Macrocytic anemia: Difference between revisions
| Line 17: | Line 17: | ||
==Overview== | ==Overview== | ||
'''Megaloblastic anemia''' is an [[anemia]] (of [[macrocytic anaemia|macrocytic]] classification) which results from inhibition of DNA synthesis in red blood cell production. It is often due to deficiency of [[vitamin B12]] and/or [[folic acid]]. It can be the result of a lack of [[intrinsic factor]] (which lack interferes with B12 absorption), causing [[pernicious anemia]], or with other antimetabolites which poison DNA production, such as chemotherapeutic agents. It is characterized by many large immature and dysfunctional red blood cells ([[megaloblasts]]) in the bone marrow, and also by hypersegmented or multisegmented [[neutrophil]]s. <ref>Babior BM, Bunn HF, Megaloblastic Anemias. Harrison’s Principles of Internal Medicine. 13th Ed. 1994: 1726-1732. ISBN 0070323704</ref> <ref>Toh BH, van Driel IR, Gleeson PA. Mechanisms of Disease: Pernicious Anemia. NEJM 1997;337:1441.</ref> | '''Macrocytosis''' is the enlargement of [[red blood cell]]s with near-constant [[haemoglobin]] concentration, and is defined by a [[mean corpuscular volume]] (MCV) of greater than 100 femtolitres (the precise criterion varies between laboratories). '''Megaloblastic anemia''' is an [[anemia]] (of [[macrocytic anaemia|macrocytic]] classification) which results from inhibition of DNA synthesis in red blood cell production. It is often due to deficiency of [[vitamin B12]] and/or [[folic acid]]. It can be the result of a lack of [[intrinsic factor]] (which lack interferes with B12 absorption), causing [[pernicious anemia]], or with other antimetabolites which poison DNA production, such as chemotherapeutic agents. It is characterized by many large immature and dysfunctional red blood cells ([[megaloblasts]]) in the bone marrow, and also by hypersegmented or multisegmented [[neutrophil]]s. <ref>Babior BM, Bunn HF, Megaloblastic Anemias. Harrison’s Principles of Internal Medicine. 13th Ed. 1994: 1726-1732. ISBN 0070323704</ref> <ref>Toh BH, van Driel IR, Gleeson PA. Mechanisms of Disease: Pernicious Anemia. NEJM 1997;337:1441.</ref> | ||
==Risk factors== | ==Risk factors== | ||
Revision as of 13:48, 21 September 2012
For patient information click here
| Macrocytic anemia | |
 | |
|---|---|
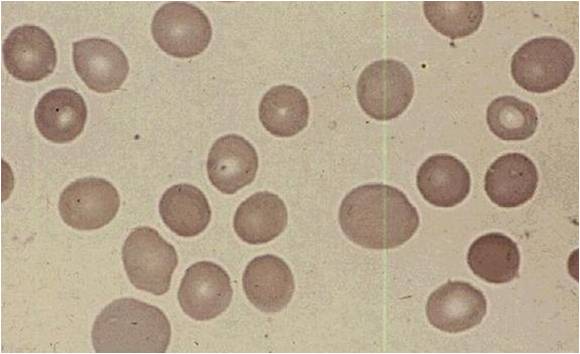
| Megaloblastic anemia blood smear | |
| ICD-10 | D51.1, D52.0, D53.1 |
| ICD-9 | 281 |
| DiseasesDB | 29507 |
| MeSH | D000749 |
Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Macrocytosis is the enlargement of red blood cells with near-constant haemoglobin concentration, and is defined by a mean corpuscular volume (MCV) of greater than 100 femtolitres (the precise criterion varies between laboratories). Megaloblastic anemia is an anemia (of macrocytic classification) which results from inhibition of DNA synthesis in red blood cell production. It is often due to deficiency of vitamin B12 and/or folic acid. It can be the result of a lack of intrinsic factor (which lack interferes with B12 absorption), causing pernicious anemia, or with other antimetabolites which poison DNA production, such as chemotherapeutic agents. It is characterized by many large immature and dysfunctional red blood cells (megaloblasts) in the bone marrow, and also by hypersegmented or multisegmented neutrophils. [1] [2]
Risk factors
- Folate deficiency
- Nutritionally deficient - Elderly, alcohol, narcotic abuse
- Those with increased demand: pregnancy, infancy, low grade hemolysis, malignancy or chronic hemodialysis.
- Pernicious Anemia (PA) is the most common cause of B12 deficiency in the US and is associated with other autoimmune disease such as Hashimoto’s, vitiligo, diabetes, adrenal insufficiency, etc. (Schmitt’s Syndrome).
- Strict Vegans and/or their infants can become B12 deficient from poor intake.
