Sarcoidosis
| Sarcoidosis | |
 | |
|---|---|
| Sarcoidosis in a Lymph Node. | |
| ICD-10 | D86 |
| ICD-9 | 135 |
| OMIM | 181000 |
| DiseasesDB | 11797 |
| MedlinePlus | 000076 |
| eMedicine | med/2063 |
| MeSH | D012507 |
|
WikiDoc Resources for Sarcoidosis |
|
Articles |
|---|
|
Most recent articles on Sarcoidosis Most cited articles on Sarcoidosis |
|
Media |
|
Powerpoint slides on Sarcoidosis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sarcoidosis at Clinical Trials.gov Clinical Trials on Sarcoidosis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sarcoidosis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sarcoidosis Discussion groups on Sarcoidosis Patient Handouts on Sarcoidosis Directions to Hospitals Treating Sarcoidosis Risk calculators and risk factors for Sarcoidosis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sarcoidosis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2] and Hilary Womble, M. D.[[3]]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
for the heart in Sarcoidosis click here
Overview
Sarcoidosis, also called sarcoid (from the Greek 'sark' and 'oid' meaning "flesh-like") or Besnier-Boeck disease, is an immune system disorder characterised by non-caseating granulomas (small inflammatory nodules) that most commonly arises in young adults. The cause of the disease is still unknown. Virtually any organ can be affected; however, granulomas most often appear in the lungs (D86.0) or the lymph nodes (D86.1). Symptoms can occasionally appear suddenly but usually appear gradually. The clinical course varies and ranges from asymptomatic disease that resolves spontaneously to a debilitating chronic condition that may lead to death.[1]
Epidemiology
Sarcoidosis most commonly affects young adults of both sexes, with a slight preponderance for women having been reported by most studies. Incidence is highest for individuals younger than 40 and peaks in the age-group from 20 to 29 years.[2]
Sarcoidosis occurs throughout the world in all races with a prevalence ranging from 1 to 40 per 100,000. The disease is most prevalent in Northern European countries, and the highest annual incidence of 60 per 100,000 is found in Sweden and Iceland. In the United States, sarcoidosis is more common in people of African descent than Caucasians, with annual incidence reported as 35.5 and 10.9 per 100,000, respectively.[3] Sarcoidosis is less commonly reported in South America, Spain and India.
The differing incidence across the world may be at least partially attributable to the lack of screening programs in certain regions of the world and the overshadowing presence of other granulolomatous diseases such as tuberculosis, that may interfere with the diagnosis of sarcoidosis where they are prevalent.[2]
There may also be racial differences in the severity of the disease. Several studies suggest that the presentation in people of African origin may be more severe than for Caucasians, who are more likely to suffer from asymptomatic disease.[4]
Signs and symptoms
Sarcoidosis is a Systemic Disease that can affect any organ. Common symptoms are vague, such as fatigue unchanged by sleep, lack of energy, weight loss, aches and pains, arthralgia, dry eyes, blurry vision, shortness of breath, a dry hacking cough or skin lesions. The cutaneous symptoms vary, and range from rashes and noduli (small bumps) to erythema nodosum or lupus pernio. It is often asymptomatic.
The combination of erythema nodosum, bilateral hilar lymphadenopathy and arthralgia is called Lofgren syndrome. This syndrome has a relatively good prognosis.
Renal, liver (including portal hypertension), heart or brain involvement may cause further symptoms and altered functioning. Manifestations in the eye include uveitis and retinal inflammation, which may result in loss of visual acuity or blindness. Sarcoidosis affecting the brain or nerves is known as neurosarcoidosis.
The combination of anterior uveitis, parotitis and fever is called uveoparotitis, and is associated with Heerfordt-Waldenstrom syndrome. (D86.8)
Investigations
Hypercalcemia (high calcium levels) and its symptoms may be the result of excessive vitamin D activation.
