 years (range 23-84 years) and 34% (n=34) were 65 years of age or older; 55% were men and 86% were Caucasian. | ||
: [[File:Denileukin02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
=====Hepatobiliary Disorders===== | =====Hepatobiliary Disorders===== | ||
*Increase in serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) from baseline occurred in 84% of subjects treated with | *Increase in [[alanine aminotransferase|serum alanine aminotransferase]] (ALT) or [[aspartate aminotransferase]] (AST) from baseline occurred in 84% of subjects treated with Denileukin (197/234). In the majority of subjects, these enzyme elevations occurred during either the first or the second cycle; enzyme elevation resolved without medical intervention and did not require discontinuation of Denileukin . | ||
=====Immunogenicity===== | =====Immunogenicity===== | ||
*An immune response to denileukin diftitox was assessed using 2 enzyme-linked immunoassays (ELISA). The first assay measured reactivity directed against intact denileukin diftitox calibrated against anti-diphtheria toxin, and the second assay measured reactivity against the IL-2 portion of the protein. An additional in vitro cell-based assay that measured the ability of antibodies in serum to protect a human IL-2R-expressing cell line from toxicity by denileukin diftitox, was used to detect the presence of neutralizing antibodies which inhibited functional activity. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to the intact fusion protein denileukin diftitox. These results are highly dependent on the sensitivity and the specificity of the assays. Additionally, the observed incidence of the antibody positivity may be influenced by several factors, including sample handling, concomitant medication, and underlying disease. For these reasons, the comparison of the incidence of antibodies to denileukin diftitox with the incidence of antibodies to other products may be misleading. | *An immune response to denileukin diftitox was assessed using 2 [[enzyme-linked immunoassays]] ([[ELISA]]). The first assay measured reactivity directed against intact denileukin diftitox calibrated against [[anti-diphtheria toxin]], and the second assay measured reactivity against the IL-2 portion of the protein. An additional in vitro cell-based assay that measured the ability of antibodies in serum to protect a human IL-2R-expressing cell line from toxicity by denileukin diftitox, was used to detect the presence of neutralizing antibodies which inhibited functional activity. The [[immunogenicity]] data reflect the percentage of patients whose test results were considered positive for antibodies to the intact [[fusion protein]] denileukin diftitox. These results are highly dependent on the sensitivity and the specificity of the assays. Additionally, the observed incidence of the antibody positivity may be influenced by several factors, including sample handling, concomitant medication, and underlying disease. For these reasons, the comparison of the incidence of antibodies to denileukin diftitox with the incidence of antibodies to other products may be misleading. | ||
*In Study 1 | *In Study 1, of 95 patients treated with denileukin diftitox, 66% tested positive for antibodies at baseline probably due to a prior exposure to [[diphtheria toxin]] or its vaccine. After 1, 2, and 3 courses of treatment, 94%, 99%, and 100% of patients tested positive, respectively. Mean titers of anti-denileukin diftitox antibodies were similarly increased in the 9 and 18 mcg/kg/day dose groups after 2 courses of treatment. Meanwhile, pharmacokinetic parameters decreased substantially ([[Cmax]]~57%, [[AUC]]~80%), and clearance increased 2- to 8-fold. | ||
*In Study 2 [see Clinical Studies (14.2)], 131 patients were assessed for binding antibodies. Of these, 51 patients (39%) had antibodies at baseline. Seventy-six percent of patients tested positive after 1 course of treatment and 97% after 3 courses of treatment. Neutralizing antibodies were assessed in 60 patients; 45%, 73%, and 97% had evidence of inhibited functional activity in the cellular assay at baseline and after 1 and 3 courses of treatment, respectively. | *In Study 2 [see Clinical Studies (14.2)], 131 patients were assessed for binding antibodies. Of these, 51 patients (39%) had antibodies at baseline. Seventy-six percent of patients tested positive after 1 course of treatment and 97% after 3 courses of treatment. Neutralizing antibodies were assessed in 60 patients; 45%, 73%, and 97% had evidence of inhibited functional activity in the cellular assay at baseline and after 1 and 3 courses of treatment, respectively. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=The following adverse reactions have been identified during postapproval use of | |postmarketing=*The following adverse reactions have been identified during postapproval use of Denileukin . Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
