COVID-19-associated acute kidney injury
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sogand Goudarzi, MD [2], Nasrin Nikravangolsefid, MD-MPH [3]
Synonyms and keywords: COVID-19-associated AKI
Overview
COVID-19 can involve many organs leading to organ failure, one of which is kidneys that manifest with mild proteinuria to advanced acute kidney injury (AKI).
Historical Perspective
- Early reports from China revealed that COVID-19 rarely involves the kidneys, as acute renal failure was not seen among COVID-19 hospitalized patients and mild BUN or creatinine rise [10.8%] and mild proteinuria [7.2%] occurred. [1]
- However, recent study found 75.4% of hospitalized patients with COVID-19 pneumonia developed hematuria, proteinuria, and AKI. But, these findings are not significantly different from other critical diseases.[2]
Pathophysiology
- Angiotensin-converting enzyme 2 (ACE2), which is a primary receptor for SARS-CoV-2 entry into cells, mostly presents in renal tubular epithelial cells as well as lungs and heart.[3]
- Despite kidney injury following COVID-19 infection is less frequent than severe lung injury, ACE2: ACE ratio is higher in the kidneys compared to the respiratory system. (1:1 in the kidneys VS 1:20 in the respiratory system)[3]
- After SARS-CoV-2 enters through the nasal cavity, it may travel to the kidneys and enters the bloodstream leading to severe inflammatory response activation and cytokine storm.
- Cytokine induced AKI may occur due to intrarenal inflammation, hyperpermeability of vessels, hypovolemia and cardiomyopathy, leading to cardiorenal syndrome type 1 that is characterized by third space volume overload such as pleural effusion, edema and intravascular volume loss (hypovolemia) and hypotension.[4]
- The major cytokine is IL-6, which induces inflammation and lung endothelial cell injury, leading to ARDS and hypoxia that subsequently cause renal tubular cell injury and AKI. [5][4]
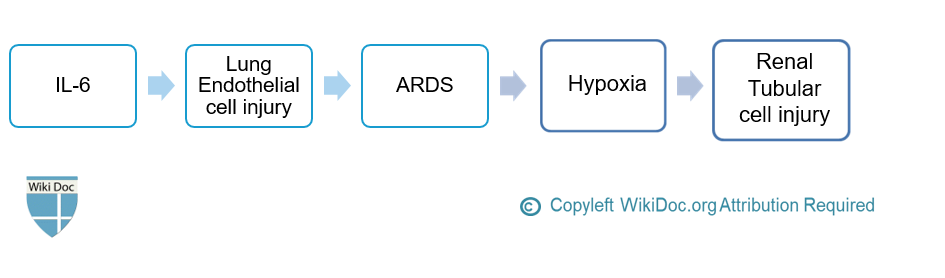
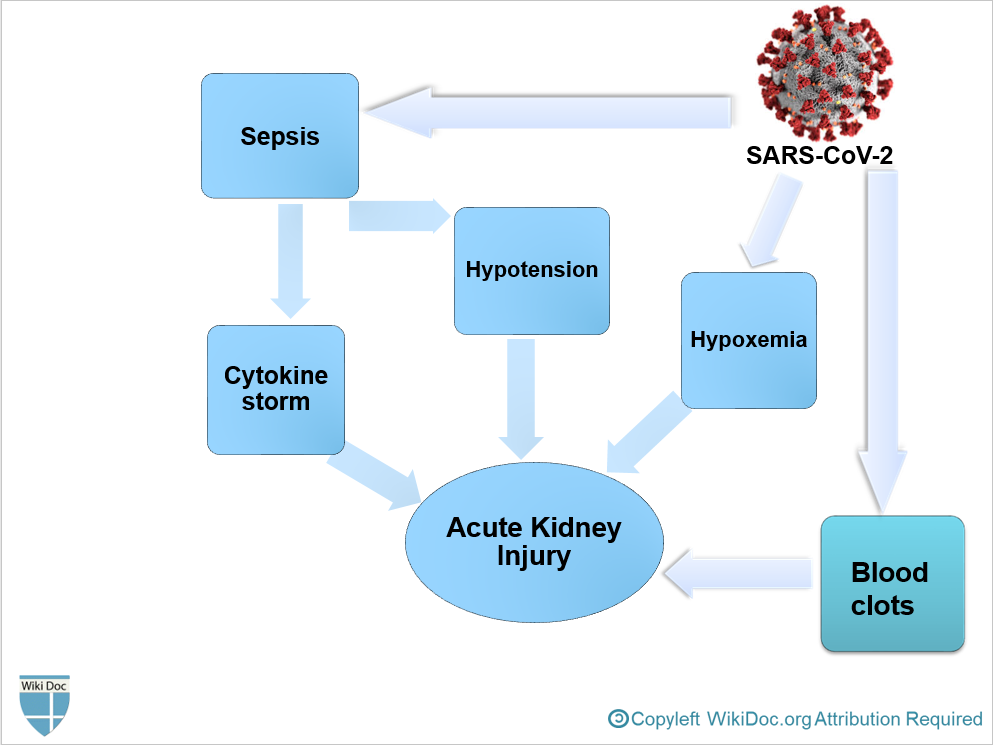
- To conclude, COVID-19-associated AKI can occur as a result of[3]:
- Sepsis and cytokine storm
- Hypovolemia and Hypotension
- Hypoxemia
- Blood clots formation due to Hypercoagulable state, leading to impaired blood flow in the renal arterioles.