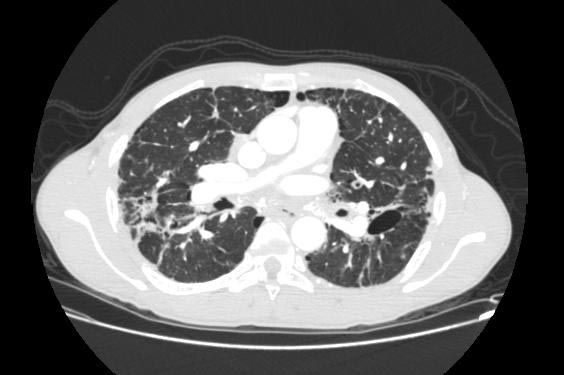
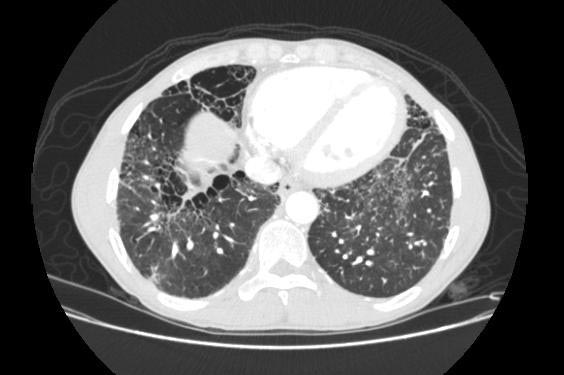
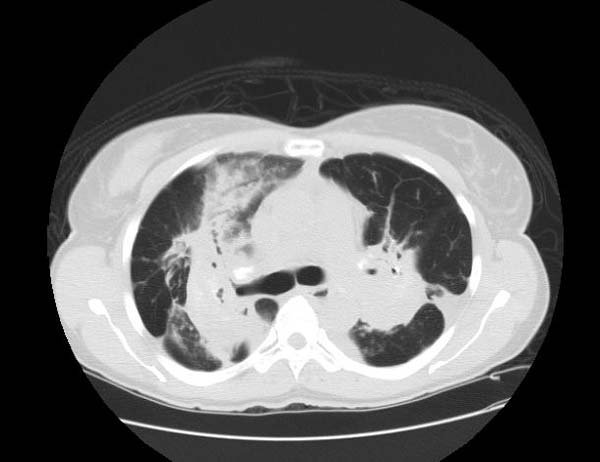
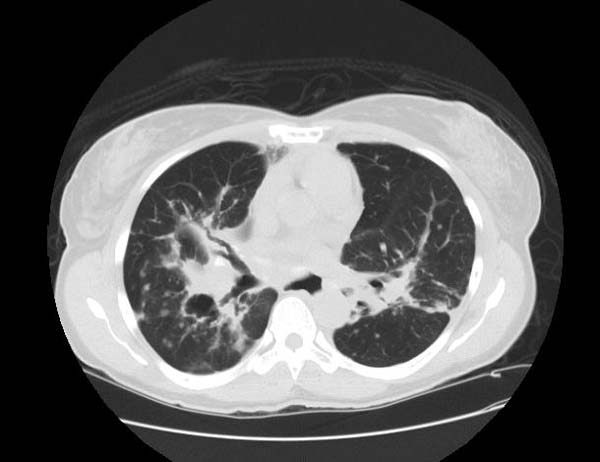
Sarcoidosis most often manifests as a restrictive disease of the lungs, causing a decrease in lung volume and decreased compliance (the ability to stretch). The disease typically limits the amount of air drawn into the lungs, but produces higher than normal expiratory flow ratios. The vital capacity (full breath in, to full breath out) is decreased, and most of this air can be blown out in the first second. This means the FEV1/FVC ratio is increased from the normal of about 80%, to 90%. Obstructive lung changes, causing a decrease in the amount of air that can be exhaled, may occur when enlarged lymph nodes in the chest compress airways or when internal inflammation or nodules impede airflow.
