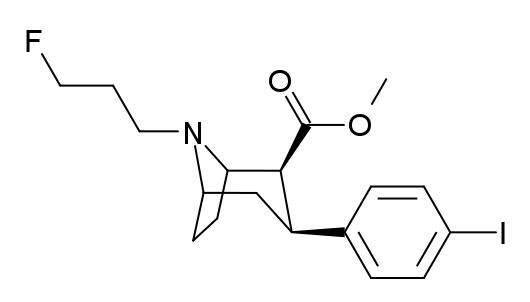
Ioflupane i-123
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ioflupane i-123 is a radiopharmaceutical that is FDA approved for the procedure of striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected Parkinsonian syndromes (PS). Common adverse reactions include hypersensitivity, injection site reactions, headache, nausea, vertigo, dry mouth and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Ioflupane i-123 is a radiopharmaceutical indicated for striatal dopamine transporter visualization using single photon emission computed tomography (SPECT) brain imaging to assist in the evaluation of adult patients with suspected Parkinsonian syndromes (PS). In these patients, ioflupane i-123 may be used to help differentiate essential tremor from tremor due to PS (idiopathic Parkinson's disease, multiple system atrophy and progressive supranuclear palsy). Ioflupane i-123 is an adjunct to other diagnostic evaluations.
Dosage
Radiation Safety
- Ioflupane i-123 emits radiation and must be handled with safety measures to minimize radiation exposure to clinical personnel and patients. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experienced in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate government agency authorized to license the use of radionuclides. Ioflupane i-123 dosing is based upon the radioactivity determined using a suitably calibrated instrument immediately prior to administration.
- To minimize radiation dose to the bladder, encourage hydration prior to and following ioflupane i-123 administration in order to permit frequent voiding. Encourage the patient to void frequently for the first 48 hours following ioflupane i-123 administration.
Thyroid Blockade Before Ioflupane i-123 Injection
- Before administration of ioflupane i-123, administer Potassium Iodide Oral Solution or Lugol's Solution (equivalent to 100 mg iodide) or potassium perchlorate (400 mg) to block uptake of iodine 123 by the patient's thyroid. Administer the blocking agent at least one hour before the dose of ioflupane i-123.
Preparation and Administration
- Use aseptic procedures and radiation shielding during preparation and administration. Inspect the ioflupane i-123 vial prior to administration and do not use it if the vial contains particulate matter or discoloration. Administer ioflupane i-123 as a slow intravenous injection (administered over a period of not less than 15 to 20 seconds) via an arm vein.
Recommended Dose
- The recommended dose is 111 to 185 MBq (3 to 5 mCi) administered intravenously.
Radiation Dosimetry
- The estimated radiation absorbed doses to an average adult from intravenous injection of ioflupane i-123 are shown in Table 1. The values are calculated assuming urinary bladder emptying at 4.8-hour intervals and appropriate thyroid blocking (iodine 123 is a known Auger electron emitter).

- The Effective Dose resulting from a ioflupane i-123 administration with an administered activity of 185 MBq (5 mCi) is 3.94 mSv in an adult.
Imaging Guidelines
- Begin SPECT imaging 3 to 6 hours following ioflupane i-123 administration. Acquire images using a gamma camera fitted with high-resolution collimators and set to a photopeak of 159 keV with a ± 10% energy window. Angular sampling should be not less than 120 views over 360 degrees. Position the subject supine with the head on an off-the-table headrest, a flexible head restraint such as a strip of tape across the chin or forehead may be used to help avoid movement, and set a circular orbit for the detector heads with the radius as small as possible (typically 11 to 15 cm).
- Experimental studies with a striatal phantom suggest that optimal images are obtained with matrix size and zoom factors selected to give a pixel size of 3.5 to 4.5 mm. Collect a minimum of 1.5 million counts for optimal images.
