Albendazole
{{DrugProjectFormSinglePage |authorTag=Adeel Jamil, M.D. [1] |genericName=Albendazole |aOrAn=a |drugClass=anthelmintic and benzimidazole |indicationType=treatment |indication=neurocysticercosis and hydatid disease |adverseReactions=abdominal pain, nausea, vomiting, and headache |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=Albendazole is indicated for the treatment of the following infections:
- Albendazole is indicated for the treatment of parenchymal neurocysticercosis due to active lesions caused by larval forms of the pork tapeworm, Taenia solium.
- Lesions considered responsive to albendazole therapy appear as nonenhancing cysts with no surrounding edema on contrast-enhanced computerized tomography. Clinical studies in patients with lesions of this type demonstrate a 74% to 88% reduction in number of cysts; 40% to 70% of albendazole-treated patients showed resolution of all active cysts.
- Albendazole is indicated for the treatment of cystic hydatid disease of the liver, lung, and peritoneum, caused by the larval form of the dog tapeworm, Echinococcus granulosus.
- This indication is based on combined clinical studies which demonstrated non-infectious cyst contents in approximately 80 to 90% of patients given Albendazole for 3 cycles of therapy of 28 days each. Clinical cure (disappearance of cysts) was seen in approximately 30% of these patients, and improvement (reduction in cyst diameter of ≥25%) was seen in an additional 40%.
- NOTE: When medically feasible, surgery is considered the treatment of choice for hydatid disease. When administering albendazole in the pre- or post-surgical setting, optimal killing of cyst contents is achieved when 3 courses of therapy have been given.
- NOTE: The efficacy of albendazole in the therapy of alveolar hydatid disease caused by Echinococcus multilocularis has not been clearly demonstrated in clinical studies.
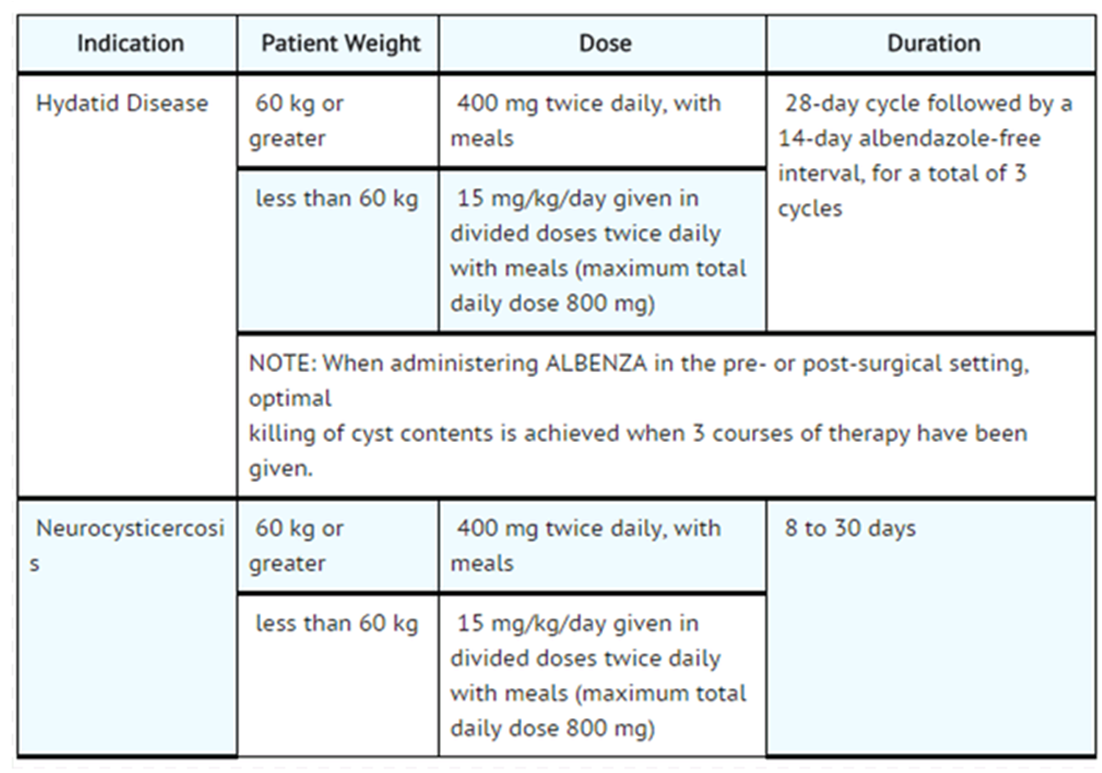
Dosage
- Dosing of Albendazole will vary, depending upon which of the following parasitic infections is being treated. In young children, the tablets should be crushed or chewed and swallowed with a drink of water.