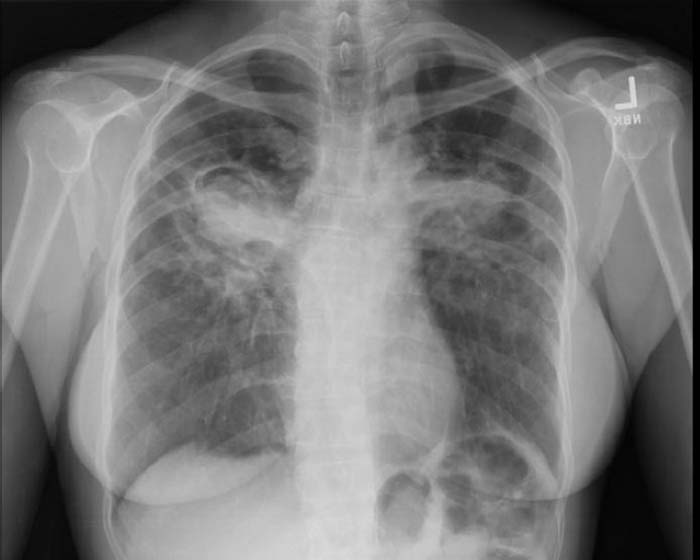
Chest X-ray changes are divided into five stages
- Stage 0 Negative chest radiograph
- Stage 1 bihilar lymphadenopathy
- Stage 2 Hilar lymphadenopathy and reticulonodular pulmonary infiltrates
- Stage 3 bilateral infiltrates
- Stage 4 fibrocystic sarcoidosis typically with upward hilar retraction, cystic & bullous changes
Because sarcoidosis can affect multiple organ systems, follow-up on a patient with sarcoidosis should always include an electrocardiogram, ophthalmologic exam, liver function tests, serum calcium and 24-hour urine calcium.
Causes and pathophysiology
No direct cause of sarcoidosis has been identified, although there have been reports of cell wall deficient bacteria that may be possible pathogens.[5] These bacteria are not identified in standard laboratory analysis. It has been thought that there may be a hereditary factor because some families have multiple members with sarcoidosis. To date, no reliable genetic markers have been identified, and an alternate hypothesis is that family members share similar exposures to environmental pathogens. There have also been reports of transmission of sarcoidosis via organ transplants.[6]
Sarcoidosis frequently causes a dysregulation of vitamin D production with an increase in extrarenal (outside the kidney) production.[7] Specifically, macrophages inside the granulomas convert vitamin D to its active form, resulting in elevated levels of the hormone 1,25-dihydroxyvitamin D and symptoms of hypervitaminosis D that may include fatigue, lack of strength or energy, irritability, metallic taste, temporary memory loss or cognitive problems. Physiological compensatory responses (e.g. suppression of the parathyroid hormone levels) may mean the patient does not develop frank hypercalcemia.
Sarcoidosis has been associated with celiac disease. Celiac disease is a condition in which there is a chronic reaction to certain protein chains, commonly referred to as glutens, found in some cereal grains. This reaction causes destruction of the villi in the small intestine, with resulting malabsorption of nutrients.
While disputed, some cases have been determined to be caused by inhalation of the dust from the collapse of the World Trade Center after the September 11, 2001 attacks.[8] See Health effects arising from the September 11, 2001 attacks for more information.
Gallium-67 citrate is useful for diagnosing suspected sarcoidosis and evaluation of treatment response. It is more sensitive than radiographic images on diagnosis of Sarcoidosis.