Image Interpretation
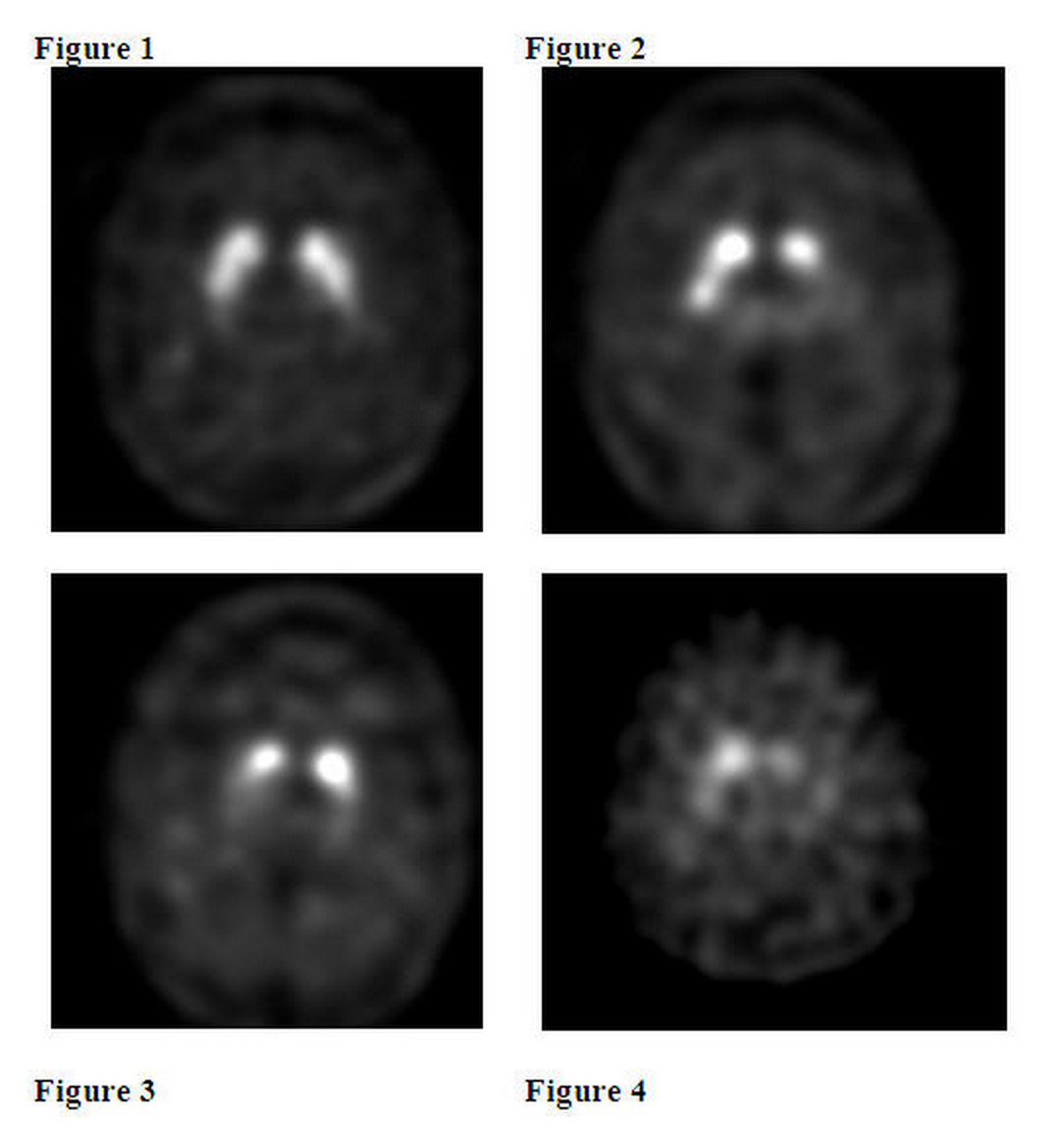
- Ioflupane i-123 images are interpreted visually, based upon the appearance of the striata. Reconstructed pixel size should be between 3.5 and 4.5 mm with slices 1 pixel thick. Optimum presentation of the reconstructed images for visual interpretation is transaxial slices parallel to the anterior commissure-posterior commissure (AC-PC) line. Determination of whether an image is normal or abnormal is made by assessing the extent (as indicated by shape) and intensity of the striatal signal. Image interpretation does not involve integration of the striatal image appearance with clinical signs and/or symptoms.
- Normal :
- In transaxial images, normal images are characterized by two symmetric comma- or crescent-shaped focal regions of activity mirrored about the median plane. Striatal activity is distinct, relative to surrounding brain tissue (Figure 1).