- Patients being treated for neurocysticercosis should receive appropriate steroid and anticonvulsant therapy as required. Oral or intravenous corticosteroids should be considered to prevent cerebral hypertensive episodes during the first week of treatment.
|offLabelAdultGuideSupport=====[[Ancylostomiasis|Ancylostomiasis-Necatoriasis]=====
- Dosing Information
- 400 mg PO as a single dose
Ascariasis
- Dosing Information
- 400 mg PO as a single dose
Capillaria infection
Clonorchiasis
Cutaneous larva migrans
Enterobiasis
- Dosing Information
- 400 mg PO as a single dose; repeat in 2 weeks
Giardiasis
HIV infection - Infection by Microsporida (disseminated or intestinal)
- Dosing Information
- 400 mg PO 2 per day until CD4+ count is greater than 200 cells/mcL for longer than 6 months after initiation of ART; add itraconazole 400 mg PO daily for disseminated disease due to Trachipleistophora or Anncaliia
Infection by Gnathostoma
Infection by Loa loa
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Albendazole in adult patients. |fdaLIADPed=There is limited information regarding FDA Labeled indications and dosage of Albendazole in pediatric patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Albendazole in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Albendazole in pediatric patients. |contraindications=* Albrendazole is contraindicated in patients with known hypersensitivity to the benzimidazole class of compounds or any components of Albendazole. |warnings=* Rare fatalities associated with the use of Albendazole have been reported due to granulocytopenia or pancytopenia. Albendazole has been shown to cause bone marrow suppression, aplastic anemia, and agranulocytosis in patients with and without underlying hepatic dysfunction. Blood counts should be monitored at the beginning of each 28-day cycle of therapy, and every 2 weeks while on therapy with albendazole in all patients. Patients with liver disease, including hepatic echinococcosis, appear to be more at risk for bone marrow suppression leading to pancytopenia, aplastic anemia, agranulocytosis, and leukopenia attributable to albendazole and warrant closer monitoring of blood counts. Albendazole should be discontinued in all patients if clinically significant decreases in blood cell counts occur.
- Albendazole should not be used in pregnant women except in clinical circumstances where no alternative management is appropriate. Patients should not become pregnant for at least 1 month following cessation of albendazole therapy. If a patient becomes pregnant while taking this drug, albendazole should be discontinued immediately. If pregnancy occurs while taking this drug, the patient should be apprised of the potential hazard to the fetus.