Causes
- SARS-CoV-2 may have a Kidney tropism. As a recent study found SARS-CoV-2 antigens in renal tubules which suggests the direct damage of SARS-CoV-2 on the kidneys. https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4. Missing or empty
|title=(help)
Epidemiology and Demographics
- AKI is frequently seen among patients with COVID-19 hospitalized in ICU, with prevalence of 0.6-29% in China "Acute Kidney Injury in COVID-19 Patients | COVID-19". and 22.2% in the USA.[8]
- The incidence of AKI in critcally ill patients with COVID-19 is estimated between 27-85%. "Acute Kidney Injury in COVID-19 Patients | COVID-19".
Risk Factors
- The most potent risk factors in the development of COVID-19 associated AKI include[9] [10]:
- Elderly
- Age>60 years
- Comorbidities
- Elderly
Natural History, Complications, and Prognosis
Natural History
- AKI is more likely to develop in the late stages of COVID-19 in critically ill patients.[11]
- Severe COVID-19 pneumonia and severe acute respiratory distress syndrome are associated with developing AKI.[2]
- If no improvement occurs during follow-up, it is contributed to higher mortality.[2]
Diagnosis
Symptoms
- Patients in the early stages of kidney failure may be asymptomatic. If left untreated, patients may progress to develop Azotemia and Uremia, which occur due to the buildup of waste materials in the blood.
- Symptoms of kidney injury include:[12]
- Nausea and Vomiting
- Weakness
- Fatigue
- Confusion
- Weight loss
- Loss of appetite
- Decrease in urine output:Oliguria or Anuria
- Fluid retention, leading edema and swelling of face and extremities
- Electrolyte imbalance; High level of Potassium which leads to cardiac arrhythmia
Laboratory Findings
- Laboratory findings of COVID-19-associated AKI include:
- Elevated BUN level
- Plasma BUN-creatinine ratio> 20 in prerenal AKI
- Plasma BUN-creatinine ratio< 15 in renal AKI or acute tubular necrosis
- Based on KDIGO definition for the diagnosis of AKI[13]:
- Elevated serum Creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or
- Elevated serum Creatinine to ≥1.5 times baseline within the previous 7 days; or
- Urine volume < 0.5 ml/kg/h for >6 hours
- Fractional excretion of sodium (FENa)
- (FENa)< 1% in prerenal AKI
- (FENa)> 2% in renal AKI or acute tubular necrosis
- Urinary sediment
- Hyaline casts in prerenal AKI
- Granular or Muddy brown casts in renal AKI or acute tubular necrosis
- Elevated BUN level
Electrocardiogram
- There are no specific ECG findings associated with AKI. However, electrolyte disturbances such as hyperkalemia might lead to various ECG abnormalities.
Treatment
Medical Therapy
- Management of AKI following COVID-19 includes treatment of infection, identifying electrolyte disorders, and intravenous fluid resuscitation.
- Treatment of AKI following COVID-19 includes:[11] [10]
- Correction of hypovolemia and hypotension by the administration of adequate intravenous fluid
- Isotonic crystalloid is recommended among all patients who develop AKI. [13]
- Correction of electrolyte disorders
- Anticoagulants in hypercoagulable conditions
- Loop diuretics
- In volume overload conditions
- Diuretics should not be used regularly as they predispose patients to volume depletion.
- Correction of hypovolemia and hypotension by the administration of adequate intravenous fluid
Interventions
- renal replacement therapy
- If AKI is unresponsive to conservative therapy
- In volume overload conditions
- Modality of choice in unstable hemodynamic status and ESRD, severe metabolic acidosis, severe hyperkalemia
- renal replacement therapy is associated with hypercoagulation.[10]
- Sequential extracorporeal therapy
- It removes cytokines.
