Pralidoxime
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pralidoxime is a cholinesterase reactivator that is FDA approved for the treatment of of poisoning by nerve agents having anticholinesterase activity. Common adverse reactions include Increased creatine kinase level.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Poisoning by Nerve Agents Having Anticholinesterase Activity
- Mild cases: headache, blurred vision, mild muscarinic signs
- Moderately Severe Cases: excessive sweating, lacrimation, salivation, diarrhea, tightness in the chest
For optimal reactivation of organophosphate-inhibited cholinesterase, atropine and pralidoxime should be administered as soon as possible after exposure. Depending on the severity of symptoms, immediately administer one atropine-containing auto-injector, followed by one pralidoxime-containing auto-injector. Atropine must be given first until its effects become apparent and only then should pralidoxime be administered. If nerve agent symptoms are still present after 15 minutes, repeat injections. If symptoms still exist after an additional 15 minutes, repeat injections for a third time. If after the third set of injections, symptoms remain, do not give any more antidotes but seek medical help.
Directions for Use: When, as described above, auto-injector use is indicated, proceed as follows:

- 1.- Remove gray safety cap.
- 2.- Place black end against outer thigh and push hard until the injector functions.
- 3.- Hold firmly in place for ten seconds, then remove. Massage the area of injection.
- 4.- Dispose of properly. Push ejected needle through a pocket flap (or other thick and conspicuous part of outer clothing). Bend needle into a hook.
- Very severe cases: Cyanosis, Respiratory Embarrassment, Coma
Initial measures should include removal of secretions, maintenance of a patent airway and, if necessary, artificial ventilation. Atropine should not be used until cyanosis has been overcome since atropine produces ventricular fibrillations in the presence of hypoxia. Morphine, theophylline, aminophylline, or succincylcholine are contraindicated. Tranquilizers of the reserpine or phenothiazine type are to be avoided.
"Pralidoxime is most effective if administered immediately after poisoning. Generally, little is accomplished if the drug is given more than 36 hours after termination of exposure. When the poison has been ingested, however, exposure may continue for some time due to slow absorption from the lower bowel, and fatal relapses have been reported after initial improvement. Continued administration for several days may be useful in such patients. Close supervision of the patient is indicated for at least 48 to 72 hours. If dermal exposure has occurred, clothing should be removed and the hair and skin washed thoroughly with sodium bicarbonate or alcohol as soon as possible. Diazepam may be given cautiously if convulsions are not controlled by atropine."
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pralidoxime in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pralidoxime in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pralidoxime FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pralidoxime in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pralidoxime in pediatric patients.
Contraindications
The pralidoxime chloride auto-injector is contraindicated in patients who are hypersensitive to any component of the product.
Warnings
Pralidoxime is not effective in the treatment of poisoning due to phosphorus, inorganic phosphates or organophosphates not having anticholinesterase activity.
Adverse Reactions
Clinical Trials Experience
Forty to 60 minutes after intramuscular injection, mild to moderate pain may be experienced at the site of injection. Pralidoxime may cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure, hyperventilation, and muscular weakness when given parenterally to normal volunteers who have not been exposed to anticholinesterase poisons. In patients it is very difficult to differentiate the toxic effects produced by atropine or the organophosphate compounds from those of the drug.
Elevations in SGOT and/or SGPT enzyme levels were observed in 1 of 6 normal volunteers given 1200 mg of pralidoxime chloride intramuscularly, and in 4 of 6 volunteers given 1800 mg intramuscularly. Levels returned to normal in about 2 weeks. Transient elevations in creatine phosphokinase were observed in all normal volunteers given the drug. A single intramuscular injection of 330 mg in 1 mL in rabbits caused myonecrosis, inflammation and hemorrhage.
When atropine and pralidoxime are used together, the signs of atropinization may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.2, 3, 4 Excitement and manic behavior immediately following recovery of consciousness have been reported in several cases. However, similar behavior has occurred in cases of organophosphate poisoning that were not treated with pralidoxime
Postmarketing Experience
There is limited information regarding Pralidoxime Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Pralidoxime Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Pralidoxime in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pralidoxime in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pralidoxime during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pralidoxime in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Pralidoxime in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Pralidoxime in geriatric settings.
Gender
There is no FDA guidance on the use of Pralidoxime with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pralidoxime with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pralidoxime in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pralidoxime in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pralidoxime in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pralidoxime in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Pralidoxime Administration in the drug label.
Monitoring
There is limited information regarding Pralidoxime Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pralidoxime and IV administrations.
Overdosage
There is limited information regarding Pralidoxime overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Pralidoxime Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Pralidoxime Mechanism of Action in the drug label.