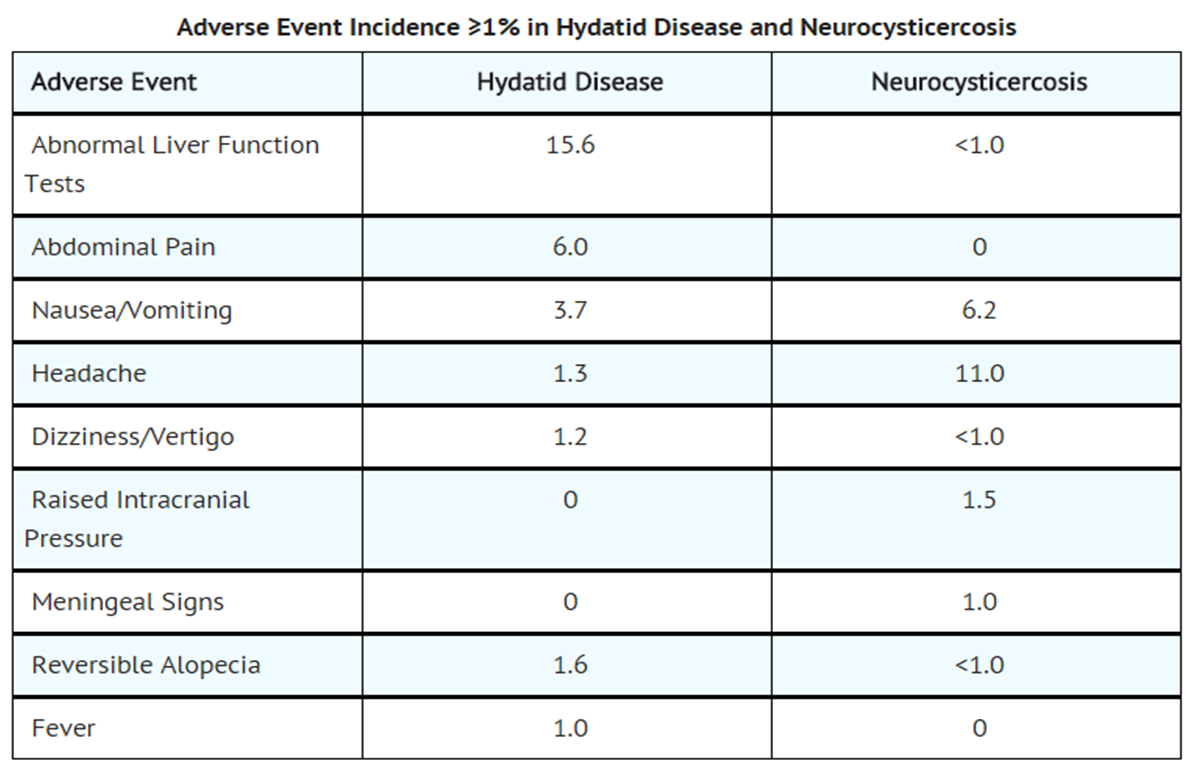
|clinicalTrials=* The adverse event profile of albendazole differs between hydatid disease and neurocysticercosis. Adverse events occurring with a frequency of ≥1% in either disease are described in the table below.
- These symptoms were usually mild and resolved without treatment. Treatment discontinuations were predominantly due to leukopenia (0.7%) or hepatic abnormalities (3.8% in hydatid disease). The following incidence reflects events that were reported by investigators to be at least possibly or probably related to albendazole.
- The following adverse events were observed at an incidence of <1%:
- Blood and Lymphatic System Disorders
- Leukopenia. There have been rare reports of granulocytopenia, pancytopenia, agranulocytosis, or thrombocytopenia. Patients with liver disease, including hepatic echinococcosis, appear to be more at risk of bone marrow suppression.
- Blood and Lymphatic System Disorders
- Immune System Disorders
- Hypersensitivity reactions, including rash and urticaria.
- Immune System Disorders
|postmarketing=* In addition to adverse events reported from clinical trials, the following events have been identified during world-wide post-approval use of Albendazole. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to Albendazole.
- Blood and Lymphatic System Disorders
- Hepatobiliary Disorders
- Skin and Subcutaneous Tissue Disorders
- Renal and Urinary Disorders
|drugInteractions=* Dexamethasone
- Steady-state trough concentrations of albendazole sulfoxide were about 56% higher when 8 mg dexamethasone was coadministered with each dose of albendazole (15 mg/kg/day) in 8 neurocysticercosis patients.
