Sandbox:Cherry: Difference between revisions
No edit summary |
|||
| Line 26: | Line 26: | ||
==Associated Conditions== | ==Associated Conditions== | ||
Revision as of 16:47, 21 December 2017
Pathophysiology prev
| https://https://www.youtube.com/watch?v=5szNmKtyBW4%7C350}} |
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief:
Overview
Cirrhosis occurs due to long term liver injury which causes an imbalance between matrix production and degradation. Early disruption of the normal hepatic matrix results in its replacement by scar tissue, which in turn has deleterious effects on cell function.
Pathophysiology
Pathology
- There are four stages of Cirrhosis as it progresses:
- Chronic nonsuppurative destructive cholangitis - inflammation and necrosis of portal tracts with lymphocyte infiltration leading to the destruction of the bile ducts.
- Development of biliary stasis and fibrosis
- Periportal fibrosis progresses to bridging fibrosis
- Increased proliferation of smaller bile ductules leading to regenerative nodule formation.
Associated Conditions
| Portal Hypertension associated conditions | |||||||||||||||||||||||||||||||||||||||||||||||
| Immunological disorders | Infections | Medication and toxins | Genetic disorders | Prothrombotic conditions | |||||||||||||||||||||||||||||||||||||||||||
| • Common variable immunodeficiency syndrome[1] • Connective tissue diseases[2] • Crohn’s disease[3] • Solid organ transplant •• Renal transplantation[4] •• Liver transplantation[5] • Hashimoto's thyroiditis[6] • Autoimmune disease[7] | • Bacterial intestinal infections • Recurrent E.coli infection[8] • Human immunodeficiency virus (HIV) infection[9] • Antiretroviral therapy[10] | • Thiopurine derivatives •• Didanosine •• Azathioprine[11] •• Cis-thioguanine[12] • Arsenicals[13] • Vitamin A[14] | • Adams-Olivier syndrome[15] • Turner syndrome[16] • Phosphomannose isomerase deficiency[17] • Familial cases[18] | • Inherited thrombophilias [19] • Myeloproliferative neoplasm[19] • Antiphospholipid syndrome[19] • Sickle cell disease[20] | |||||||||||||||||||||||||||||||||||||||||||
Gross Pathology
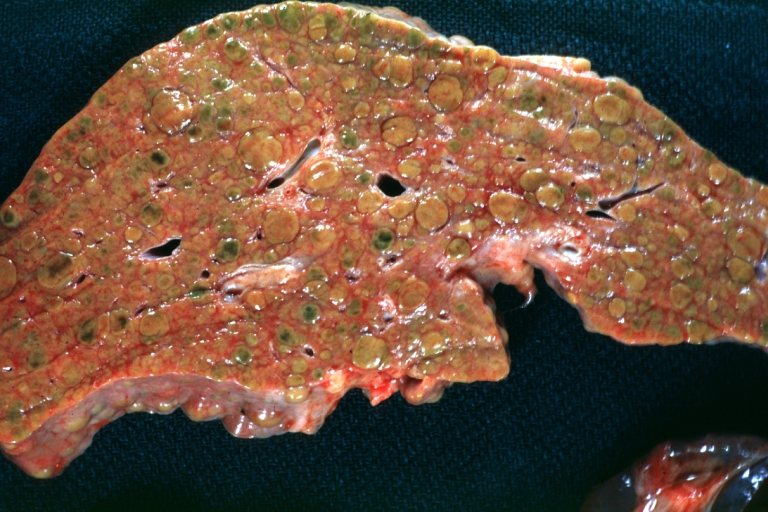
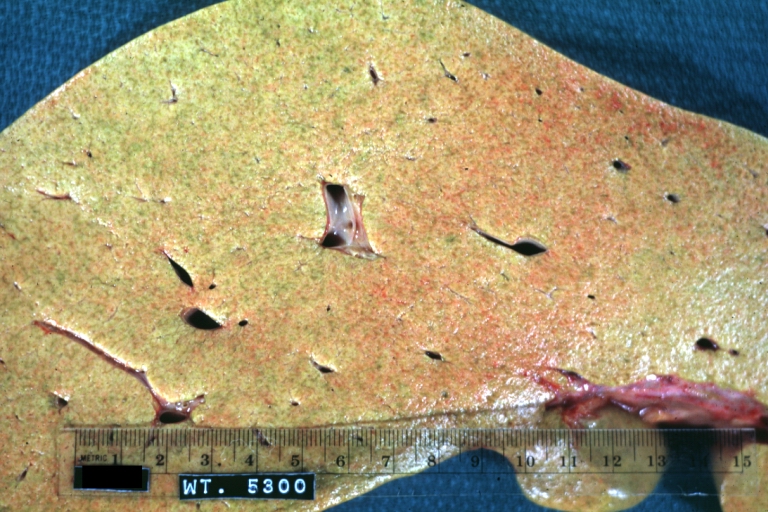
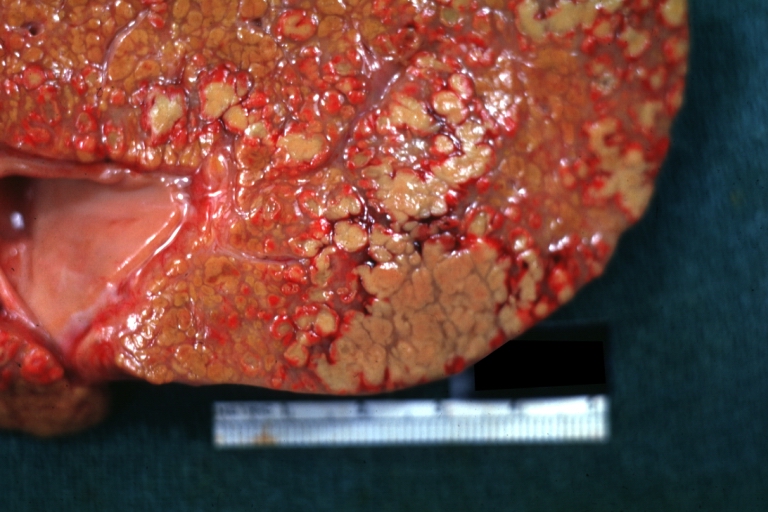
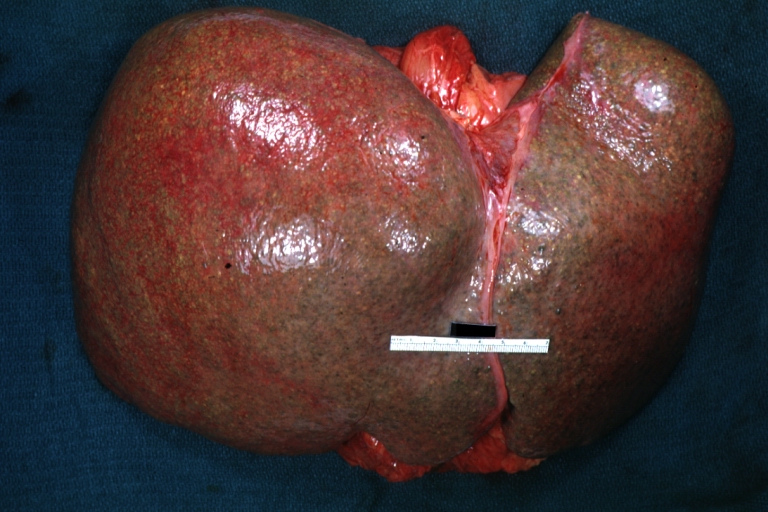
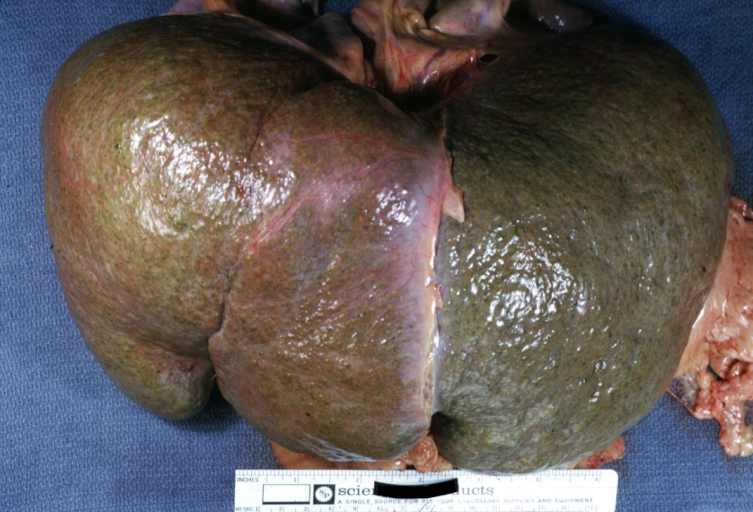
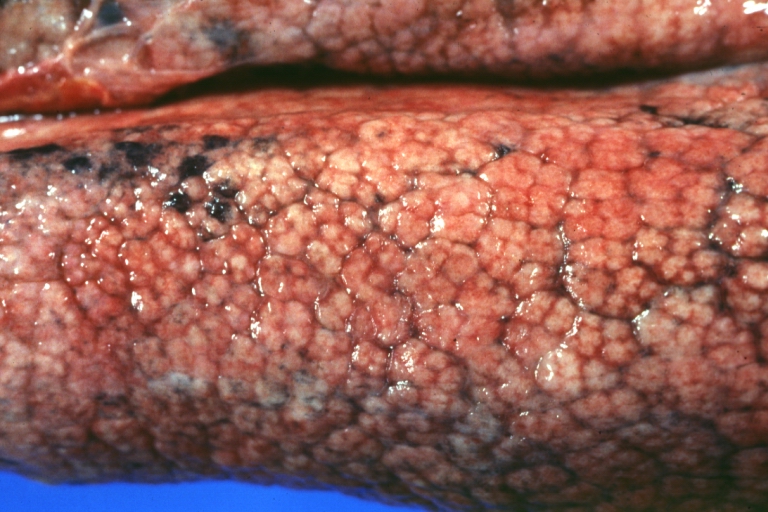
Macroscopically, the liver may initially be enlarged, but with progression of the disease, it becomes smaller. Its surface is irregular, the consistency is firm, and the color is often yellow (if associates steatosis). Depending on the size of the nodules there are three macroscopic types: micronodular, macronodular and mixed cirrhosis.
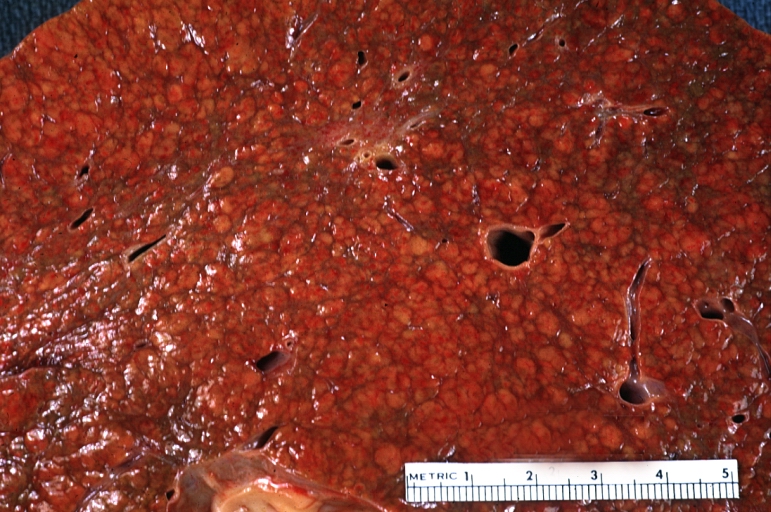
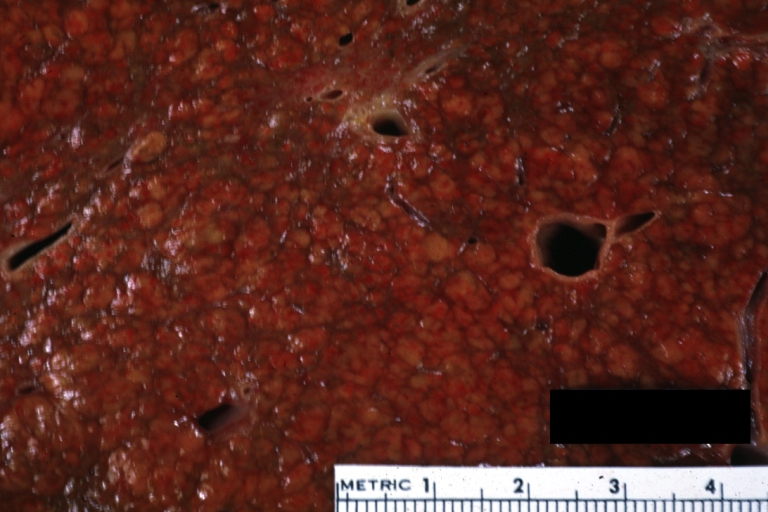
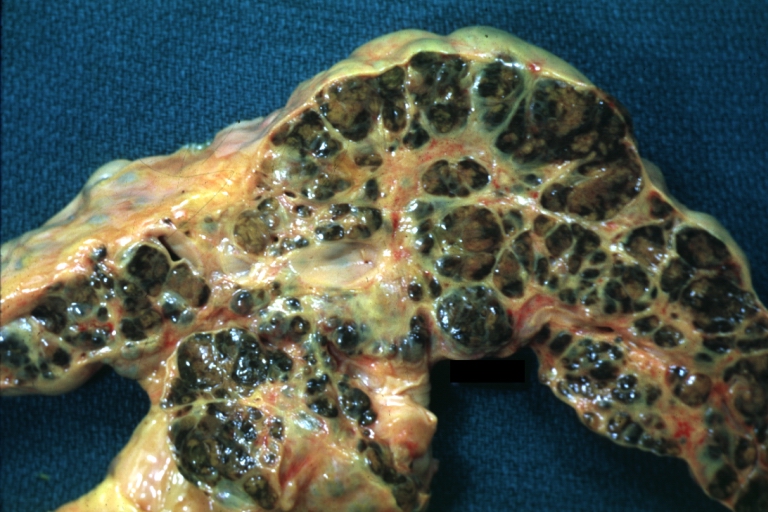
- In the micronodular form (Laennec's cirrhosis or portal cirrhosis) regenerating nodules are under 3 mm.
- In macronodular cirrhosis (post-necrotic cirrhosis), the nodules are larger than 3 mm.
- The mixed cirrhosis consists of a variety of nodules with different sizes.