- Abnormal :
- Abnormal DaTscan images fall into at least one of the following three categories (all are considered abnormal).
- Activity is asymmetric, e.g. activity in the region of the putamen of one hemisphere is absent or greatly reduced with respect to the other. Activity is still visible in the caudate nuclei of both hemispheres resulting in a comma or crescent shape in one and a circular or oval focus in the other. There may be reduced activity between at least one striatum and surrounding tissues (Figure 2).
- Activity is absent in the putamen of both hemispheres and confined to the caudate nuclei. Activity is relatively symmetric and forms two roughly circular or oval foci. Activity of one or both is generally reduced (Figure 3).
- Activity is absent in the putamen of both hemispheres and greatly reduced in one or both caudate nuclei. ::*Activity of the striata with respect to the background is reduced (Figure 4).
Dosage forms and strength
- Single-use vials containing 185 MBq (5 mCi) in 2.5 mL sterile solution for intravenous injection [74 MBq (2 mCi) per mL at calibration time].
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ioflupane i-123 in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioflupane i-123 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Ioflupane i-123 is not indicated for use in children. The safety and efficacy of ioflupane i-123 have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Ioflupane i-123 in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Ioflupane i-123 in pediatric patients.
Contraindications
- Ioflupane i-123 is contraindicated in patients with known hypersensitivity to the active substance or to any of the excipients, or to iodine.
Warnings
Hypersensitivity Reactions
- hypersensitivity reactions have been reported following ioflupane i-123 administration. The reactions have generally consisted of skin erythema and pruritis and have either resolved spontaneously or following the administration of corticosteroids and anti-histamines. Prior to administration, question the patient for a history of prior reactions to ioflupane i-123. If the patient is known or strongly suspected of having had a hypersensitivity reaction to ioflupane i-123, the decision to administer ioflupane i-123 should be based upon an assessment of the expected benefits compared to the potential hypersensitivity risks. Have anaphylactic and hypersensitivity treatment measures available prior to ioflupane i-123 administration and, following administration, observe patients for symptoms or signs of a hypersensitivity reaction.
Thyroid Accumulation
- The ioflupane i-123 injection may contain up to 6% of free iodide (iodine 123). To decrease thyroid accumulation of iodine 123, block the thyroid gland before administration of ioflupane i-123. Avoid the use of Potassium Iodide Oral Solution or Lugol's Solution in patients who are sensitive to such products. Failure to block thyroid uptake of iodine 123 may result in an increased long term risk for thyroid neoplasia.
Adverse Reactions
Clinical Trials Experience
- The data from clinical studies reflect exposure to ioflupane i-123 in 942 subjects with a mean age of 66 years (range 25 to 90 years). Among these subjects, 42% were women and 99% Caucasian. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of ioflupane i-123 cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical trials, no serious adverse reactions were reported. Other adverse reactions occurred at a rate of 1% or less and the reported events consisted of headache, nausea, vertigo, dry mouth or dizziness. These reactions were of mild to moderate severity.
Postmarketing Experience
- Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. In the postmarketing experience, hypersensitivity reactions have been reported. The reactions generally related to rash and pruritis within minutes of ioflupane i-123 administration. The reactions either resolved spontaneously or following the administration of corticosteroids and antihistamines. Injection site pain has also been reported.
Drug Interactions
- The ioflupane within ioflupane i-123 binds to the dopamine transporter. Drugs that bind to the dopamine transporter with high affinity may interfere with the image obtained following ioflupane i-123 administration. These potentially interfering drugs consist of: amoxapine, amphetamine, benztropine, bupropion, buspirone, cocaine, mazindol, methamphetamine, methylphenidate, norephedrine, phentermine, phenylpropanolamine, selegiline, and sertraline. Selective serotonin reuptake inhibitors (paroxetine and citalopram) may increase or decrease ioflupane binding to the dopamine transporter. Whether discontinuation of these drugs prior to ioflupane i-123 administration may minimize the interference with a ioflupane i-123 image is unknown.
- The impact of dopamine agonists and antagonists upon ioflupane i-123 imaging results has not been established.