- Praziquantel
- In the fed state, praziquantel (40 mg/kg) increased mean maximum plasma concentration and area under the curve of albendazole sulfoxide by about 50% in healthy subjects (n = 10) compared with a separate group of subjects (n = 6) given albendazole alone. Mean Tmax and mean plasma elimination half-life of albendazole sulfoxide were unchanged. The pharmacokinetics of praziquantel were unchanged following coadministration with albendazole (400 mg).
- Cimetidine
- Albendazole sulfoxide concentrations in bile and cystic fluid were increased (about 2-fold) in hydatid cyst patients treated with cimetidine (10 mg/kg/day) (n = 7) compared with albendazole (20 mg/kg/day) alone (n = 12). Albendazole sulfoxide plasma concentrations were unchanged 4 hours after dosing.
- Theophylline
- The pharmacokinetics of theophylline (aminophylline 5.8 mg/kg infused over 20 minutes) were unchanged following a single oral dose of albendazole (400 mg) in 6 healthy subjects.
|FDAPregCat=C |useInPregnancyFDA=* Albendazole has been shown to be teratogenic (to cause embryotoxicity and skeletal malformations) in pregnant rats and rabbits. The teratogenic response in the rat was shown at oral doses of 10 and 30 mg/kg/day (0.10 times and 0.32 times the recommended human dose based on body surface area in mg/m2, respectively) during gestation days 6 to 15 and in pregnant rabbits at oral doses of 30 mg/kg/day (0.60 times the recommended human dose based on body surface area in mg/m2) administered during gestation days 7 to 19. In the rabbit study, maternal toxicity (33% mortality) was noted at 30 mg/kg/day. In mice, no teratogenic effects were observed at oral doses up to 30 mg/kg/day (0.16 times the recommended human dose based on body surface area in mg/m2), administered during gestation days 6 to 15.
- There are no adequate and well-controlled studies of albendazole administration in pregnant women. Albendazole should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
|useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Template:Levofloxacin in women who are pregnant.
|useInLaborDelivery=There is no FDA guidance on use of Levofloxacin during labor and delivery. |useInNursing=* Albendazole is excreted in animal milk. It is not known whether it is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when albendazole is administered to a nursing woman. |useInPed=* Experience in children under the age of 6 years is limited. In hydatid disease, infection in infants and young children is uncommon, but no problems have been encountered in those who have been treated. In neurocysticercosis, infection is more frequently encountered. In 5 published studies involving pediatric patients as young as 1 year, no significant problems were encountered, and the efficacy appeared similar to the adult population. |useInGeri=* Experience in patients 65 years of age or older is limited. The number of patients treated for either hydatid disease or neurocysticercosis is limited, but no problems associated with an older population have been observed. |useInGender=There is no FDA guidance on the use of albendazole with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of albendazole with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of albendazole in patients with hepatic impairment. |useInHepaticImpair=There is no FDA guidance on the use of albendazole in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of albendazole in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of albendazole in patients who are immunocompromised. |administration=* Oral |monitoring=* White Blood Cell Count
- Albendazole has been shown to cause occasional (less than 1% of treated patients) reversible reductions in total white blood cell count. Rarely, more significant reductions may be encountered including granulocytopenia, agranulocytosis, or pancytopenia. Blood counts should be performed at the start of each 28-day treatment cycle and every 2 weeks during each 28-day cycle in all patients. Patients with liver disease, including hepatic echinococcosis, appear to be more at risk of bone marrow suppression and warrant closer monitoring of blood counts. Albendazole should be discontinued in all patients if clinically significant decreases in blood cell counts occur.
- Liver Function
- In clinical trials, treatment with albendazole has been associated with mild to moderate elevations of hepatic enzymes in approximately 16% of patients. These elevations have generally returned to normal upon discontinuation of therapy. There have also been case reports of acute liver failure of uncertain causality and hepatitis.