Thyroid conditions: [[hyperthyroidism]], [[thyroiditis]], [[thyrotoxicosis]], and [[hypothyroidism]]. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=* No formal drug-drug interaction studies have been conducted with | |drugInteractions=* No formal drug-drug interaction studies have been conducted with Denileukin | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* It is not known whether | |useInPregnancyFDA=* It is not known whether Denileukin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Animal reproduction studies have not been conducted with Denileukin . Denileukin should be given to a pregnant woman only if clearly needed. | ||
|useInPregnancyAUS=* There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of | |useInPregnancyAUS=* There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Denileukin in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of | |useInLaborDelivery=There is no FDA guidance on use of Denileukin during labor and delivery. | ||
|useInNursing= | |useInNursing=*It is not known whether Denileukin is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Denileukin , a decision should be made whether to discontinue nursing or to discontinue Denileukin , taking into account the importance of the drug to the mother. | ||
|useInPed= | |useInPed=Safety and effectiveness in pediatric patients have not been established. | ||
|useInGeri= | |useInGeri=*Clinical studies of Denileukin did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. | ||
|useInGender=There is no FDA guidance on the use of | |useInGender=There is no FDA guidance on the use of Denileukin with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of | |useInRace=There is no FDA guidance on the use of Denileukin with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of | |useInRenalImpair=There is no FDA guidance on the use of Denileukin in patients with [[renal impairment]]. | ||
|useInHepaticImpair=There is no FDA guidance on the use of | |useInHepaticImpair=There is no FDA guidance on the use of Denileukin in patients with [[hepatic impairment]]. | ||
|useInReproPotential=There is no FDA guidance on the use of | |useInReproPotential=There is no FDA guidance on the use of Denileukin in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of | |useInImmunocomp=There is no FDA guidance one the use of Denileukin in patients who are [[immunocompromised]]. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* Intravenous | ||
|monitoring=*Infusion reactions: Immediately stop and permanently discontinue Denileukin for serious infusion reactions. Monitor patients following infusion. | |||
* | *[[Capillary leak syndrome]]: Monitor weight, [[edema]], [[blood pressure]] and [[serum albumin]] levels. | ||
| | *[[Visual Acuity|Loss of Visual Acuity]] and [[color blindness|Color Vision]]: Monitor visual acuity and color vision. | ||
*Laboratory Tests: Monitor [[serum albumin]] levels prior to the initiation of each treatment course. Delay administration of Denileukin until [[serum albumin]] levels are at least 3.0 g/dL | |||
* | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of Denileukin in the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=*Doses of approximately twice the recommended dose (31 mcg/kg/day) resulted in moderate-to-severe [[nausea]], [[vomiting]], [[fever]], [[chills]] and/or persistent [[asthenia]]. | ||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 477002950 | |||
| IUPAC_name = Diphtheria toxin-Interleukin-2 fusion protein | |||
| image = | |||
==== | <!--Clinical data--> | ||
| tradename = Ontak | |||
| Drugs.com = {{drugs.com|monograph|denileukin_diftitox}} | |||
| MedlinePlus = a611024 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> C | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = Intravenous | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = 70-80 min | |||
| excretion = | |||
=== | <!--Identifiers--> | ||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 173146-27-5 | |||