Use in Specific Populations
Pregnancy
- It is not known whether ioflupane i-123 can cause fetal harm or increase the risk of pregnancy loss when administered to a pregnant woman. Animal reproductive and developmental toxicity studies have not been conducted with ioflupane i-123. Prior to the administration of ioflupane i-123 to women of childbearing potential, assess for the presence of pregnancy. Ioflupane i-123 should be given to a pregnant woman only if clearly needed.
- Like all radiopharmaceuticals, ioflupane i-123 has a potential to cause fetal harm. The likelihood of fetal harm depends on the stage of fetal development, and the magnitude of the radionuclide dose. Administration of ioflupane i-123 at a dose of 185 MBq (5 mCi) results in an absorbed radiation dose to the uterus of 0.3 rad (3.0 mGy). Radiation doses greater than 15 rad (150 mGy) have been associated with congenital anomalies but doses under 5 rad (50 mGy) generally have not. Radioactive iodine products cross the placenta and can permanently impair fetal thyroid function.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ioflupane i-123 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ioflupane i-123 during labor and delivery.
Nursing Mothers
- It is not known whether ioflupane i-123 is excreted into human milk. However, iodine 123 is excreted into human milk. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to interrupt nursing after administration of ioflupane i-123 or not to administer ioflupane i-123, taking into account the importance of the drug to the mother. Based on the physical half-life of iodine 123 (13.2 hours), nursing women may consider interrupting nursing and pumping and discarding breast milk for 6 days after ioflupane i-123 administration in order to minimize risks to a nursing infant.
Pediatric Use
- Ioflupane i-123 is not indicated for use in children. The safety and efficacy of ioflupane i-123 have not been established in pediatric patients.
Geriatic Use
- In the two principal clinical studies, 45% of the subjects were aged 65 and over. There were no differences in response compared to younger subjects that would require a dose adjustment. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Ioflupane i-123 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ioflupane i-123 with respect to specific racial populations.
Renal Impairment
- The effect of renal or hepatic impairment upon ioflupane i-123 imaging has not been established. Ioflupane i-123 is excreted by the kidney and patients with severe renal impairment may have increased radiation exposure and altered ioflupane i-123 images.
Hepatic Impairment
- The effect of renal or hepatic impairment upon ioflupane i-123 imaging has not been established.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ioflupane i-123 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ioflupane i-123 in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous.
Monitoring
- There is limited information regarding drug monitoring.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- The clinical consequence of overdose with ioflupane i-123 has not been reported. It is unknown whether or not ioflupane is dialyzable. Due to the small quantity of ioflupane in each vial, overdosage with ioflupane is not expected to result in pharmacologic effects. The major risks of overdose relates predominantly to increased radiation exposure, with the long-term risks for neoplasia. In case of overdosage of radioactivity, frequent urination and defecation should be encouraged to minimize radiation exposure to the patient; care should be taken to avoid contamination from the radioactivity eliminated by the patient.
Pharmacology
Mechanism of Action
- The active drug substance in ioflupane i-123 is N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-[123I]iodophenyl)nortropane or ioflupane I 123. In vitro, ioflupane binds reversibly to the human recombinant dopamine transporter (DaT) (Ki = 0.62 nM; IC50 = 0.71 nM). Autoradiography of post-mortem human brain slices exposed to radiolabeled ioflupane shows concentration of the radiolabel in striatum (caudate nucleus and putamen). The specificity of the binding of ioflupane I 125 to dopamine transporter was demonstrated by competition studies with the DaT inhibitor GBR 12909 (a dopamine reuptake inhibitor), the serotonin reuptake inhibitor citalopram, and the norepinephrine reuptake inhibitor desipramine in post-mortem human brain slices exposed to radiolabeled ioflupane. Citalopram reduced binding in the neocortex and thalamus with only minor effects in the striatum. This indicated that the binding in the cortex and thalamus is mainly to the serotonin reuptake sites. Desipramine showed no effect on the level of striatal binding of ioflupane I 125, but reduced extrastriatal binding by 60 to 85%. The binding of ioflupane I 125 to the striatum was abolished in the presence of high concentrations of GBR 12909, indicating selectivity of ioflupane binding for the pre-synaptic DaT.