- Liver function tests (transaminases) should be performed before the start of each treatment cycle and at least every 2 weeks during treatment. If hepatic enzymes exceed twice the upper limit of normal, consideration should be given to discontinuing albendazole therapy based on individual patient circumstances. Restarting albendazole treatment in patients whose hepatic enzymes have normalized off treatment is an individual decision that should take into account the risk/benefit of further albendazole usage. Laboratory tests should be performed frequently if albendazole treatment is restarted.
- Patients with abnormal liver function test results are at increased risk for hepatotoxicity and bone marrow suppression. Therapy should be discontinued if liver enzymes are significantly increased or if clinically significant decreases in blood cell counts occur.
|IVCompat=There is limited information regarding IV Compatibility albendazole in the drug label. |overdose=* Significant toxicity and mortality were shown in male and female mice at doses exceeding 5,000 mg/kg; in rats, at estimated doses between 1,300 and 2,400 mg/kg; in hamsters, at doses exceeding 10,000 mg/kg; and in rabbits, at estimated doses between 500 and 1,250 mg/kg. In the animals, symptoms were demonstrated in a dose-response relationship and included diarrhea, vomiting, tachycardia, and respiratory distress.
- One overdosage has been reported with Albendazole in a patient who took at least 16 grams over 12 hours. No untoward effects were reported. In case of overdosage, symptomatic therapy and general supportive measures are recommended.
|drugBox=
| |

| |
Albendazole
| |
| Systematic (IUPAC) name | |
| Methyl [5-(propylthio)-1H-benzoimidazol-2-yl]carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | P02 Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 265.333 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | <5%[1] |
| Protein binding | 70%[1] |
| Metabolism | Hepatic[1] |
| Half life | 8-12 hours[1] |
| Excretion | Urine, faeces[1] |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
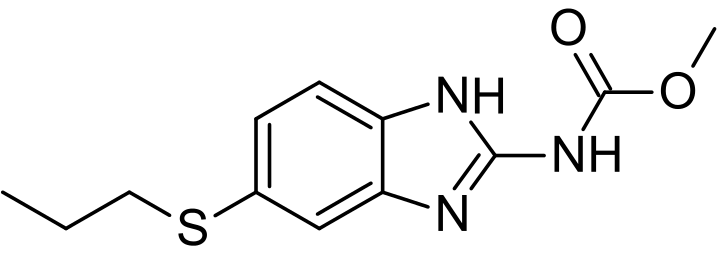
|mechAction=* As a vermicidal, albendazole causes degenerative alterations in the intestinal cells of the worm by binding to the colchicine-sensitive site of tubulin, thus inhibiting its polymerization or assembly into microtubules. The loss of the cytoplasmic microtubules leads to impaired uptake of glucose by the larval and adult stages of the susceptible parasites, and depletes their glycogen stores. Degenerative changes occur in the endoplasmic reticulum, the mitochondria of the germinal layer, and the subsequent release of lysosome. |structure=* Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34. It has the following chemical structure:

|PD=There is limited information regarding pharmacodynamics of albendazole in the drug label. |PK=====Absorption and Metabolism=====
- Albendazole is poorly absorbed from the gastrointestinal tract due to its low aqueous solubility. Albendazole concentrations are negligible or undetectable in plasma as it is rapidly converted to the sulfoxide metabolite prior to reaching the systemic circulation. The systemic anthelmintic activity has been attributed to the primary metabolite, albendazole sulfoxide. Oral bioavailability appears to be enhanced when albendazole is coadministered with a fatty meal (estimated fat content 40 g) as evidenced by higher (up to 5-fold on average) plasma concentrations of albendazole sulfoxide as compared to the fasted state.