| ATC_prefix = L01 | |||
| ATC_suffix = XX29 | |||
| ATC_supplemental = | |||
| PubChem = | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00004 | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = 25E79B5CTM | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201550 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = NA | |||
<!--Chemical data--> | |||
| C=2560 | H=4042 | N=678 | O=799 | S=17 | |||
| molecular_weight = 57647.3 g/mol | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction=*Denileukin diftitox is a fusion protein designed to direct the [[cytocidal action]] of [[diphtheria toxin]] to cells which express the [[IL-2 receptor]]. Ex vivo studies report that after binding to the [[IL-2 receptor]] on the cell surface, denileukin diftitox is internalized by [[receptor-mediated endocytosis]]. The fusion protein is subsequently cleaved, releasing [[diphtheria toxin]] enzymatic and translocation domains from the IL-2 fragment, resulting in the [[inhibition of protein synthesis]] and ultimately, cell death. | |||
|mechAction=* | |||
<!--Structure--> | <!--Structure--> | ||
|structure=* | |structure=* Denileukin , is a recombinant DNA-derived cytotoxic protein composed of the amino acid sequences for [[diphtheria toxin]] fragments A and B (Met1-Thr387)-His and the sequences for human [[interleukin-2]] (IL-2; Ala1-Thr133). It is produced in an E. coli expression system and has a molecular weight of 58 kD. [[Neomycin]] is used in the fermentation process but is undetectable in the final product. Denileukin is supplied in single use vials as a sterile, frozen solution intended for intravenous (IV) administration. Each 2 mL vial of Denileukin contains 300 mcg of recombinant denileukin diftitox in a sterile solution of citric acid (20 mM), [[EDTA]] (0.05 mM) and polysorbate 20 (<1%) in Water for Injection, USP. The solution has a pH range of 6.9 to 7.2. | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of | |PD=There is limited information regarding <i>Pharmacodynamics</i> of Denileukin in the drug label. | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK=*Pharmacokinetic parameters associated with denileukin diftitox were determined over a range of doses (3 to 31 mcg/kg/day) in patients with [[lymphoma]]. Denileukin diftitox was administered as an IV infusion following the schedule used in the clinical trials. Following the first dose, denileukin diftitox displayed 2-compartment behavior with a distribution phase (half-life approximately 2 to 5 minutes) and a terminal phase (half-life approximately 70 to 80 minutes). Systemic exposure was variable but proportional to dose. Mean clearance was approximately 0.6 to 2.0 mL/min/kg and the [[volume of distribution|mean volume of distribution]] was similar to that of circulating blood (0.06 to 0.09 L/kg). The mean clearance increased approximately 2- to 8-fold from course 1 to course 3 corresponding to a decrease in exposure of approximately 75%. No accumulation was evident between the first and fifth doses. Gender and age have no effect on pharmacokinetics of denileukin diftitox. | ||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*There have been no studies to assess the carcinogenic potential of denileukin diftitox. Denileukin diftitox showed no evidence of [[mutagenicity]] in the [[Ames test]] and the [[chromosomal aberration assay]]. There have been no studies to assess the effect of denileukin diftitox on [[fertility]]. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies= | ||
=====Study 1: Placebo Controlled Study in CTCL (Stage Ia to III) Patients===== | |||
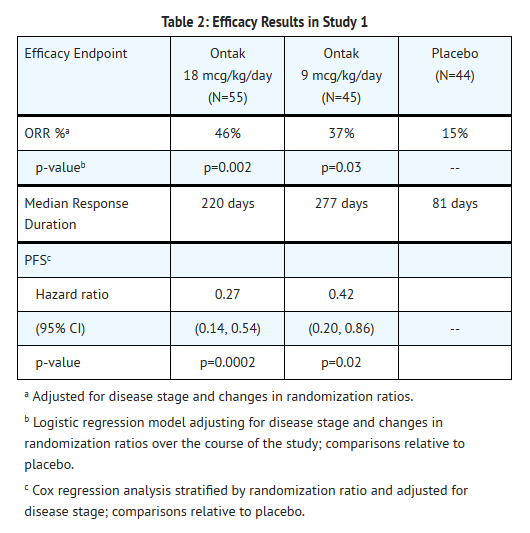
*The safety and efficacy of Denileukin were evaluated in a randomized, double-blind, placebo-controlled, 3-arm trial in patients with Stage Ia to III CD25(+) CTCL. Eligible patients were required to have expression of CD25 on ≥ 20% of biopsied malignant cells by [[immunohistochemistry]] . Patients were randomized to receive 0, 9 or 18 mcg/kg/day Denileukin via intravenous infusion days 1-5 of each 21-day cycle, for up to 8 cycles. Randomization was stratified by disease stage (≤IIa vs. ≥IIb). The main efficacy outcome was objective response rate (ORR), using a Weighted Skin Severity Index, in conjunction with assessment of lymph node involvement and percentage of abnormal blood lymphocytes. A total of 144 patients were randomized: 44 patients to placebo, 45 patients to 9 mcg/kg/day Denileukin and 55 patients to 18 mcg/kg/day Denileukin . Randomization for the study was carried out at 1:1:1 for the first 73 patients, 4:1:4 for the next 31 patients, and 1:4:4 for the remaining 40 patients. The median age of patients was 59 years (range 23 to 84 years); 34% were ≥ 65 years. Fifty-five percent were men and 86% were Caucasian. Sixty-seven percent had early stage disease (≤ IIa). Patients had received a median of 2 anti-CTCL therapies (range 0 to 6) prior to study entry. Results for objective response rate (ORR) and progression-free survival (PFS) are shown in the table below. | |||