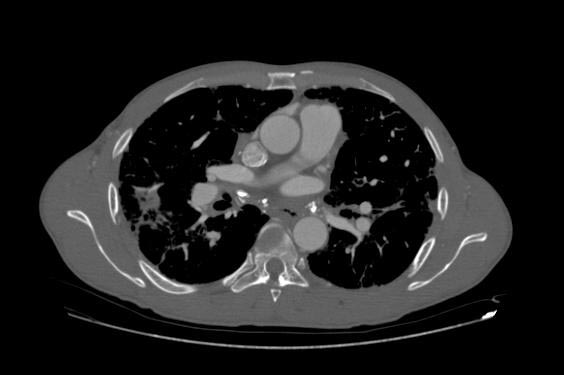
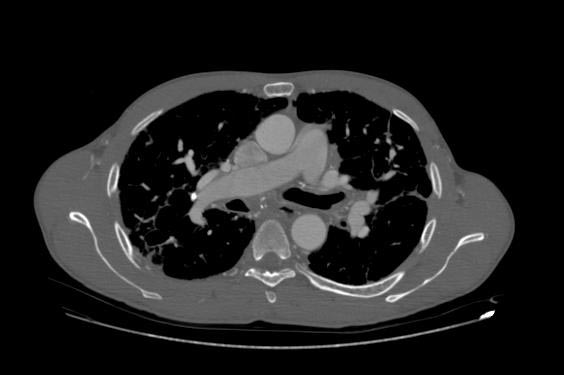
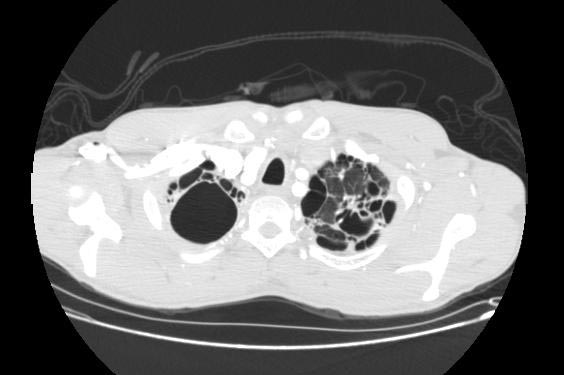
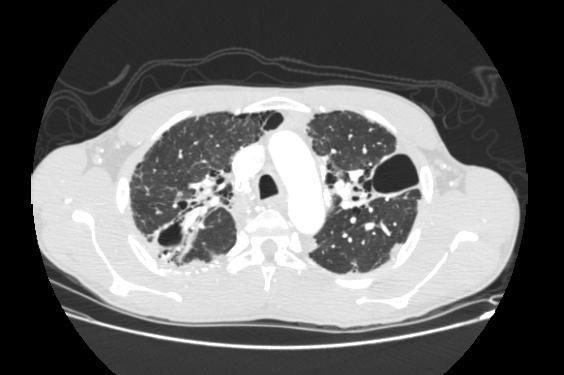
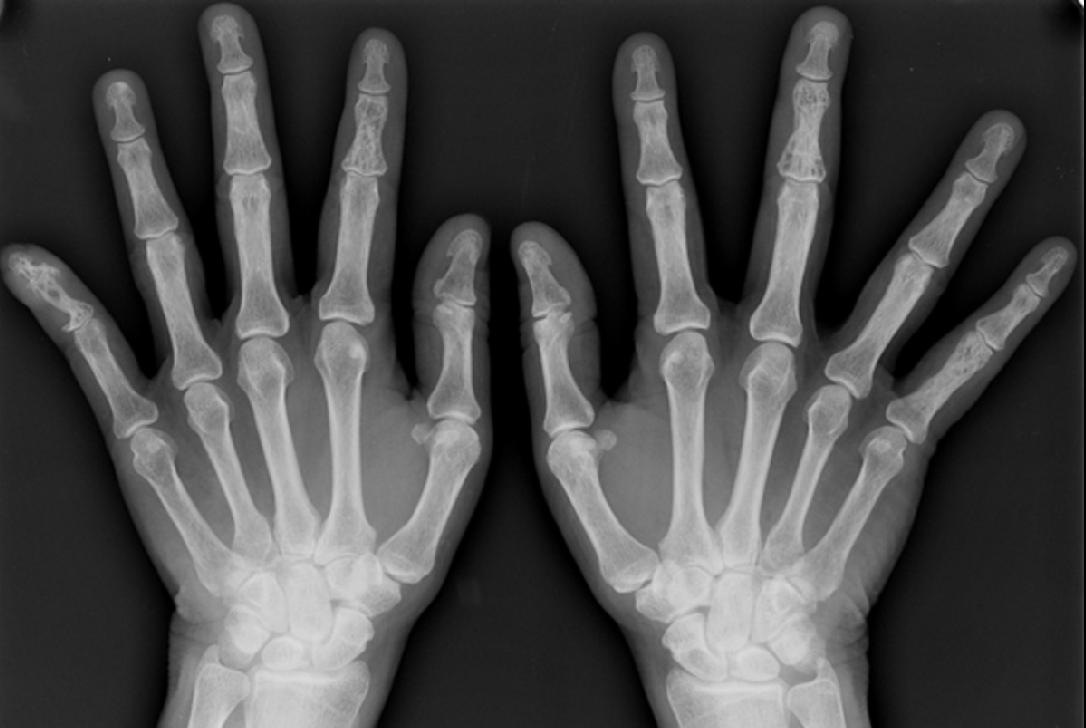
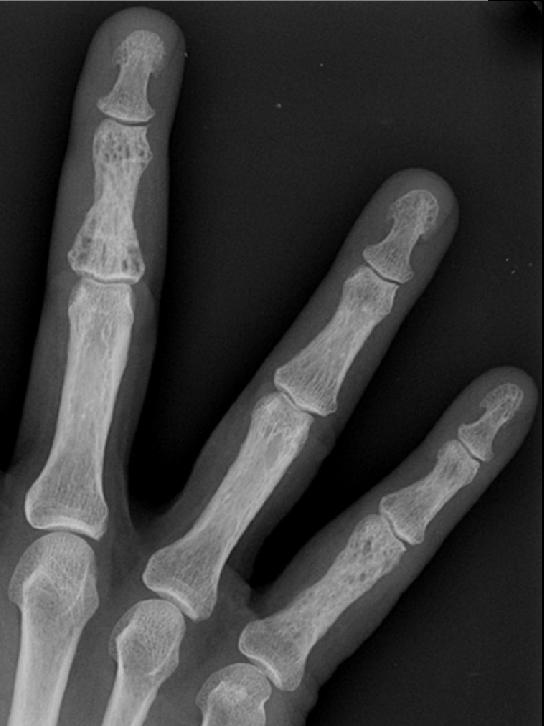
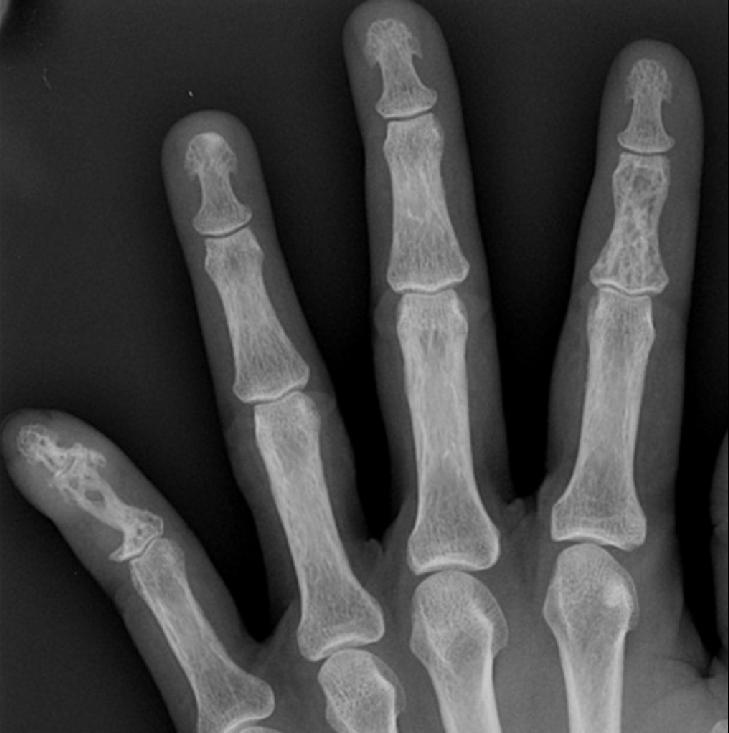
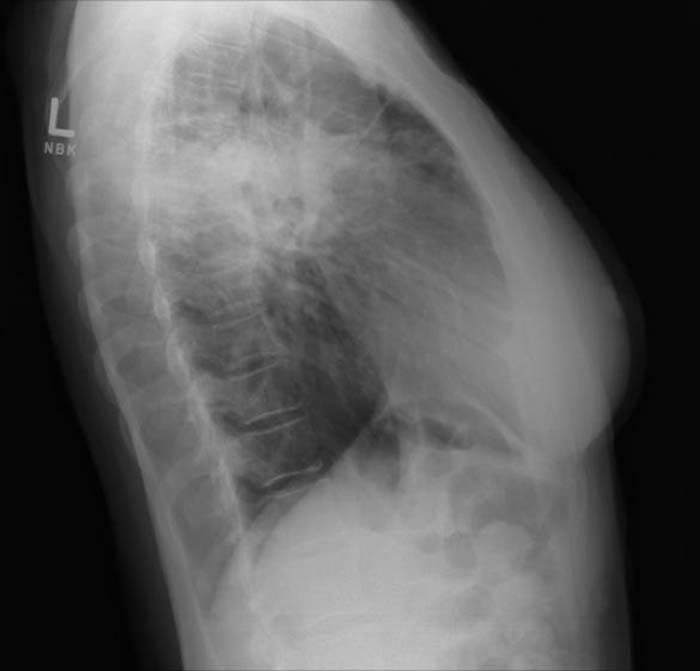
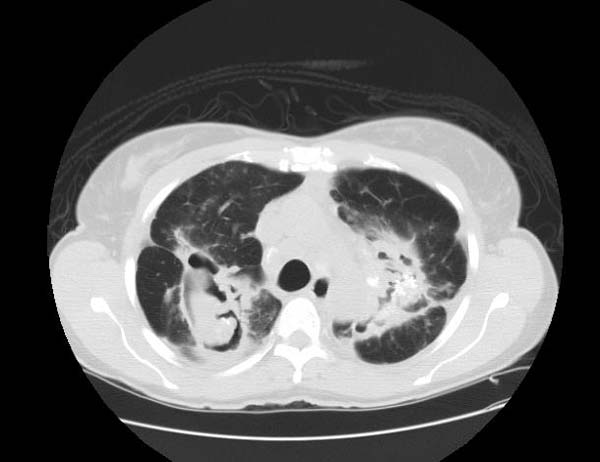
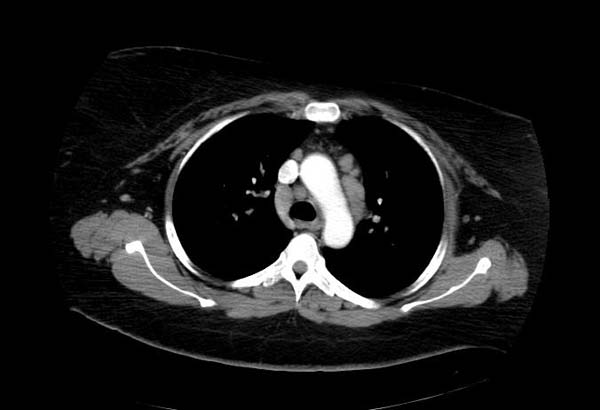