Gross Pathology
| ||
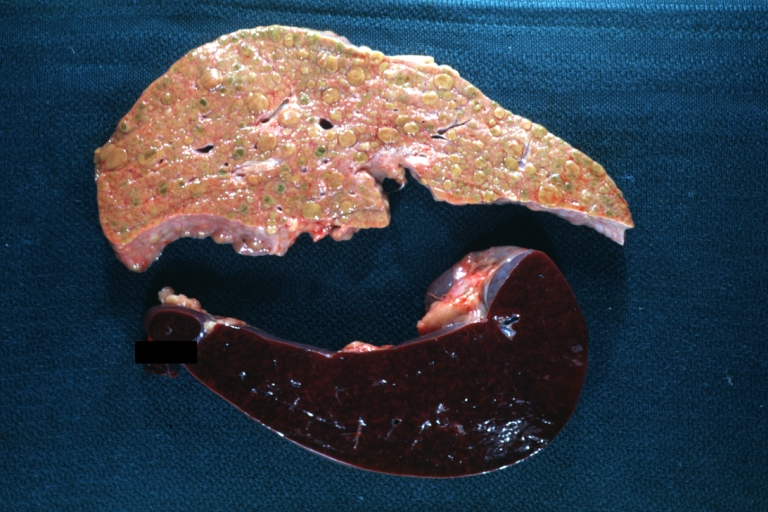
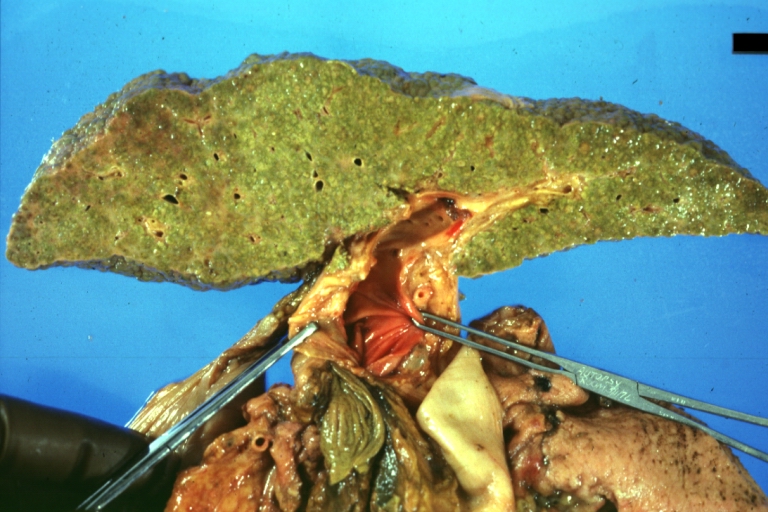
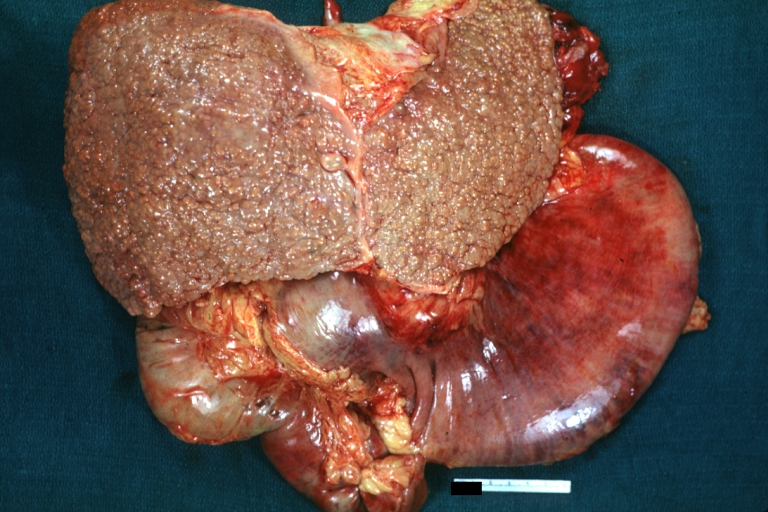
CirrhosisOn gross pathology there are two types of cirrhosis:
|
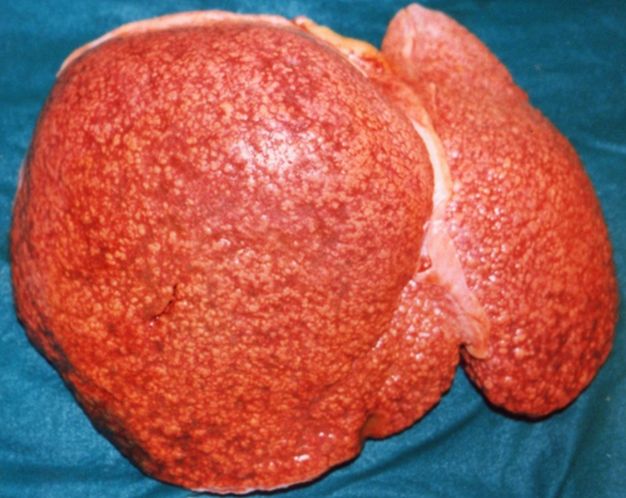 |
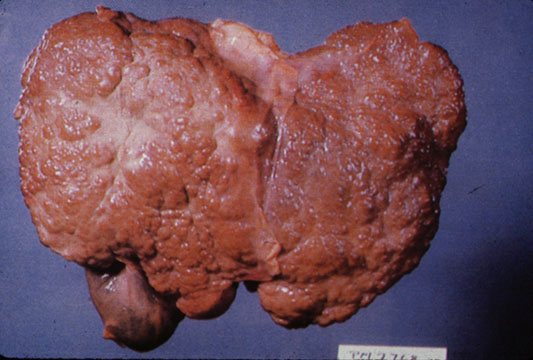 |
SplenomegalyOn gross pathology, diffuse enlargement and congestion of the spleen are characteristic findings of splenomegaly. |
 | |
Esophageal VaricesOn gross pathology, prominent, congested, and tortoise veins in the lower parts of esophagus are characteristic findings of esophageal varices. |
 | |
-
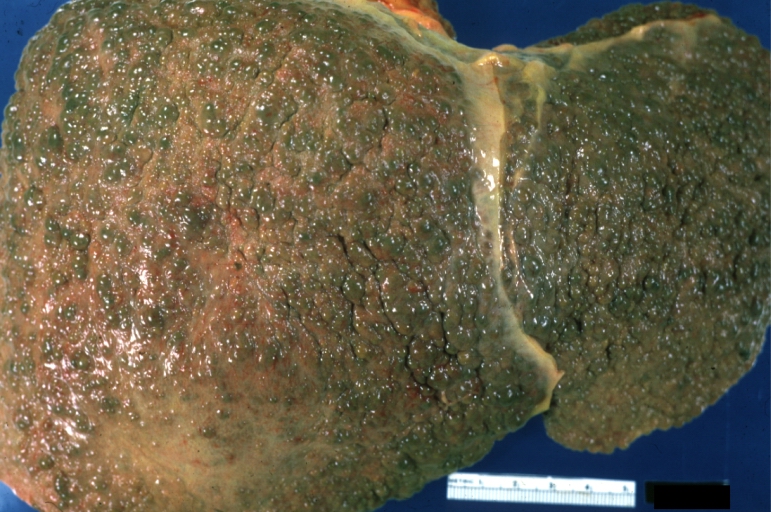
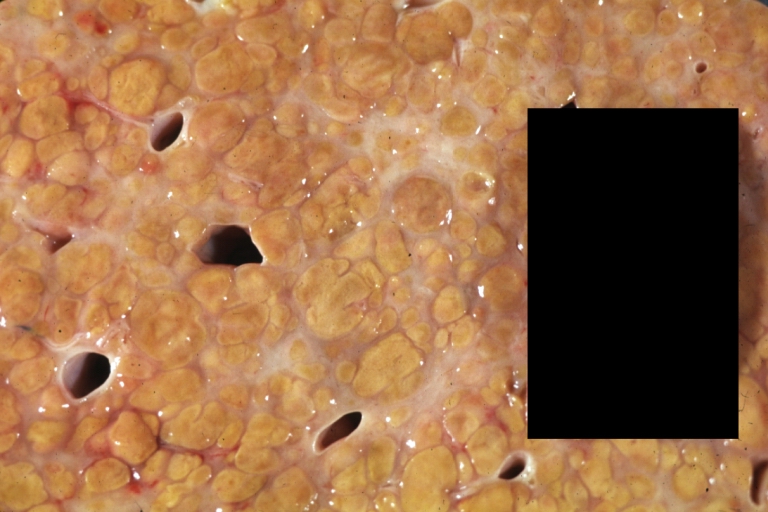
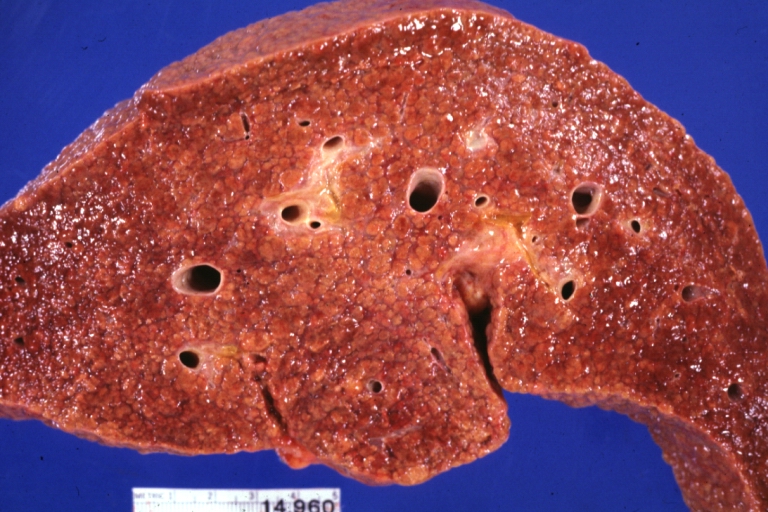
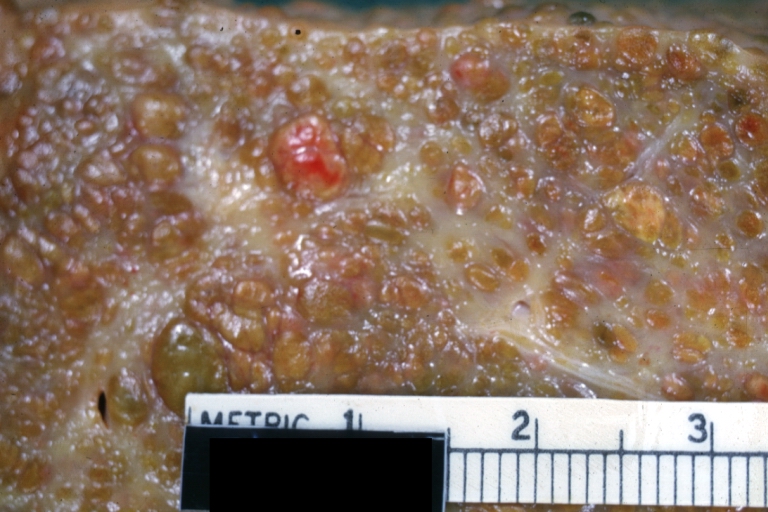
Cirrhosis: Gross, external view of micronodular cirrhosis
-
Cirrhosis: Gross, cut section of previous one (an excellent example)