: [[File:Denileukin03.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
=====Study 2: Dose Evaluation Study in CTCL (Stage IIb to IVa) Patients===== | |||
*A randomized, double-blind study was conducted to evaluate doses of 9 or 18 mcg/kg/day in 71 patients with recurrent or persistent, Stage Ib to IVa [[cutaneous T-cell lymphoma|CTCL]]. Entry to this study required demonstration of [[CD25|CD25 expression]] on at least 20% of the cells in any relevant tumor tissue sample (skin biopsy) or circulating cells. Tumor biopsies were not evaluated for expression of other IL-2 receptor subunit components ([[CD122]]/CD132). Denileukin was administered as an IV infusion daily for 5 days every 3 weeks. Patients received a median of 6 courses of Denileukin therapy (range 1 to 11). The study population had received a median of 5 prior therapies (range 1 to 12) with 63% of patients entering the trial with Stage IIb or more advanced stage disease. The median age of patients was 64 years (range 26 to 91 years); 49% were ≥ 65 years. Fifty-two percent were men and 75% were Caucasian. | |||
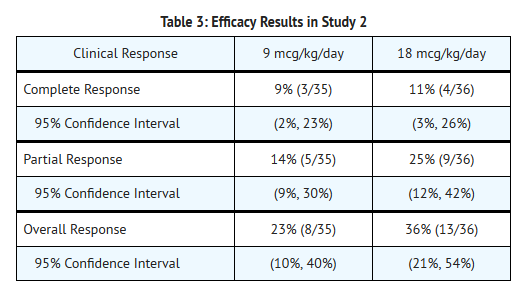
*Overall, 30% (95% CI: 18-41%) of patients treated with Denileukin experienced an objective tumor response (50% reduction in tumor burden which was sustained for ≥6 weeks; Table 3). Seven patients (10%) achieved a complete response and 14 patients (20%) achieved a partial response. The overall median duration of response, measured from first day of response, was 4 months with a median duration for complete response of 9 months and for partial response of 4 months. | |||
: [[File:Denileukin04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=*Denileukin is supplied as 150 mcg/ml, sterile, frozen solution (300 mcg in 2 mL) in a sterile single-use vial. | ||
*NDC 62856-603-01, 6 vials in a package. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo= | |storage=*Store frozen at or below -10°C (14°F) | ||
|fdaPatientInfo=*Advise patients to report: | |||
:*[[Fever]], [[chills]], breathing problems, [[chest pain]], [[tachycardia]], and [[urticaria]] following infusion. | |||
:*[[weight gain|Rapid weight gain]], [[edema]], and [[orthostatic hypotension]] following infusion. Instruct patients to weigh themselves daily. | |||
:*[[Visual loss]], including [[loss of color vision]]. | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol- | |alcohol=* Alcohol-Denileukin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* ® | |brandNames=*Ontak ® | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
| Line 303: | Line 239: | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Denileukin05.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=Denileukin06.png | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Chemotherapeutic agents]] | |||
Latest revision as of 19:41, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS INFUSION REACTIONS, CAPILLARY LEAK SYNDROME AND LOSS OF VISUAL ACUITY.
|
Overview
Denileukin diftitox is an antineoplastic agent that is FDA approved for the treatment of patients with persistent or recurrent cutaneous T-cell lymphoma whose malignant cells express the CD25 component of the IL-2 receptor. There is a Black Box Warning for this drug as shown here. Common adverse reactions include peripheral edema, pruritus, rash, diarrhea, loss of appetite, nausea, Vomiting,Increased liver enzymes, arthralgia, backache, myalgia, asthenia, headache, cough, dyspnea,fatigue, fever, pain, rigor..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cutaneous T-cell lymphoma
- Denileukin ® is indicated for the treatment of patients with persistent or recurrent cutaneous T-cell lymphoma whose malignant cells express the CD25 component of the IL-2 receptor
Dosing Schedule and Administration
- Premedicate with an antihistamine and acetaminophen prior to each Denileukin infusion.