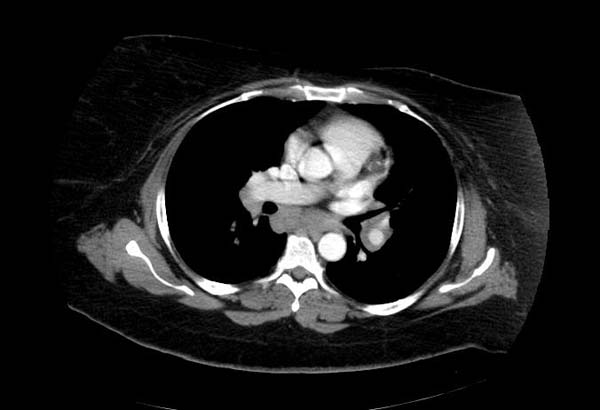
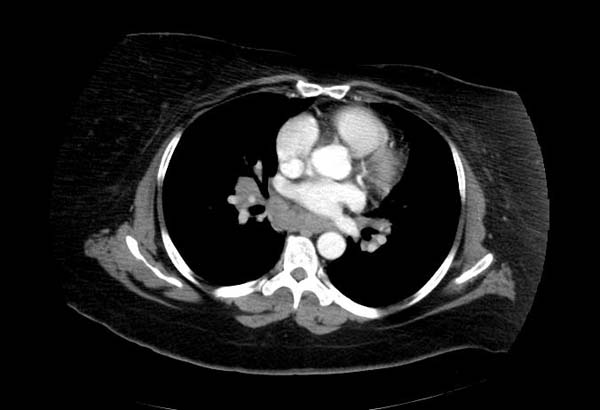
Image Examples
Treatment
Corticosteroids, most commonly prednisone, have been the standard treatment for many years. In some patients, this treatment can slow or reverse the course of the disease, but other patients unfortunately do not respond to steroid therapy. The use of corticosteroids in mild disease is controversial because in many cases the disease remits spontaneously. Additionally, corticosteroids have many recognized dose- and duration-related side effects (which can be reduced through the use of alternate-day dosing for those on chronic prednisone therapy [9]), and their use is generally limited to severe, progressive, or organ-threatening disease. The influence of corticosteroids or other immunosuppressants on the natural history is unclear.