-
Cirrhosis: Gross, close-up image
-
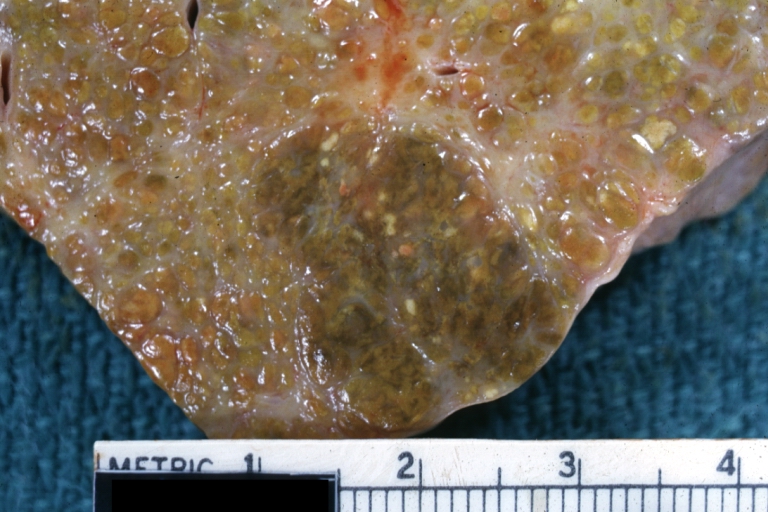
Macronodular cirrhosis and hepatoma
-
Cirrhosis: Gross, close-up, natural color (an excellent example)
-
Cirrhosis: Gross, close-up (an excellent example)
-
Cirrhosis: Gross, close-up view
-
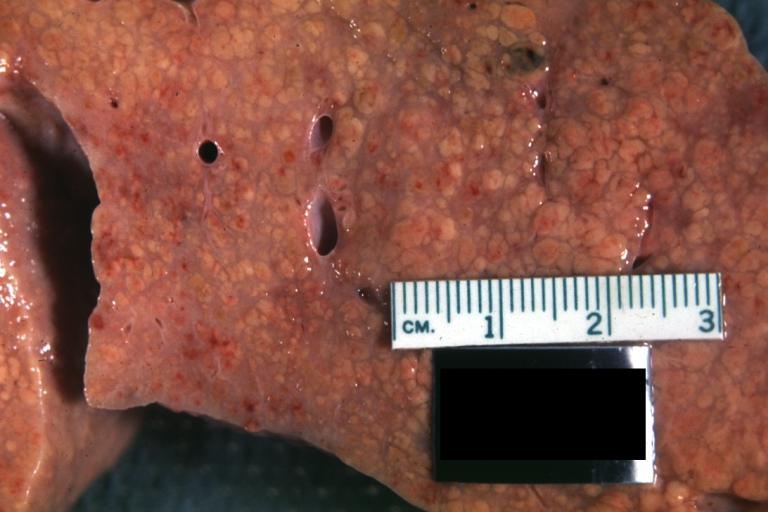
Micronodular cirrhosis: Gross, external view (an excellent example)
-
Micronodular cirrhosis: Gross, close-up image
-
Micronodular cirrhosis: Gross (an excellent example)
-
Macronodular cirrhosis: Gross, natural color (perfect color for cirrhosis), close-up, an excellent example
-
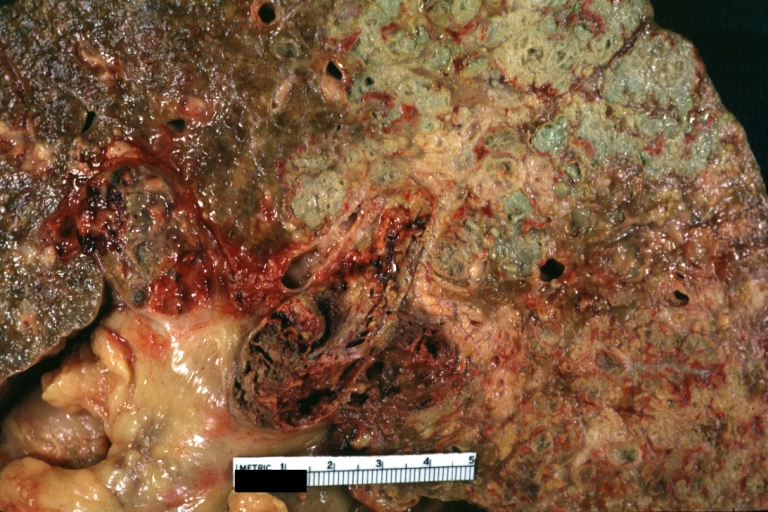
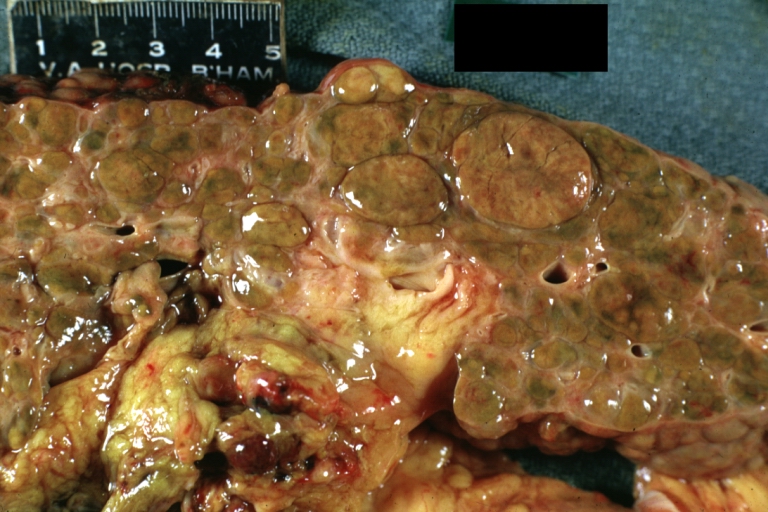
Cirrhosis with portocaval shunt: Gross, severe cirrhosis with extensive liver necrosis due to thrombosis of portocaval shunt (well shown)
-
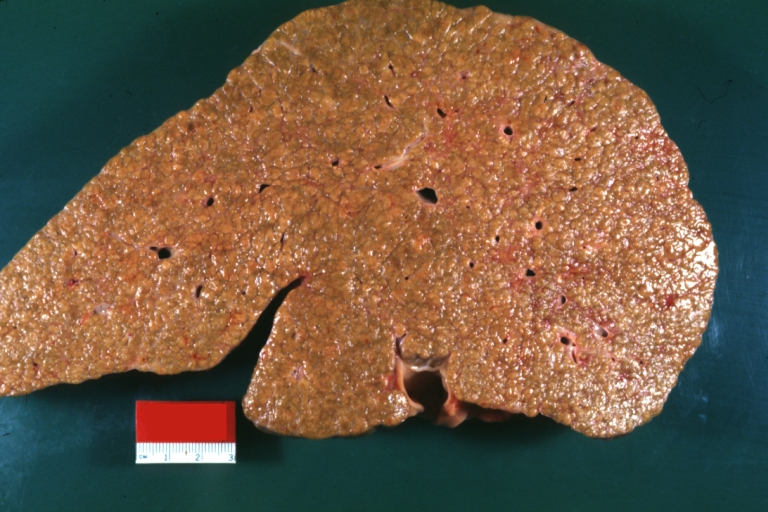
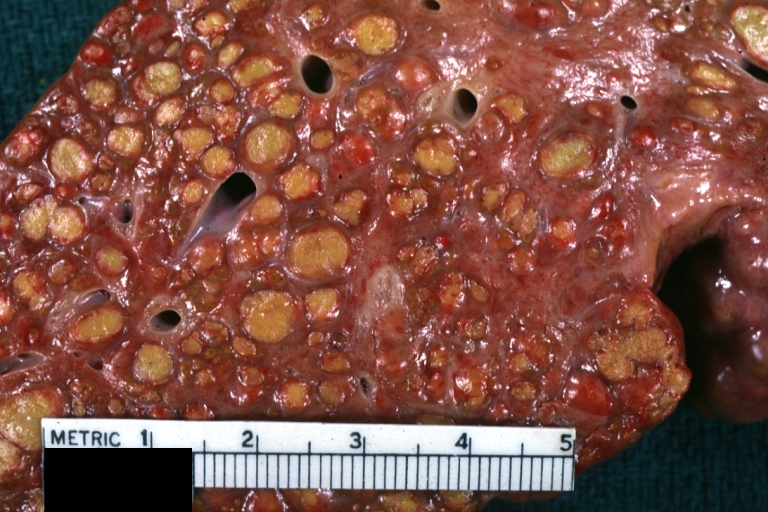