- Administer at 9 or 18 mcg/kg/day by intravenous infusion over 30-60 minutes for 5 consecutive days every 21 days for 8 cycles.
- Do not administer as a bolus injection.
- Withhold administration of Denileukin if serum albumin levels are less than 3.0 g/dL.
- Discontinue for adverse infusion reactions.
Preparation and Administration
- Thaw vials in the refrigerator at 2 to 8°C (36 to 46°F) for not more than 24 hours or at room temperature for 1 to 2 hours.
- Bring Denileukin to room temperature, before preparing the dose.
- Mix the solution in the vial by gentle swirling; do not shake.
- Visually inspect for particulate matter and discoloration prior to administration, whenever solution and container permit. Use only if the solution is clear, colorless and without visible particulate matter. After thawing, a haze may be visible which should clear when the solution is at room temperature.
- Do not refreeze Denileukin after thawing.
- Prepare and hold diluted Denileukin in plastic syringes or soft plastic IV bags. Do not use glass containers.
- Maintain concentration of Denileukin at 15 mcg/mL or higher.
- during all steps in the preparation of the solution for IV infusion.
- Withdraw the calculated dose from the vial(s) and inject it into an empty IV infusion bag. Do not add more than 9 mL of sterile saline without preservative to the IV bag for each 1 mL of Denileukin .
- Do not mix Denileukin with other drugs.
- Do not administer Denileukin through an in-line filter.
- Administer prepared solutions of Denileukin within 6 hours, using a syringe pump or IV infusion bag.
- Discard unused portions of Denileukin immediately.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Denileukin diftitox in adult patients.
Non–Guideline-Supported Use
Chronic lymphoid leukemia
- 18 micrograms/kilogram/day intravenously infused over 60 minutes for 5 days, every 21 days, for a maximum of 8 cycles [1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Safety and effectiveness in pediatric patients have not been established.
Non–Guideline-Supported Use
Safety and effectiveness in pediatric patients have not been established.
Contraindications
None.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS INFUSION REACTIONS, CAPILLARY LEAK SYNDROME AND LOSS OF VISUAL ACUITY.
|
Infusion Reactions
- Infusion reactions, defined as symptoms occurring within 24 hours of infusion and resolving within 48 hours of the last infusion in that course, were reported in 70.5% (165/234) of Denileukin -treated patients across 3 clinical studies utilizing the approved doses and schedule. Serious infusion reactions were reported in 8.1% (19/234) of Denileukin -treated patients. There have been post-marketing reports of infusion reactions resulting in death.
- For patients completing at least 4 courses of Denileukin treatment in Study 1 , the incidence of infusion reactions was lower in the 3rd and 4th cycles as compared to the 1st and 2nd cycles of Denileukin .
- Resuscitative equipment should be available during Denileukin administration. Immediately stop and permanently discontinue Denileukin for serious infusion reactions.
Capillary Leak Syndrome
- Capillary leak syndrome was defined as the occurrence of at least 2 of the following 3 symptoms (hypotension, edema, serum albumin <3.0 g/dL) at any time during Denileukin therapy. These symptoms were not required to occur simultaneously to be characterized as capillary leak syndrome. As defined, capillary leak syndrome was reported in 32.5% (76/234) of Denileukin -treated patients. Among these 76 patients with capillary leak syndrome, one-third required hospitalization or medical intervention to prevent hospitalization. There have been post-marketing reports of capillary leak syndrome resulting in death.
- The onset of symptoms in patients with capillary leak syndrome may be delayed, occurring up to 2 weeks following infusion. Symptoms may persist or worsen after the cessation of Denileukin .
- Regularly assess patients for weight gain, new onset or worsening edema, hypotension (including orthostatic changes) and monitor serum albumin levels prior to the initiation of each course of therapy and more often as clinically indicated. Withhold Denileukin for serum albumin levels of less than 3.0 g/dL .