Severe symptoms are generally treated with steroids, and steroid-sparing agents such as azathioprine and methotrexate are often used. Rarely, cyclophosphamide has also been used. As the granulomas are caused by collections of immune system cells, particularly T cells, there has been some early indications of success using immunosuppressants, interleukin-2 inhibitors or anti-tumor necrosis factor-alpha treatment (such as infliximab). Unfortunately, none of these have provided reliable treatment and there can be significant side effects such as an increased risk of reactivating latent tuberculosis.
Avoidance of sunlight and Vitamin D foods may be helpful in patients who are susceptible to developing hypercalcemia.
Case Examples
Case #1
Clinical Summary
This 33-year-old white female was admitted for evaluation of abnormal findings on a chest x-ray. She was asymptomatic and a physical examination revealed no significant abnormalities. Laboratory results indicated hypercalcemia and elevated gamma globulin. Radiographic examination showed enlarged subcarinal, hilar, and right paratracheal lymph nodes. A right paratracheal lymph node was biopsied. Special stains for acid-fast bacilli and fungi were negative and a diagnosis of sarcoidosis was made.
Histopathological Findings






See also
References
- ↑ http://linkinghub.elsevier.com/retrieve/pii/S0954-6111(07)00366-6. Missing or empty
|title=(help) - ↑ 2.0 2.1 Baughman RP, Lower EE, du Bois RM. Sarcoidosis. The Lancet 2003/3/29;361(9363):1111-8.
- ↑ Henke, C. E., G. Henke, L. R. Elveback, C. M. Beard, D. J. Ballard and L. T. Kurland. 1986. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am. J. Epidemiol. 123:840–845.
- ↑ "American Thoracic Society: Statement on sarcoidosis. Am J Respir Crit Care Med 1999;160:736-755.
- ↑ Almenoff PL, Johnson A, Lesser M, Mattman LH. Growth of acid fast L forms from the blood of patients with sarcoidosis. Thorax 1996;51:530-3. PMID 8711683.
- ↑ Padilla ML, Schilero GJ, Teirstein AS. Donor-acquired sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:18-24. PMID 12002380.
- ↑ Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med 1981;305:440-3. PMID 6894783.
- ↑ New York Times article, May 24, 2007
- ↑ "Dosing Considerations"
External links
- The Foundation for Sarcoidosis Research
- Pathology Images of Sarcoidosis and Other Granulomatous Diseases
- Microscopy of granulomas in sarcoidosis
- Template:GPnotebook
- The Sarcoidosis Community is The Foundation for Sarcoidosis Research's online community
- Dorothy P. and Richard P. Simmons Center for Interstitial Lung Disease
- MedPix Pulmonary Sarcoid
da:Sarkoidose de:Sarkoidose eo:Sarkoidozo eu:Sarkoidosia he:סרקואידוזיס lb:Sarkoidos nl:Sarcoïdose no:Sarkoidose nn:sarkoidose sq:Sarkoidoza fi:Sarkoidoosi sv:Sarkoidos