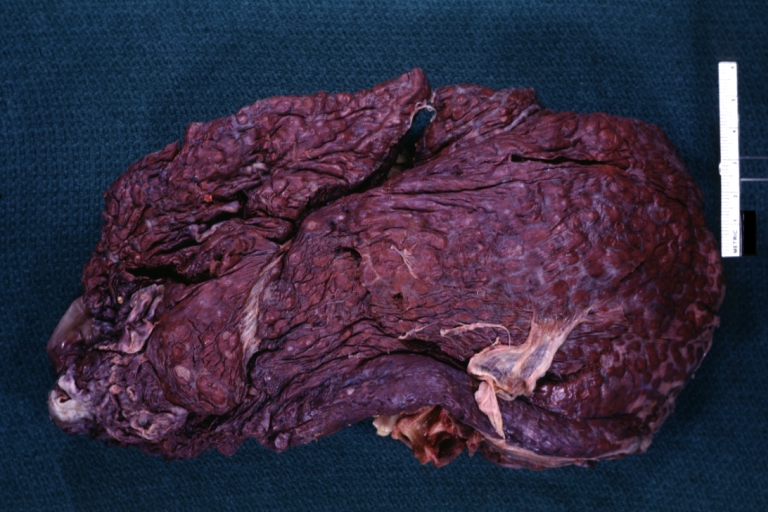
Endstage cirrhosis: Gross, natural color, close-up (an excellent example)
-
Endstage cirrhosis: Gross, natural color, close-up view is an excellent example for nodules of yellow-orange liver tissue and broad irregular bands of fibrosis
-
Endstage cirrhosis: Gross, natural color, close-up cut surface, very well shown nodules of yellow and necrotic opaque liver tissue with broad and irregular bands of fibrosis (an excellent example)
-
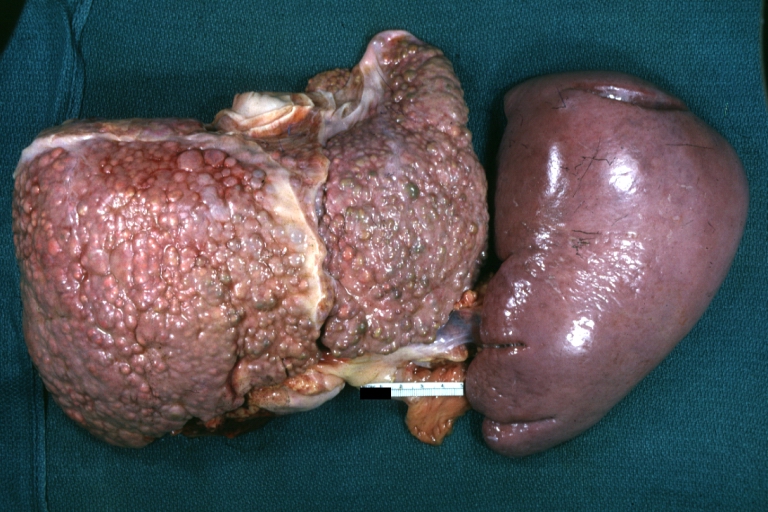
Macronodular cirrhosis: Gross, natural color, external view of liver and very enlarged spleen (liver has variable size nodules up to about 2 cm)
-
Macronodular cirrhosis: Gross, natural color, cut surface, large irregular bands of fibrosis with variable size liver cell nodules up to about 8 mm and all necrotic appears to be an end stage liver disease.