- Patients with malabsorptive disorders such as blind loops/bacterial overgrowth, Sprue, Whipple’s and Crohn’s can malabsorb folate and B12. D.Latum is a competitor for B12 absorption. This entity is most commonly found in Scandinavia.
Pathophysiology and Etiology
Biochemical Review
- Folate is important in the production of various building blocks necessary for the production of biologic macromolecules. By combining with carbon moieties, tetrahydrofolate (THF) becomes methelenetetrahydofolate. This molecule is then able to donate carbon moieties to form purines, dTMP, and methionine. Of note, Vitamin B12 is also a cofactor in the production of methionine.
- THF is the resulting molecule after donation of carbon moieties except in the synthesis of dTMP from dUMP. DHF (dihydrofolate) results from this reaction. DHF reductase must act on DHF to participate in reactions again.
- The two metabolically active forms of Vitamin B12 are Methycobalamin and Adenosylcobalamin. The former is important in methionine synthesis. Methionine is necessary for the production of choline phospholipids. Adenosylcobalamin is necessary to convert methylmalonyl CoA to succinyl-CoA. Interruption of this reaction eventually leads to nonphysiologic fatty acid production and abnormal neuronal lipid production.
- B12 deficiency also leads to folate metabolism derangement. Tissue folate levels are reduced in the setting of Vitamin B12 deficiency through a complicated biochemical pathway. This is known as the “folate trap hypothesis” and explains why large doses of folate will help the hematological manifestations. The mechanism of the neurologic manifestations remains independent of folate metabolism.
Body Stores
Folate
- Folate has minimum daily requirement of 50 mcg per day this requirement can increase substantially in settings such as pregnancy.
- Total body stores are approximately 5-20mg with half held in the liver. The serum folate level is not a reliable index of tissue folate levels.
- Serum folate levels can go up or down despite normal tissue levels depending on dietary intake and EtOH intake. The RBC (red blood cell) folate level is a better measure of tissue folate stores.
Vitamin B12
- The minimum daily requirement for B12 is 2.5 mcg.
- About 4mg is stored in the body with half in the liver.
- Obviously, it takes much longer to become B12 (3-6 years) versus folate (3 months) if intake ceased abruptly.
- The test for B12 is variable.
Diagnosis
Laboratory diagnosis
- Measurement of homocysteine and Methyl malanoic acid can help confirm and differentiate from folate deficiency.
- Both are elevated in B12 deficiency while only homocysteine is elevate in folate deficiency.
- If PA is based on low serum level, Anti-IF antibodies are confirmatory but only present in 50%.
- Anti-pareital cell Abs are more sensitive (90%) but less specific.
Schilling Test
- Dietary B12 is bound to factors that are cleaved off by acid leaving B12 free to bind to R factors secreted by the saliva and gastric juice.
- B12 bound to R factor is not absorbed, but instead requires the alkaline pancreatic secretions and proteases in the duodenum to be freed from R factor.
- Free B12 can then bind to IF where it is transported to the ileum where a specific receptor then takes up the complex. Therefore, normal B12 absorption and action are dependent of 5 things:
- Dietary intake
- Acid in the stomach
- Pancreatic secretions
- Secretion of IF by Gastric parietal cells
- An ileum that can absorb the IF-B12 complex
- The Schilling test is designed to test the different components of this system.
- First, 1mg IM B12 is given to saturate transcobalmin.
- Radiolabeled B12 is then given orally.
- If it can be absorbed, then >9% will be excreted in the urine in 24 hours and the rest is eliminated in the feces undetected.
- Renal insufficiency causes a falsely low level.
- It may be spuriously normal in patients without gastrectomy.
- Since, acid is required to free dietary B12 from binding factors.
- Radiolabeled b12 is not bound to these factors. Therefore, persons with impaired acid and pepsin production can absorb too little dietary B12, but will absorb free B12 normally.
- Part two of the Schilling test is administering radiolabeled b12 with OF to see if the problem corrects. This should differentiate PA from those with intestinal malabsorption. Remember, B12 deficiency affects the intestinal mucosal cells and causes malabsorption. Therefore, Part II should only be done 4 weeks after replacement, giving the mucosa time to regenerate in PA.
- If Part II is abnormal, Part III is basically a repeat of Part I after a course of antibiotics/vermicides.
- If this is abnormal, an malabsorptive cause is implicated.
Causes
Normal B12 absorption and action are dependent of 5 things: dietary intake, acid in the stomach, pancreatic secretions, secretion of IF by Gastric parietal cells, an ileum that can absorb the IF-B12 complex
- Deficient intake
- Deficient intrinsic factor (pernicious anaemia or gastrectomy)
- Bilogical competition for B12 by diverticulosis, fistula, intestinal anastomosis, achlorhydria and infection by the marine parasite Diphyllobothrium latum
- Selective B12 malabsorption (congenital and drug-induced)
- Chronic pancreatitis
- Ileal resection and bypass
- Folate Deficiency:
- Deficient intake.