Visual Loss
- Loss of visual acuity, usually with loss of color vision, with or without retinal pigment mottling has been reported following administration of Denileukin . Recovery was reported in some of the affected patients; however, most patients reported persistent visual impairment.
CD25 Tumor Expression and Evaluation
- Confirm that the patient's malignant cells express CD25 prior to administration of Denileukin . A testing service for the assay of CD25 expression in tumor biopsy samples is available. For information on this service call 877-873-4724.
Laboratory Monitoring/Hypoalbuminemia
- Monitor serum albumin levels prior to the initiation of each treatment course. Withhold administration of Denileukin if serum albumin levels are less than 3.0 g/dL
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the label:
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Safety data are available for 3 clinical studies in which 234 patients received Denileukin at 9 mcg/kg (n=80) or 18 mcg/kg (n=154) at the recommended schedule. Of these studies, 1 was placebo-controlled and dose-ranging (Study 1, 100 Denileukin -treated patients), one was a dose-comparison of 9 and 18 mcg/kg (Study 2, n=71), and the third was a single-arm study using 18 mcg/kg (n=63); all studies were limited to adult patients with CTCL. The median age of patients across the clinical studies was 60 years (range 23-91 years) and 36% (n=85) were 65 years of age or older; 55% were men and 85% were Caucasian.
- Across all 3 studies, the most common adverse reactions in Denileukin -treated patients (≥20%) were pyrexia, nausea, fatigue, rigors, vomiting, diarrhea, headache, peripheral edema, cough, dyspnea and pruritus. The most common serious adverse reactions were capillary leak syndrome (11.1%), infusion reactions (8.1%), and visual changes including loss of visual acuity (4%). Denileukin was discontinued in 28.2% (66/234) of patients due to adverse reactions.
- The data described in Table 1 reflect exposure to Denileukin in 100 patients administered as a single agent at the recommended dosing schedule in the randomized placebo-controlled trial (Study 1). The median number of Denileukin cycles was 7 (range 1-10) for the 9 mcg/kg cohort and 6 (range 1-11) for the 18 mcg/kg cohort. The median age of patients was 59 years (range 23-84 years) and 34% (n=34) were 65 years of age or older; 55% were men and 86% were Caucasian.
Hepatobiliary Disorders
- Increase in serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) from baseline occurred in 84% of subjects treated with Denileukin (197/234). In the majority of subjects, these enzyme elevations occurred during either the first or the second cycle; enzyme elevation resolved without medical intervention and did not require discontinuation of Denileukin .
Immunogenicity
- An immune response to denileukin diftitox was assessed using 2 enzyme-linked immunoassays (ELISA). The first assay measured reactivity directed against intact denileukin diftitox calibrated against anti-diphtheria toxin, and the second assay measured reactivity against the IL-2 portion of the protein. An additional in vitro cell-based assay that measured the ability of antibodies in serum to protect a human IL-2R-expressing cell line from toxicity by denileukin diftitox, was used to detect the presence of neutralizing antibodies which inhibited functional activity. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to the intact fusion protein denileukin diftitox. These results are highly dependent on the sensitivity and the specificity of the assays. Additionally, the observed incidence of the antibody positivity may be influenced by several factors, including sample handling, concomitant medication, and underlying disease. For these reasons, the comparison of the incidence of antibodies to denileukin diftitox with the incidence of antibodies to other products may be misleading.
- In Study 1, of 95 patients treated with denileukin diftitox, 66% tested positive for antibodies at baseline probably due to a prior exposure to diphtheria toxin or its vaccine. After 1, 2, and 3 courses of treatment, 94%, 99%, and 100% of patients tested positive, respectively. Mean titers of anti-denileukin diftitox antibodies were similarly increased in the 9 and 18 mcg/kg/day dose groups after 2 courses of treatment. Meanwhile, pharmacokinetic parameters decreased substantially (Cmax~57%, AUC~80%), and clearance increased 2- to 8-fold.
- In Study 2 [see Clinical Studies (14.2)], 131 patients were assessed for binding antibodies. Of these, 51 patients (39%) had antibodies at baseline. Seventy-six percent of patients tested positive after 1 course of treatment and 97% after 3 courses of treatment. Neutralizing antibodies were assessed in 60 patients; 45%, 73%, and 97% had evidence of inhibited functional activity in the cellular assay at baseline and after 1 and 3 courses of treatment, respectively.