-
Macronodular cirrhosis: Gross, natural color view of frontal sections of liver and spleen showing a contracted macronodular liver and an enlarged spleen as large as the liver
-
Macronodular cirrhosis: Gross, natural color slab of liver
-
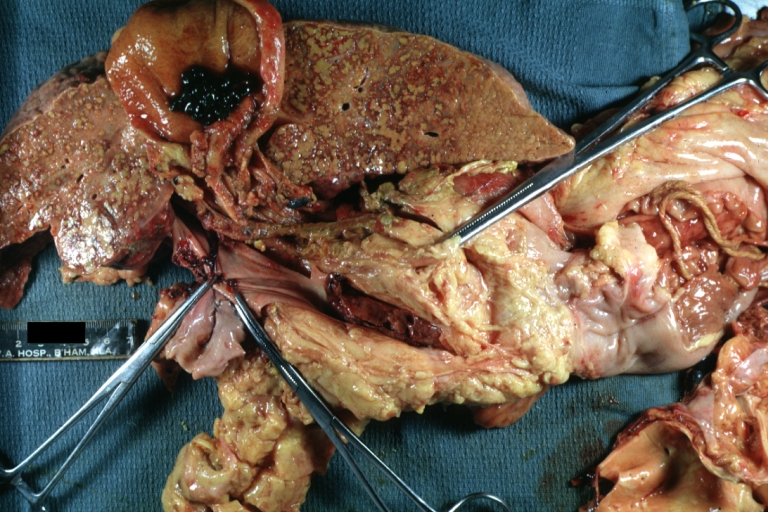
Fatty change and early cirrhosis: Gross, natural color, rather close-up image showing typical fatty color, and in lighting at lower right of micrography micronodularity is evident (quite good example)
-
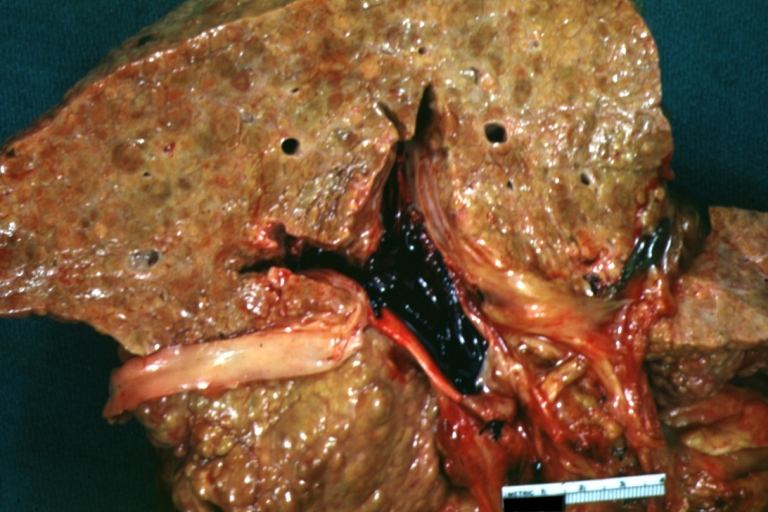
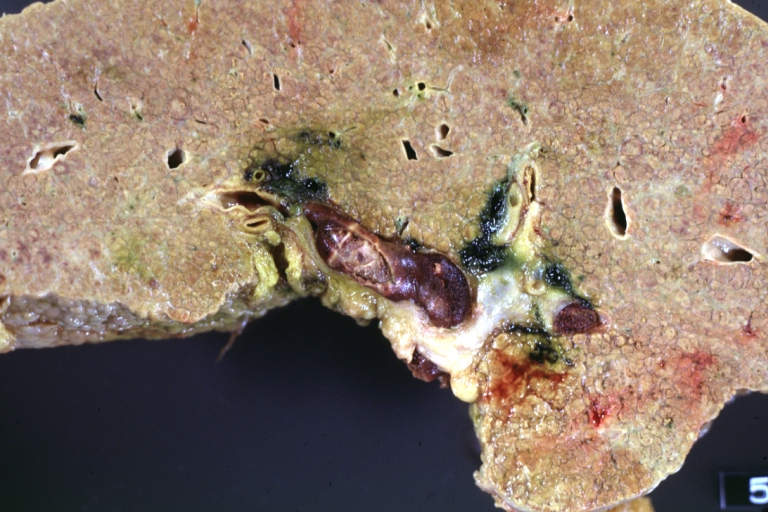
Cirrhosis with portal vein thrombosis: Gross, natural color, sectioned liver with portal vein exposed and filled with red thrombus. A good example of end stage cirrhosis.
-
Endstage cirrhosis with lobular necrosis: Gross, natural color, very close-up view (an excellent example of alcoholic cirrhosis)
-
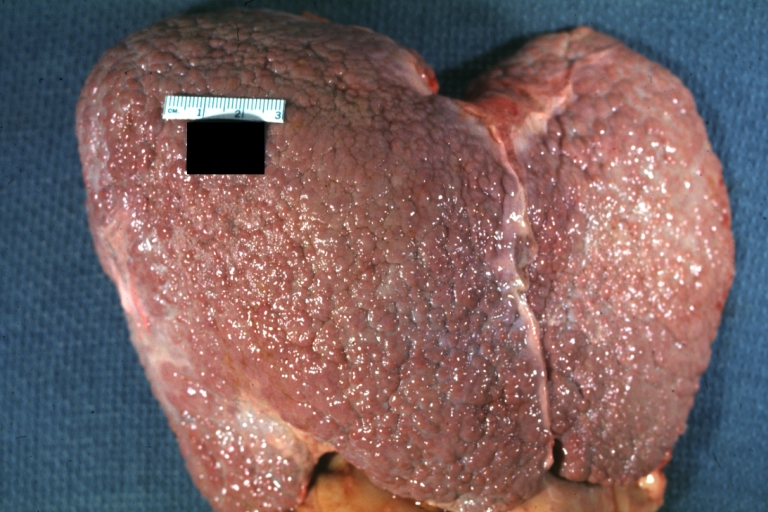
Micronodular cirrhosis: Gross, natural color view of whole liver through capsule with obvious cirrhosis (note to quite large liver)
-
Micronodular cirrhosis: Gross, natural color, view of whole liver showing external surface typical cirrhotic liver (history of alcoholism)
-
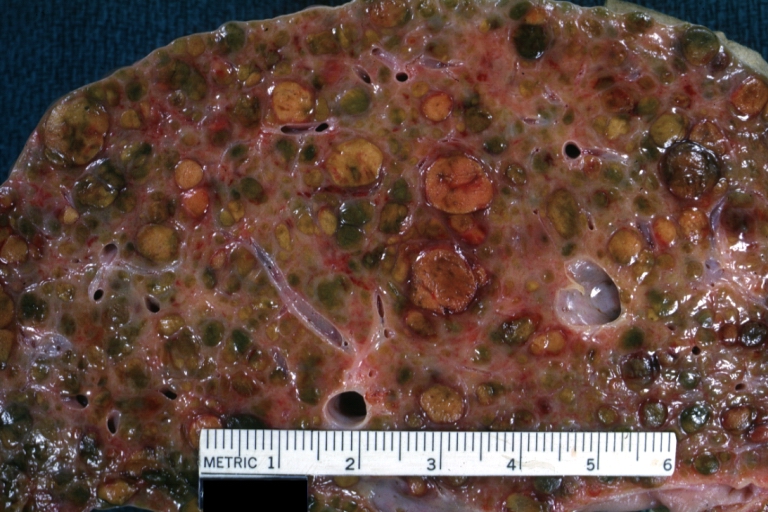
Lung: Idiopathic Interstitial Fibrosis: Gross, natural color, an excellent photo of lung cirrhosis (close-up view)
-
Endstage cirrhosis: Gross, natural color, slice of liver. Portal vein is opened to show size and patency.