Prevention
- Patients with COVID-19 should be evaluated for intravascular volume status based on physical examination and fluid balance.[10]
- BUN, serum creatinine, and electrolytes such as sodium, potassium and bicarbonate should be monitored frequently every 48 hours or more in high risk patients.[10]
- Isotonic saline is recommended as a prevention strategy for patients who are at increased risk for AKI by expanding intravascular volume. [13]
References
- ↑ Wang, Luwen; Li, Xun; Chen, Hui; Yan, Shaonan; Li, Dong; Li, Yan; Gong, Zuojiong (2020). "Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China". American Journal of Nephrology. 51 (5): 343–348. doi:10.1159/000507471. ISSN 0250-8095.
- ↑ Jump up to: 2.0 2.1 2.2 Pei, Guangchang; Zhang, Zhiguo; Peng, Jing; Liu, Liu; Zhang, Chunxiu; Yu, Chong; Ma, Zufu; Huang, Yi; Liu, Wei; Yao, Ying; Zeng, Rui; Xu, Gang (2020). "Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia". Journal of the American Society of Nephrology. 31 (6): 1157–1165. doi:10.1681/ASN.2020030276. ISSN 1046-6673.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Malha, Line; Mueller, Franco B.; Pecker, Mark S.; Mann, Samuel J.; August, Phyllis; Feig, Peter U. (2020). "COVID-19 and the Renin-Angiotensin System". Kidney International Reports. 5 (5): 563–565. doi:10.1016/j.ekir.2020.03.024. ISSN 2468-0249.
- ↑ Jump up to: 4.0 4.1 4.2 Ronco C, Reis T (2020). "Kidney involvement in COVID-19 and rationale for extracorporeal therapies". Nat Rev Nephrol. 16 (6): 308–310. doi:10.1038/s41581-020-0284-7. PMC 7144544 Check
|pmc=value (help). PMID 32273593 Check|pmid=value (help). - ↑ Husain-Syed F, Slutsky AS, Ronco C (2016). "Lung-Kidney Cross-Talk in the Critically Ill Patient". Am J Respir Crit Care Med. 194 (4): 402–14. doi:10.1164/rccm.201602-0420CP. PMID 27337068.
- ↑ Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D (2006). "Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes". J Am Soc Nephrol. 17 (11): 3067–75. doi:10.1681/ASN.2006050423. PMID 17021266.
- ↑ Perico L, Benigni A, Remuzzi G (2020). "Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade". Nephron. 144 (5): 213–221. doi:10.1159/000507305. PMC 7179544 Check
|pmc=value (help). PMID 32203970 Check|pmid=value (help). - ↑ Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; et al. (2020). "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area". JAMA. doi:10.1001/jama.2020.6775. PMC 7177629 Check
|pmc=value (help). PMID 32320003 Check|pmid=value (help). - ↑ Rabb H (2020). "Kidney diseases in the time of COVID-19: major challenges to patient care". J Clin Invest. 130 (6): 2749–2751. doi:10.1172/JCI138871. PMC 7259985 Check
|pmc=value (help). PMID 32250968 Check|pmid=value (help). - ↑ Jump up to: 10.0 10.1 10.2 10.3 10.4 Selby NM, Forni LG, Laing CM, Horne KL, Evans RD, Lucas BJ; et al. (2020). "Covid-19 and acute kidney injury in hospital: summary of NICE guidelines". BMJ. 369: m1963. doi:10.1136/bmj.m1963. PMID 32457068 Check
|pmid=value (help). - ↑ Jump up to: 11.0 11.1 11.2 Ronco C, Reis T, Husain-Syed F (2020). "Management of acute kidney injury in patients with COVID-19". Lancet Respir Med. doi:10.1016/S2213-2600(20)30229-0. PMC 7255232 Check
|pmc=value (help). PMID 32416769 Check|pmid=value (help). - ↑ Skorecki K, Green J, Brenner BM (2005). "Chronic renal failure". In Kasper DL, Braunwald E, Fauci AS, et al. Harrison's Principles of Internal Medicine (16th ed.). New York, NY: McGraw-Hill. pp. 1653–63. ISBN 978-0-07-139140-5.
- ↑ Jump up to: 13.0 13.1 13.2 Khwaja A (2012). "KDIGO clinical practice guidelines for acute kidney injury". Nephron Clin Pract. 120 (4): c179–84. doi:10.1159/000339789. PMID 22890468.