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Denileukin . Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Thyroid conditions: hyperthyroidism, thyroiditis, thyrotoxicosis, and hypothyroidism.
Drug Interactions
- No formal drug-drug interaction studies have been conducted with Denileukin
Use in Specific Populations
Pregnancy
- It is not known whether Denileukin can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Animal reproduction studies have not been conducted with Denileukin . Denileukin should be given to a pregnant woman only if clearly needed.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Denileukin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Denileukin during labor and delivery.
Nursing Mothers
- It is not known whether Denileukin is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from Denileukin , a decision should be made whether to discontinue nursing or to discontinue Denileukin , taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Denileukin did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Denileukin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Denileukin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Denileukin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Denileukin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Denileukin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Denileukin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Infusion reactions: Immediately stop and permanently discontinue Denileukin for serious infusion reactions. Monitor patients following infusion.
- Capillary leak syndrome: Monitor weight, edema, blood pressure and serum albumin levels.
- Loss of Visual Acuity and Color Vision: Monitor visual acuity and color vision.
- Laboratory Tests: Monitor serum albumin levels prior to the initiation of each treatment course. Delay administration of Denileukin until serum albumin levels are at least 3.0 g/dL
IV Compatibility
There is limited information regarding IV Compatibility of Denileukin in the drug label.
Overdosage
- Doses of approximately twice the recommended dose (31 mcg/kg/day) resulted in moderate-to-severe nausea, vomiting, fever, chills and/or persistent asthenia.
Pharmacology
Denileukin diftitox
| |
| Systematic (IUPAC) name | |
| Diphtheria toxin-Interleukin-2 fusion protein | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 57647.3 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 70-80 min |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous |
Mechanism of Action
- Denileukin diftitox is a fusion protein designed to direct the cytocidal action of diphtheria toxin to cells which express the IL-2 receptor. Ex vivo studies report that after binding to the IL-2 receptor on the cell surface, denileukin diftitox is internalized by receptor-mediated endocytosis. The fusion protein is subsequently cleaved, releasing diphtheria toxin enzymatic and translocation domains from the IL-2 fragment, resulting in the inhibition of protein synthesis and ultimately, cell death.
Structure
- Denileukin , is a recombinant DNA-derived cytotoxic protein composed of the amino acid sequences for diphtheria toxin fragments A and B (Met1-Thr387)-His and the sequences for human interleukin-2 (IL-2; Ala1-Thr133). It is produced in an E. coli expression system and has a molecular weight of 58 kD. Neomycin is used in the fermentation process but is undetectable in the final product. Denileukin is supplied in single use vials as a sterile, frozen solution intended for intravenous (IV) administration. Each 2 mL vial of Denileukin contains 300 mcg of recombinant denileukin diftitox in a sterile solution of citric acid (20 mM), EDTA (0.05 mM) and polysorbate 20 (<1%) in Water for Injection, USP. The solution has a pH range of 6.9 to 7.2.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Denileukin in the drug label.
Pharmacokinetics
- Pharmacokinetic parameters associated with denileukin diftitox were determined over a range of doses (3 to 31 mcg/kg/day) in patients with lymphoma. Denileukin diftitox was administered as an IV infusion following the schedule used in the clinical trials. Following the first dose, denileukin diftitox displayed 2-compartment behavior with a distribution phase (half-life approximately 2 to 5 minutes) and a terminal phase (half-life approximately 70 to 80 minutes). Systemic exposure was variable but proportional to dose. Mean clearance was approximately 0.6 to 2.0 mL/min/kg and the mean volume of distribution was similar to that of circulating blood (0.06 to 0.09 L/kg). The mean clearance increased approximately 2- to 8-fold from course 1 to course 3 corresponding to a decrease in exposure of approximately 75%. No accumulation was evident between the first and fifth doses. Gender and age have no effect on pharmacokinetics of denileukin diftitox.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- There have been no studies to assess the carcinogenic potential of denileukin diftitox. Denileukin diftitox showed no evidence of mutagenicity in the Ames test and the chromosomal aberration assay. There have been no studies to assess the effect of denileukin diftitox on fertility.