- Following administration of ioflupane i-123 to humans, radioactive decay of the iodine 123 emits gamma radiation which can be detected externally using gamma detectors, allowing visualization of the brain striata through SPECT imaging.
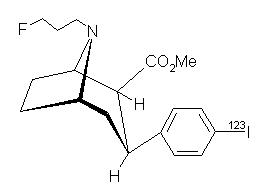
Structure
- ioflupane i-123 [Ioflupane I 123 Injection] is a sterile, pyrogen-free radiopharmaceutical for intravenous injection. The clear and colorless solution is supplied in single-use vials in which each milliliter contains 0.07 to 0.13 µg ioflupane, 74 MBq (2 mCi) of iodine 123 (as ioflupane I 123) at calibration time, 5.7 mg acetic acid, 7.8 mg sodium acetate and 0.05 mL (5%) ethanol. The pH of the solution is between 4.2 and 5.2. Ioflupane I 123 has the following structural formula:
Physical Characteristics
- Iodine 123 is a cyclotron-produced radionuclide that decays to 123Te by electron capture and has a physical half-life of 13.2 hours. The photon that is useful for detection and imaging studies is listed in Table 2.

External Radiation
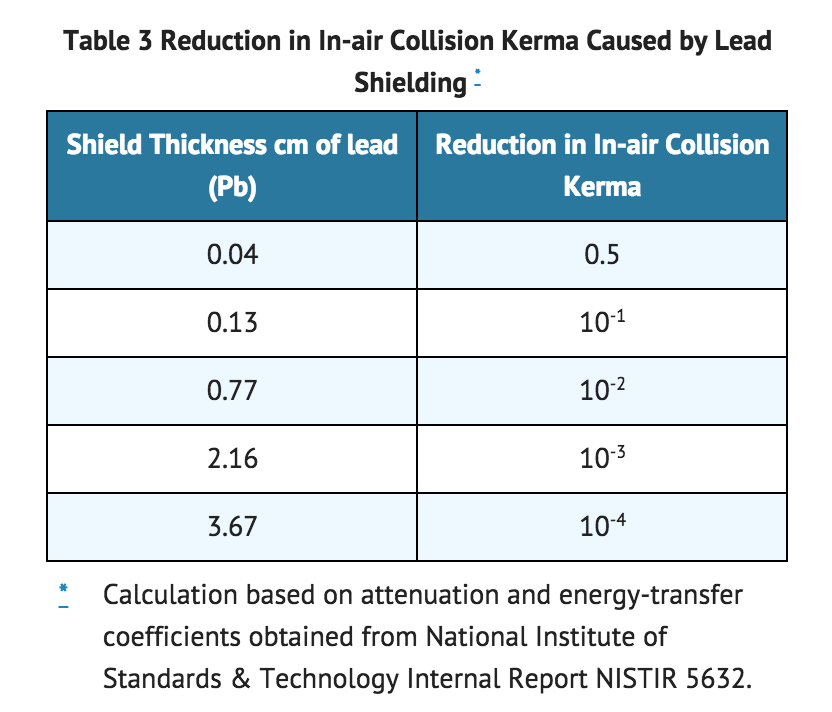
- The specific gamma-ray constant for iodine 123 is 1.6 R/mCi-hr at 1 cm. The first half-value thickness of lead (Pb) for iodine 123 is 0.04 cm. The relative transmission of radiation emitted by the radionuclide that results from interposition of various thicknesses of Pb is shown in Table 3 (e.g., the use of 2.16 cm Pb will decrease the external radiation exposure by a factor of about 1,000).
Pharmacodynamics
- As ioflupane i-123 contains a very small quantity of ioflupane, no ioflupane pharmacologic effects are expected.
Pharmacokinetics
- The pharmacokinetics of ioflupane I 123 were studied by monitoring radioactivity following intravenous injection; only 5% of the administered radioactivity remained in whole blood at 5 minutes post-injection. Uptake in the brain reached approximately 7% of injected radioactivity at 10 minutes post-injection and decreased to 3% after 5 hours; striata to background ratios were relatively constant between 3 and 6 hours post-injection. About 30% of the whole brain radioactivity was attributed to striatal uptake. By 48 hours post-injection, approximately 60% of the injected radioactivity has been excreted in the urine, with fecal excretion estimated to be approximately 14%.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Studies on reproductive toxicity have not been conducted. Ioflupane showed no evidence of mutagenic potential in in vitro or in vivo mutagenicity studies. Studies to assess the carcinogenic potential of ioflupane have not been performed.