- Increased needs: pregnancy, infant, rapid cellular proliferation, and cirrhosis
- Malabsorption (congenital and drug-induced)
- Intestinal and jejunal resection
- Combined Dieficiency (Tropical Sprue): Vitamin B12 & Folate.
- Inherited DNA Synthesis Disorders: Deficient thiamine and factors (e.g. enzymes) responsible for folate metabolism.
- Toxins and Drugs:
- Folic acid antagonists (methotrexate)
- Purine antagonists (6-mercaptopurine)
- Pyrimidine antagonists (cytosine arabinoside)
- In general:
- Nutritional defects (folic acid or vitamin B12 which is mainly from animal sources; vegans may require supplementation)
- Chronic liver diseases
- Alcoholism
- Pregnancy
- Decreased production of intrinsic factor (this disease entity is called pernicious anemia)
- Intestinal malabsorption (due to an enteritis, celiac disease or other causes).
- Fish tapeworm infestation (Diphyllobothrium latum)
- Failure to replicate chromosomes due to lack of thymidine.
- Lesch-Nyhan Syndrome
- Cytotoxic drugs interfering with DNA synthesis
- intestinal flora disruption due to antibiotic use
Classification
Megaloblastic anemias (DNA replication disorders)
- Commonest cause of macrocytic anemia.
- In megaloblastic anemias cells are larger because they cannot produce DNA quickly enough to divide at the right time as they grow, and thus grow too large before division.
- Causes for the DNA synthetic problem range from lack of certain vitamins needed to produce DNA (notably folate and B12), to poisons or inhibitors of DNA replication, such as some kinds of antiviral drugs and chemotherapeutic agents.
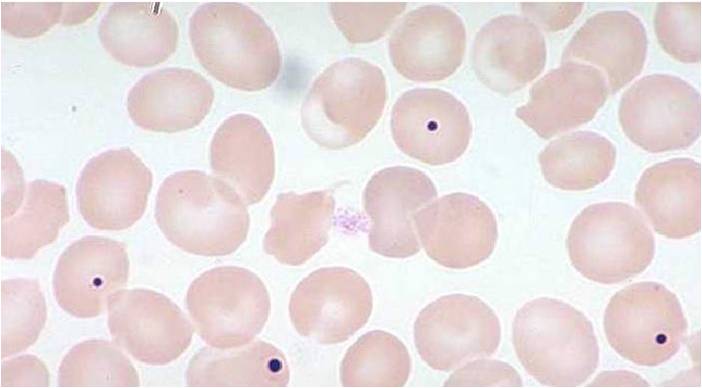
- The pathognomonic findings of megaloblastic anemia are: megaloblasts in bone marrow, ovalocytes in the (peripheral) blood smear, and hypersegmented neutrophils.
Non megaloblastic macrocytic anemias
- Non megaloblastic macrocytic anemias, are disorders associated with increased red cell membrane surface area
- It is commonly associated with pathologies of the liver and spleen which produce codocytes or "target cells" which have a central collection of hemoglobin surrounded by a pallor (a thin area) then followed by a thicker collection of hemoglobin at the rim of the cell.
Alcohol
- Round macrocytes which are not codocytes are produced in chronic alcoholism (which produces a mild macrocytosis even in the absence of vitamin deficiency), apparently as a direct toxic effect of alcohol specifically on the bone marrow.
Association with rapid red cell turnover and reticulocytosis
Mild macrocytocis is a common finding associated with rapid blood restoration or production, since in general, "fresh" or newly-produced red cells (reticulocytes) are larger than the mean (average) size, due to slow shrinkage of normal cells over a normal red cell circulating lifetime. Thus, chronic obstructive pulmonary disease (COPD), in which which red cells are rapidly produced in response to low oxygen levels in the blood, often produces mild macrocytosis. Also, rapid blood replacement from the marrow after a traumatic blood loss, or rapid red blood cell turnover from rapid hemolysis, also often produces mild macrocytosis in the associated anemia. ==Diagnosis==
History and Symptoms
- The major manifestations of Folate or B12 deficiency are related to the anemia and gastrointestinal dysfunction. Only B12 deficiency causes neurologic dysfunction. Constitutional symptoms related to anemia such as fatigue, dyspnea, lightheadedess, and anorexia occur. High output cardiac failure and angina are also consequences.
Symptoms mostly related to GI mucosal abnormalities. Tend to be worse in folate rather than B12 deficiency. Diarrhea, cheilosis and glossitis can be noted.
- The classic picture of B12 deficiency is subacute combined degeneration of the dorsal columns. Specific for B12 deficiency, the patient will present with a broad based gait, ataxic, irritable, forgetful with numbness or paresthesias. Rhomberg and Babinski’s can be noted. Dementia may progress to frank “Megaloblastic Madness”. Remember, hematological abnormalities can occur without neurologic manifestations in B12 deficiency.