Clinical Studies
Study 1: Placebo Controlled Study in CTCL (Stage Ia to III) Patients
- The safety and efficacy of Denileukin were evaluated in a randomized, double-blind, placebo-controlled, 3-arm trial in patients with Stage Ia to III CD25(+) CTCL. Eligible patients were required to have expression of CD25 on ≥ 20% of biopsied malignant cells by immunohistochemistry . Patients were randomized to receive 0, 9 or 18 mcg/kg/day Denileukin via intravenous infusion days 1-5 of each 21-day cycle, for up to 8 cycles. Randomization was stratified by disease stage (≤IIa vs. ≥IIb). The main efficacy outcome was objective response rate (ORR), using a Weighted Skin Severity Index, in conjunction with assessment of lymph node involvement and percentage of abnormal blood lymphocytes. A total of 144 patients were randomized: 44 patients to placebo, 45 patients to 9 mcg/kg/day Denileukin and 55 patients to 18 mcg/kg/day Denileukin . Randomization for the study was carried out at 1:1:1 for the first 73 patients, 4:1:4 for the next 31 patients, and 1:4:4 for the remaining 40 patients. The median age of patients was 59 years (range 23 to 84 years); 34% were ≥ 65 years. Fifty-five percent were men and 86% were Caucasian. Sixty-seven percent had early stage disease (≤ IIa). Patients had received a median of 2 anti-CTCL therapies (range 0 to 6) prior to study entry. Results for objective response rate (ORR) and progression-free survival (PFS) are shown in the table below.
Study 2: Dose Evaluation Study in CTCL (Stage IIb to IVa) Patients
- A randomized, double-blind study was conducted to evaluate doses of 9 or 18 mcg/kg/day in 71 patients with recurrent or persistent, Stage Ib to IVa CTCL. Entry to this study required demonstration of CD25 expression on at least 20% of the cells in any relevant tumor tissue sample (skin biopsy) or circulating cells. Tumor biopsies were not evaluated for expression of other IL-2 receptor subunit components (CD122/CD132). Denileukin was administered as an IV infusion daily for 5 days every 3 weeks. Patients received a median of 6 courses of Denileukin therapy (range 1 to 11). The study population had received a median of 5 prior therapies (range 1 to 12) with 63% of patients entering the trial with Stage IIb or more advanced stage disease. The median age of patients was 64 years (range 26 to 91 years); 49% were ≥ 65 years. Fifty-two percent were men and 75% were Caucasian.
- Overall, 30% (95% CI: 18-41%) of patients treated with Denileukin experienced an objective tumor response (50% reduction in tumor burden which was sustained for ≥6 weeks; Table 3). Seven patients (10%) achieved a complete response and 14 patients (20%) achieved a partial response. The overall median duration of response, measured from first day of response, was 4 months with a median duration for complete response of 9 months and for partial response of 4 months.
How Supplied
- Denileukin is supplied as 150 mcg/ml, sterile, frozen solution (300 mcg in 2 mL) in a sterile single-use vial.
- NDC 62856-603-01, 6 vials in a package.
Storage
- Store frozen at or below -10°C (14°F)
Images
Drug Images
{{#ask: Page Name::Denileukin diftitox |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Denileukin diftitox |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients to report:
- Fever, chills, breathing problems, chest pain, tachycardia, and urticaria following infusion.
- Rapid weight gain, edema, and orthostatic hypotension following infusion. Instruct patients to weigh themselves daily.
- Visual loss, including loss of color vision.
Precautions with Alcohol
- Alcohol-Denileukin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Ontak ®
Look-Alike Drug Names
There is limited information regarding Denileukin diftitox Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Frankel AE, Surendranathan A, Black JH, White A, Ganjoo K, Cripe LD (2006). "Phase II clinical studies of denileukin diftitox diphtheria toxin fusion protein in patients with previously treated chronic lymphocytic leukemia". Cancer. 106 (10): 2158–64. doi:10.1002/cncr.21851. PMID 16586495.
{{#subobject:
|Page Name=Denileukin diftitox
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Denileukin diftitox |Label Name=Denileukin05.png
}}
{{#subobject:
|Label Page=Denileukin diftitox |Label Name=Denileukin06.png
}}