Animal Toxicology and/or Pharmacology
- Single- and repeated-dose intravenous toxicity studies have been performed using ioflupane in rats, rabbits, and dogs. Additionally, single-dose acute toxicity studies have been performed in cynomolgus monkeys. No mortality or other toxicity was observed at doses up to 5,500 times the maximum clinical dose of ioflupane i-123; at doses greater than 1,500 times the maximum clinical dose, pharmacological responses such as mydriasis and hyperactivity were seen in some species.
Clinical Studies
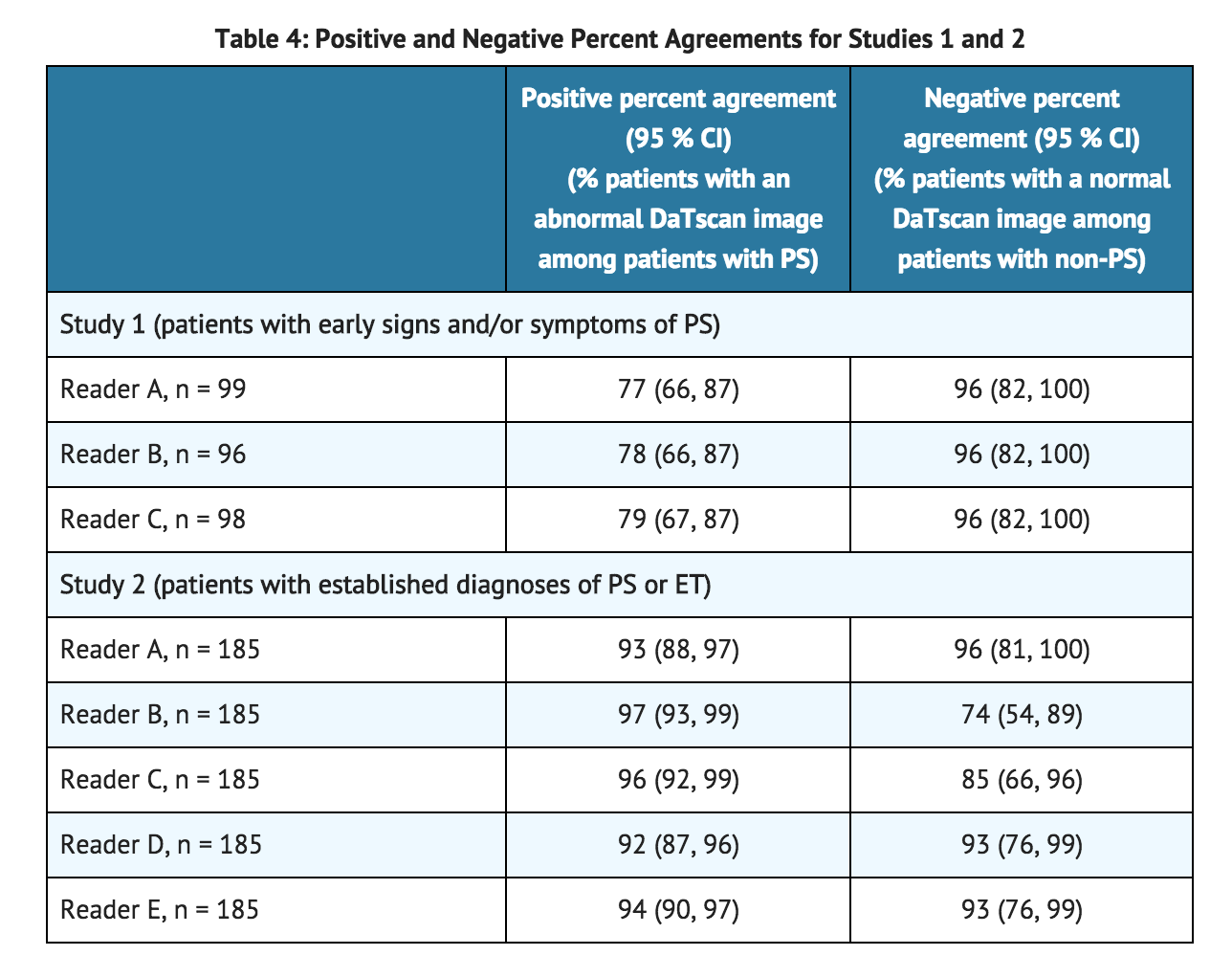
- The safety and efficacy of ioflupane i-123 were evaluated in two multicenter, single-arm studies (Study 1 and Study 2) that evaluated 284 adult patients with tremor. In the studies, ioflupane i-123 image outcomes were compared to a reference clinical diagnostic standard of "PS" or "non-PS". The reference clinical diagnostic standard for "PS" consisted of the following diagnoses: Parkinson's disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP). These three conditions have been associated with dopaminergic neurodegeneration and ioflupane i-123 imaging was not designed to distinguish among the conditions. The reference clinical diagnostic standard for "non-PS" consisted of an essential tremor (ET) diagnosis or other non-PS diagnosis. Three to 6 hours after ioflupane i-123 administration, subjects underwent SPECT imaging with a variety of multi-headed cameras or a multi-detector single-slice systems. The median administered activity evaluated in clinical studies was 173 MBq (4.7 mCi) [range, 88 to 287 MBq (2.4 to 7.8 mCi)].
- Ioflupane i-123 images were evaluated by readers blinded to clinical information. Study 1 readers had no other role in patient assessment; Study 2 readers included site investigators. The reference clinical diagnostic standards were the clinical diagnoses established by a consensus panel of movement disorder specialists that evaluated data inclusive through 36 months of follow-up (Study 1) or the investigator-determined baseline clinical diagnosis (Study 2). Study 1 consisted of patients with early features of Parkinsonism; patients with features suggestive of MSA or PSP were excluded. Study 2 consisted of patients with clinically established diagnosis of PS (PD, MSA, PSP) or ET.