- Maximal plasma concentrations of albendazole sulfoxide are typically achieved 2 to 5 hours after dosing and are on average 1.31 mcg/mL (range 0.46 to 1.58 mcg/mL) following oral doses of albendazole (400 mg) in 6 hydatid disease patients, when administered with a fatty meal. Plasma concentrations of albendazole sulfoxide increase in a dose-proportional manner over the therapeutic dose range following ingestion of a fatty meal (fat content 43.1 g). The mean apparent terminal elimination half-life of albendazole sulfoxide typically ranges from 8 to 12 hours in 25 normal subjects, as well as in 14 hydatid and 8 neurocysticercosis patients.
- Following 4 weeks of treatment with albendazole (200 mg three times daily), 12 patients’ plasma concentrations of albendazole sulfoxide were approximately 20% lower than those observed during the first half of the treatment period, suggesting that albendazole may induce its own metabolism.
Distribution
- Albendazole sulfoxide is 70% bound to plasma protein and is widely distributed throughout the body; it has been detected in urine, bile, liver, cyst wall, cyst fluid, and cerebral spinal fluid (CSF). Concentrations in plasma were 3- to 10-fold and 2- to 4-fold higher than those simultaneously determined in cyst fluid and CSF, respectively. Limited in vitro and clinical data suggest that albendazole sulfoxide may be eliminated from cysts at a slower rate than observed in plasma.
Metabolism and Excretion
- Albendazole is rapidly converted in the liver to the primary metabolite, albendazole sulfoxide, which is further metabolized to albendazole sulfone and other primary oxidative metabolites that have been identified in human urine. Following oral administration, albendazole has not been detected in human urine. Urinary excretion of albendazole sulfoxide is a minor elimination pathway with less than 1% of the dose recovered in the urine. Biliary elimination presumably accounts for a portion of the elimination as evidenced by biliary concentrations of albendazole sulfoxide similar to those achieved in plasma.
|nonClinToxic=There is limited information regarding nonclinical toxicology of Estradiol valerate and estradiol valerate/dienogest in the drug label. |howSupplied=* Albendazole is supplied as 200 mg, white to off-white, circular, biconvex, bevel-edged, film coated TILTAB tablet embossed "ap" and "550". They are supplied as follows:
- Bottles of 2 NDC 52054-550-22
- Bottles of 28 NDC 52054-550-28
- ALBENZA and TILTAB are registered trademarks of GlaxoSmithKline, used with permission.
- Manufactured by:
- GlaxoSmithKline
- Mississauga, Ontario
- L5N 6L4 Canada
- Distributed by:
- Amedra Pharmaceuticals, LLC
- Horsham, PA 19044
- LB# 799-03 Rev. February, 2013
|storage=* Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. |fdaPatientInfo=* Patients should be advised that:
- Some people, particularly young children, may experience difficulties swallowing the tablets whole. In young children, the tablets should be crushed or chewed and swallowed with a drink of water.
- Albendazole may cause fetal harm, therefore, women of childbearing age should begin treatment after a negative pregnancy test.
- Women of childbearing age should be cautioned against becoming pregnant while on albendazole or within 1 month of completing treatment.
- During albendazole therapy, because of the possibility of harm to the liver or bone marrow, routine (every 2 weeks) monitoring of blood counts and liver function tests should take place.
- Albendazole should be taken with food.
|alcohol=Alcohol-Albendazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=* Albenza® }} {{#subobject:
|Page Name=Albendazole |Pill Name=ALBENZA_NDC_520540550.jpg |Drug Name=ALBENZA |Pill Ingred=ALBENDAZOLE[ALBENDAZOLE]|+sep=; |Pill Imprint=ap;550 OR SB;5500 |Pill Dosage=200 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=12 |Pill Scoring=1 |Pill Image= |Drug Author=Amedra Pharmaceuticals LLC |NDC=520540550
}}
{{#subobject:
|Label Page=Albendazole |Label Name=Albendazole package01.png
}}
{{#subobject:
|Label Page=Albendazole |Label Name=Albendazole package02.png
}}