Hematological findings
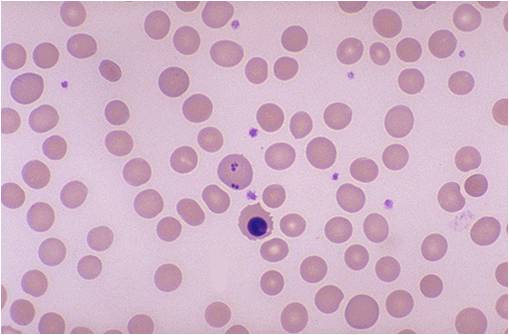
MCV is often >110. Hct can often be as low as 15. Elevated LDH and bilirubin are seen since dyserythopoesis leads to destruction of >90% of RBC precursors. Hypersegmentation of PMNs is quite sensitive (>5% with 5 or more lobes or >1% with 6 lobes). Reticulocyte, WBC and platelets are low to normal. In one series of patients with B12 deficiency, 64% had a MCV greater than 100, and only 29% had anemia. In general the blood film can point towards vitamin deficiency:
- Decreased red blood cell (RBC) count and hemoglobin levels
- Increased mean corpuscular volume (MCV >95 fl often >110) and mean corpuscular hemoglobin (MCH)
- The reticulocyte count is normal
- The platelet count may be reduced.
- Neutrophil granulocytes may show multisegmented nuclei ("senile neutrophil"). This is thought to be due to decreased production and a compensatory prolonged lifespan for circulating neutrophils.
- Anisocytosis (increased variation in RBC size) and poikilocytosis (abnormally shaped RBCs).
- Macrocytes (larger than normal RBCs) are present.
- Ovalocytes (oval shaped RBCs) are present.
- Bone marrow (not normally checked in a patient suspected of megaloblastic anemia) shows megaloblastic hyperplasia.
- Howell-Jolly bodies (chromosomal remnant) also present.
Blood chemistries will also show:
- Increased homocysteine and methylmalonic acid in B12 deficiency
- Increased homocysteine in folate defiency
Analysis
The Schilling test was performed in the past to determine the nature of the vitamin B12 deficiency, but due to the lack of available radioactive B12, it is now largely a historical artifact. Vitamin BTemplate:Ssub is a necessary prosthetic group to the enzyme methylmalonyl-coenzyme A mutase. BTemplate:Ssub deficiency leads to dysfunction of this enzyme and a buildup of its substrate, methylmalonic acid, the elevated level of which can be detected in the urine and blood. Since the level of methylmalonic acid is not elevated in folic acid deficiency, this test provides a one tool in differentiating the two. However, since the test for elevated methylmalonic acid is not specific enough, the gold standard for the diagnosis of B12 deficiency is a low blood level of B12. Unlike the Shilling test, which often included B12 with intrinsic factor, a low level of blood B12 gives no indication as to the etiology of the low B12, which may result from a number of mechanisms.
Treatment
- Folate is administered 1mg QD. Higher doses may be required in malabsorptive syndromes. It is empirically given to those with SCD and those on HD.
- B12 must be given as a load then maintenance. Most advocate 1000 mcg IM Qweek x4 then 100mcg/month.
- LDH falls in 2 days. Hypokalemia requiring replacement can occur in the acute phase as new cells are being generated rapidly.
- A reticulocytosis begins in 3-5 days and peaks in 10 days. The HCT will rise within 10days. If it does not, suspect another disorder. Hypersegmented PMNs disappear in 10-14 days.
- Neurologic abnormalities may take up to 6 months to resolve if ever. The longer the disease has been present, the worse is the prognosis for recovery.
- Persons with PA have a 2x risk of gastric CA (in some studies). Screen for occult blood.
el:Μακροκυτταρική αναιμία sq:Anemi makrocitike
References
- ↑ Babior BM, Bunn HF, Megaloblastic Anemias. Harrison’s Principles of Internal Medicine. 13th Ed. 1994: 1726-1732. ISBN 0070323704
- ↑ Toh BH, van Driel IR, Gleeson PA. Mechanisms of Disease: Pernicious Anemia. NEJM 1997;337:1441.
- ↑ Sailer, Christian, Wasner, Susanne. Differential Diagnosis Pocket. Hermosa Beach, CA: Borm Bruckmeir Publishing LLC, 2002:77 ISBN 1591032016
- ↑ Kahan, Scott, Smith, Ellen G. In A Page: Signs and Symptoms. Malden, Massachusetts: Blackwell Publishing, 2004:68 ISBN 140510368X
gl:Anemia megaloblástica he:אנמיה מגלובלסטית it:Anemia megaloblastica sl:Megaloblastna anemija sr:Мегалобластна анемија