-
Endstage cirrhosis: Gross, natural color, severe cirrhosis with bile stasis
-
Portal Vein Thrombosis with cirrhosis: Gross, close-up, micronodular cirrhosis with portal vein thrombosis
-
Lung: Hematite: Gross, natural color, external view of "pulmonary cirrhosis" with typical hematite color
-
Gross, natural color of liver and stomach view from external surfaces, micronodular cirrhosis and hemorrhagic gastritis (as the surgeon would see these in natural color)
Microscopic Pathology
Microscopically, cirrhosis is characterized by regeneration nodules surrounded by fibrous septa. In these nodules, regenerating hepatocytes are disorderly disposed. Portal tracts, central veins and the radial pattern of hepatocytes are absent. Fibrous septa are important and may present inflammatory infiltrate (lymphocytes, macrophages). If it is a secondary biliary cirrhosis, biliary ducts are damaged, proliferated or distended - bile stasis. These dilated ducts contain inspissated bile which appears as bile casts or bile thrombi (brown-green, amorphous). Bile retention may be found also in the parenchyma, as the so called "bile lakes".[25]
Microscopic Pathology
| |
CirrhosisRobbins definition of microscopic histopathological findings in cirrhosis includes (all three is needed for diagnosis):[26] |
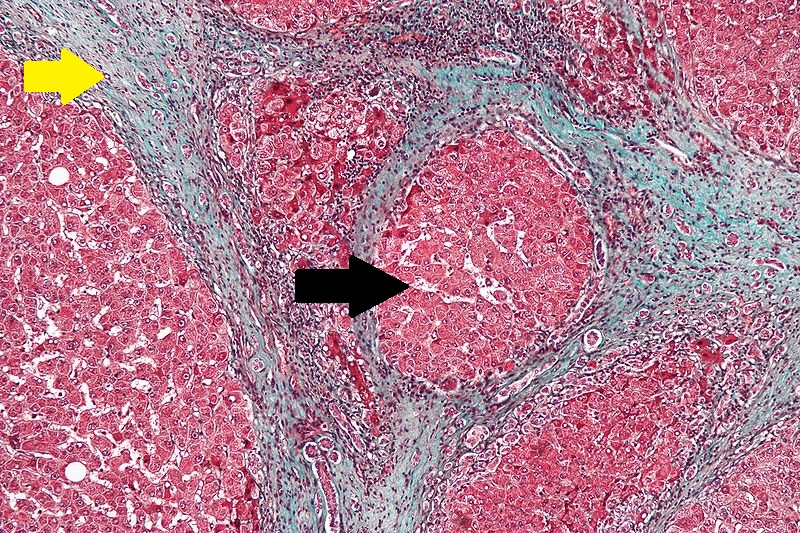 |
Esophageal varicesThe main microscopic histopathological findings in esophageal varices are:
|
 |
Hepatic amyloidosisThe main microscopic histopathological findings in hepatic amyloidosis is amorphous extracellular pink stuff on H&E staining. |
 |
Congestive hepatopathyThe main microscopic histopathological findings in congestive hepatopathy (due to heart failure or Budd-Chiari syndrome) are:
|
 |
Chronic active hepatitis - Cirrhosis
{{#ev:youtube|CzKGvWZrUpU}}
Micronodular cirrhosis
{{#ev:youtube|CV8OYeIUXko}}
Primary biliary cirrhosis
{{#ev:youtube|Jj8ozr_IttM}}
References
- ↑ Fuss IJ, Friend J, Yang Z, He JP, Hooda L, Boyer J, Xi L, Raffeld M, Kleiner DE, Heller T, Strober W (2013). "Nodular regenerative hyperplasia in common variable immunodeficiency". J. Clin. Immunol. 33 (4): 748–58. doi:10.1007/s10875-013-9873-6. PMC 3731765. PMID 23420139.
- ↑ Vaiphei K, Bhatia A, Sinha SK (2011). "Liver pathology in collagen vascular disorders highlighting the vascular changes within portal tracts". Indian J Pathol Microbiol. 54 (1): 25–31. doi:10.4103/0377-4929.77319. PMID 21393872.
- ↑ De Boer NK, Tuynman H, Bloemena E, Westerga J, Van Der Peet DL, Mulder CJ, Cuesta MA, Meuwissen SG, Van Nieuwkerk CM, Van Bodegraven AA (2008). "Histopathology of liver biopsies from a thiopurine-naïve inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia". Scand. J. Gastroenterol. 43 (5): 604–8. doi:10.1080/00365520701800266. PMID 18415755.
- ↑ Allison MC, Mowat A, McCruden EA, McGregor E, Burt AD, Briggs JD, Junor BJ, Follett EA, MacSween RN, Mills PR (1992). "The spectrum of chronic liver disease in renal transplant recipients". Q. J. Med. 83 (301): 355–67. PMID 1438671.
- ↑ Gane E, Portmann B, Saxena R, Wong P, Ramage J, Williams R (1994). "Nodular regenerative hyperplasia of the liver graft after liver transplantation". Hepatology. 20 (1 Pt 1): 88–94. PMID 8020909.
- ↑ Imai Y, Minami Y, Miyoshi S, Kawata S, Saito R, Noda S, Tamura S, Nishikawa M, Tajima K, Tarui S (1986). "Idiopathic portal hypertension associated with Hashimoto's disease: report of three cases". Am. J. Gastroenterol. 81 (9): 791–5. PMID 2944377.
- ↑ Li X, Gao W, Chen J, Tang W (2000). "[Non-cirrhotic portal hypertension associated with autoimmune disease]". Zhonghua Wai Ke Za Zhi (in Chinese). 38 (2): 101–3. PMID 11831999.
- ↑ Kono K, Ohnishi K, Omata M, Saito M, Nakayama T, Hatano H, Nakajima Y, Sugita S, Okuda K (1988). "Experimental portal fibrosis produced by intraportal injection of killed nonpathogenic Escherichia coli in rabbits". Gastroenterology. 94 (3): 787–96. PMID 3276575.
- ↑ Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC (2014). "Idiopathic portal hypertension: natural history and long-term outcome". Hepatology. 59 (6): 2276–85. doi:10.1002/hep.26904. PMID 24155091.
- ↑ Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S, Garcia-Samaniego J, Soriano V (2008). "Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome". Antivir. Ther. (Lond.). 13 (1): 103–7. PMID 18389904.
- ↑ Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A, Duclos B, Seksik P, Mary JY, Colombel JF (2007). "Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine". Gut. 56 (10): 1404–9. doi:10.1136/gut.2006.114363. PMC 2000290. PMID 17504943.
- ↑ Calabrese E, Hanauer SB (2011). "Assessment of non-cirrhotic portal hypertension associated with thiopurine therapy in inflammatory bowel disease". J Crohns Colitis. 5 (1): 48–53. doi:10.1016/j.crohns.2010.08.007. PMID 21272804.
- ↑ Nevens F, Fevery J, Van Steenbergen W, Sciot R, Desmet V, De Groote J (1990). "Arsenic and non-cirrhotic portal hypertension. A report of eight cases". J. Hepatol. 11 (1): 80–5. PMID 2398270.
- ↑ Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C (1991). "Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases". Gastroenterology. 100 (6): 1701–9. PMID 2019375.
- ↑ Girard M, Amiel J, Fabre M, Pariente D, Lyonnet S, Jacquemin E (2005). "Adams-Oliver syndrome and hepatoportal sclerosis: occasional association or common mechanism?". Am. J. Med. Genet. A. 135 (2): 186–9. doi:10.1002/ajmg.a.30724. PMID 15832360.
- ↑ Roulot D (2013). "Liver involvement in Turner syndrome". Liver Int. 33 (1): 24–30. doi:10.1111/liv.12007. PMID 23121401.