- Among the 99 patients in Study 1, 44% were female, 42% were aged 65 or over and all were Caucasian; among the 185 patients in Study 2, 35% were female, 48% were aged 65 or over and 99% were Caucasian. Among the patients in Study 1, the baseline clinical diagnoses consisted of: probable PD (44%), possible PD (31%), "benign" PD (6%), possible ET (11%), and other diagnoses (7%). Among the patients in Study 2, the baseline clinical diagnoses consisted of: PD (70%), ET (15%), MSA (10%), and PSP (5%).
- Table 4 shows the positive percent agreement and negative percent agreement of the ioflupane i-123 image results with the reference clinical diagnostic standard. Positive percent agreement represents the percent of patients with abnormal ioflupane i-123 images among all the patients with a clinical diagnostic reference standard of PS. The negative percent agreement represents the percent of patients with normal ioflupane i-123 images among the patients with a non-PS clinical diagnostic reference standard.
- The effectiveness of ioflupane i-123 as a screening or confirmatory test and for monitoring disease progression or response to therapy has not been established.
How Supplied
- Ioflupane i-123 is supplied in 10-mL glass vials containing a total volume of 2.5 mL of solution with a total radioactivity of 185 MBq (5 mCi) at calibration time. Each vial is enclosed in a lead container of appropriate thickness.
NDC 17156-210-01
Storage
- Store DaTscan at 20° to 25°C (68° to 77°F). This product does not contain a preservative. Store DaTscan within the original lead container or equivalent radiation shielding.
- Do not use DaTscan (Ioflupane I 123 Injection) preparations after the expiration date and time stated on the label.
Images
Drug Images
{{#ask: Page Name::Ioflupane i-123 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ioflupane i-123 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Instruct patients to inform their physician or healthcare provider if they:
- have reduced renal or hepatic function.
- are sensitive to DaTscan.
- are sensitive to Potassium Iodide Oral Solution or Lugol's Solution.
- may be pregnant, are trying to become pregnant, or are breast feeding.
- Instruct patients to increase their level of hydration prior to and after receiving DaTscan and to void frequently for the first 48 hours following DaTscan administration.
Precautions with Alcohol
- Alcohol-Ioflupane i-123 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DATSCAN®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.