Structure
There is limited information regarding Pralidoxime Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pralidoxime Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Pralidoxime Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Pralidoxime Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pralidoxime Clinical Studies in the drug label.
How Supplied
There is limited information regarding Pralidoxime How Supplied in the drug label.
Storage
There is limited information regarding Pralidoxime Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pralidoxime |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pralidoxime |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pralidoxime Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pralidoxime interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pralidoxime Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Pralidoxime Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
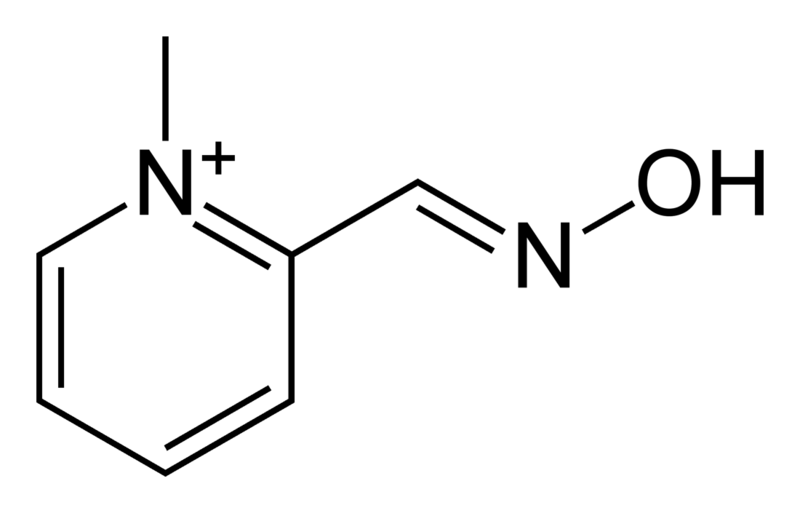
| Formula | C7H9N2O+ |
| Molar mass | 137.159 g/mol |
|
WikiDoc Resources for Pralidoxime |
|
Articles |
|---|
|
Most recent articles on Pralidoxime Most cited articles on Pralidoxime |
|
Media |
|
Powerpoint slides on Pralidoxime |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pralidoxime at Clinical Trials.gov Clinical Trials on Pralidoxime at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pralidoxime
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pralidoxime Discussion groups on Pralidoxime Patient Handouts on Pralidoxime Directions to Hospitals Treating Pralidoxime Risk calculators and risk factors for Pralidoxime
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pralidoxime |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Pralidoxime belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to combat poisoning by organophosphates or acetylcholinesterase inhibitors (nerve gas), in conjunction with atropine. Pralidoxime is most commonly in the form of Pralidoxime Chloride, also known as 2-PAM CL (or just 2-PAM by the military).
In a normal nicotinic synaptic junction, including motor end plates and preganglionic fibers, acetylcholine (ACh) is released from the presynaptic axon terminal into the synaptic cleft. The ACh then diffuses through the synaptic cleft and binds to nicotinic receptors on the postsynaptic membrane. This induces a subsequent action potential (AP) that continues through the postganglionic cell, or induces contraction in the motor end plate.
In order to prevent overstimulation or saturation of the synapse, or both, an enzyme known as acetylcholinesterase breaks down the neurotransmitter ACh. By removing the ACh, the synapse is brought to a state where it is ready for subsequent activation. Saturation of the synapse occurs when there is an excess of acetylcholine in the synaptic cleft, which inhibits further nerve transmission as the nicotinic receptors are full. Agents which inhibit acetylcholinesterase will lead to a build-up of ACh in the cleft.
Mechanism of action
Organophosphates inhibit cholinesterase by phosphorylation of the enzyme. Pralidoxime reactivates the cholinesterase by removing the phosphoryl group that is bound to the ester group. In this reaction both the organophosphate and the pralidoxime are mutually inactivated. These products undergo rapid metabolism, leading to the removal of the organophosphate.
Paradoxically, pralidoxime in doses above the optimal dose is itself an inhibitor of cholinesterase, and therefore can also produce the same symptoms as the toxins themselves. However, unlike organophosphates, pralidoxime binding is reversible, hence some protection is extended to the cholinesterase enzyme, this although has a negligible effect on the pharmacology of pralidoxime.
Dosage
Recommended dosages, according to online sources, seem to be:
- Adults: 30 mg/kg (Typically 1.5 - 2 g), administered either by intravenous therapy or intramuscular injection
- Children: 50 mg/kg
Interactions
When atropine and pralidoxime are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, succinylcholine, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning.
Contraindications
There are no known absolute contraindications for the use of pralidoxime. Relative contraindications include known hypersensitivity to the drug and other situations in which the risk of its use clearly outweighs possible benefit.
See Also
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Antidotes