- ↑ de Lonlay P, Seta N (2009). "The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib". Biochim. Biophys. Acta. 1792 (9): 841–3. doi:10.1016/j.bbadis.2008.11.012. PMID 19101627.
- ↑ Sarin SK, Mehra NK, Agarwal A, Malhotra V, Anand BS, Taneja V (1987). "Familial aggregation in noncirrhotic portal fibrosis: a report of four families". Am. J. Gastroenterol. 82 (11): 1130–3. PMID 3499813.
- ↑ 19.0 19.1 19.2 Bayan K, Tüzün Y, Yilmaz S, Canoruc N, Dursun M (2009). "Analysis of inherited thrombophilic mutations and natural anticoagulant deficiency in patients with idiopathic portal hypertension". J. Thromb. Thrombolysis. 28 (1): 57–62. doi:10.1007/s11239-008-0244-8. PMID 18685811.
- ↑ Kumar S, Joshi R, Jain AP (2007). "Portal hypertension associated with sickle cell disease". Indian J Gastroenterol. 26 (2): 94. PMID 17558079.
- ↑ <CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)>
- ↑ "www.meddean.luc.edu".
- ↑ Amadalvarez - Own work, <"https://creativecommons.org/licenses/by-sa/4.0" title="Creative Commons Attribution-Share Alike 4.0">CC BY-SA 4.0, <"https://commons.wikimedia.org/w/index.php?curid=49669333">Link
- ↑ <http://wellcomeimages.org/indexplus/obf_images/29/b4/13f38971164f946a97f9d32ddd93.jpg>Gallery: <"http://wellcomeimages.org/indexplus/image/L0074357.html"><"http://creativecommons.org/licenses/by/4.0> CC BY 4.0, <"https://commons.wikimedia.org/w/index.php?curid=36297209">
- ↑ Pathology atlas, "cirrhosis".
- ↑ Mitchell, Richard (2012). Pocket companion to Robbins and Cotran pathologic basis of disease. Philadelphia, PA: Elsevier Saunders. ISBN 978-1416054542.
- ↑ "File:Cirrhosis high mag.jpg - Libre Pathology".
- ↑ "Esophageal varices - Libre Pathology".
- ↑ "File:Hepatic amyloidosis - high mag.jpg - Libre Pathology".
- ↑ "File:2 CEN NEC 1 680x512px.tif - Libre Pathology".
the end
-
Portal HTN results from the combination of the following:
- Structural disturbances associated with advanced liver disease account for 70% of total hepatic vascular resistance.
- Functional abnormalities such as endothelial dysfunction and increased hepatic vascular tone account for 30% of total hepatic vascular resistance.
Pathogenesis of Cirrhosis due to Alcohol:
- More than 66 percent of all American adults consume alcohol.
- Cirrhosis due to alcohol accounts for approximately forty percent of mortality rates due to cirrhosis.
- Mechanisms of alcohol-induced damage include:
- Impaired protein synthesis, secretion, glycosylation
- Ethanol intake leads to elevated accumulation of intracellular triglycerides by:
- Lipoprotein secretion
- Decreased fatty acid oxidation
- Increased fatty acid uptake
- Alcohol is converted by Alcohol dehydrogenase to acetaldehyde.
- Due to the high reactivity of acetaldehyde, it forms acetaldehyde-protein adducts which cause damage to cells by:
- Trafficking of hepatic proteins
- Interrupting microtubule formation
- Interfering with enzyme activities
- Damage of hepatocytes leads to the formation of reactive oxygen species that activate Kupffer cells.[1]
- Kupffer cell activation leads to the production of profibrogenic cytokines that stimulates stellate cells.
- Stellate cell activation leads to the production of extracellular matrix and collagen.
- Portal triads develop connections with central veins due to connective tissue formation in pericentral and periportal zones, leading to the formation of regenerative nodules.
- Shrinkage of the liver occurs over years due to repeated insults that lead to:
- Loss of hepatocytes
- Increased production and deposition of collagen
Pathology
- There are four stages of Cirrhosis as it progresses:
- Chronic nonsuppurative destructive cholangitis - inflammation and necrosis of portal tracts with lymphocyte infiltration leading to the destruction of the bile ducts.
- Development of biliary stasis and fibrosis
- Periportal fibrosis progresses to bridging fibrosis
- Increased proliferation of smaller bile ductules leading to regenerative nodule formation.
Causes
| Drugs and Toxins | Infections | Autoimmune | Metabolic | Biliary obstruction(Secondary bilary cirrhosis) | Vascular | Miscellaneous |
|---|---|---|---|---|---|---|
| Alcohol | Hepatitis B | Primary Biliary Cirrhosis | Wilson's disease | Cystic fibrosis | Chronic RHF | Sarcoidosis |
| Methotrexate | Hepatitis C | Autoimmune hepatitis | Hemochromatosis | Biliary atresia | Budd-Chiari syndrome | Intestinal
bypass operations for obesity |
| Isoniazid | Schistosoma japonicum | Primary Sclerosing Cholangitis | Alpha-1 antitrypsin deficiency | Bile duct strictures | Veno-occlusive disease | Cryptogenic: unknown |
| Methyldopa | Porphyria | Gallstones | ||||
| Glycogen storage diseases (such as Galactosaemia, Abetalipoproteinaemia) |
Cirrhosis
Pathophysiology [2][3][4][5][6][1]
- When an injured issue is replaced by a collagenous scar, it is termed as fibrosis.
- When fibrosis of the liver reaches an advanced stage where distortion of the hepatic vasculature also occurs, it is termed as cirrhosis of the liver.
- The cellular mechanisms responsible for cirrhosis are similar regardless of the type of initial insult and site of injury within the liver lobule.
- Viral hepatitis involves the periportal region, whereas involvement in alcoholic liver disease is largely pericentral.
- If the damage progresses, panlobular cirrhosis may result.
- Cirrhosis involves the following steps: [7]
- Inflammation
- Hepatic stellate cell activation
- Angiogenesis
- Fibrogenesis
- Kupffer cells are hepatic macrophages responsible for Hepatic Stellate cell activation during injury.
- The hepatic stellate cell (also known as the perisinusoidal cell or Ito cell) plays a key role in the pathogenesis of liver fibrosis/cirrhosis.
- Hepatic stellate cells(HSC) are usually located in the subendothelial space of Disse and become activated to a myofibroblast-like phenotype in areas of liver injury.
- Collagen and non collagenous matrix proteins responsible for fibrosis are produced by the activated Hepatic Stellate Cells(HSC).
- Hepatocyte damage causes the release of lipid peroxidases from injured cell membranes leading to necrosis of parenchymal cells.
- Activated HSC produce numerous cytokines and their receptors, such as PDGF and TGF-f31 which are responsible for fibrogenesis.
- The matrix formed due to HSC activation is deposited in the space of Disse and leads to loss of fenestrations of endothelial cells, which is a process called capillarization.
- Cirrhosis leads to hepatic microvascular changes characterised by [8]
- formation of intra hepatic shunts (due to angiogenesis and loss of parenchymal cells)
- hepatic endothelial dysfunction
- The endothelial dysfunction is characterised by [9]
- insufficient release of vasodilators, such as nitric oxide due to oxidative stress
- increased production of vasoconstrictors (mainly adrenergic stimulation and activation of endothelins and RAAS)
- Fibrosis eventually leads to formation of septae that grossly distort the liver architecture which includes both the liver parenchyma and the vasculature. A cirrhotic liver compromises hepatic sinusoidal exchange by shunting arterial and portal blood directly into the central veins (hepatic outflow). Vascularized fibrous septa connect central veins with portal tracts leading to islands of hepatocytes surrounded by fibrous bands without central veins.[10][11][12]
- The formation of fibrotic bands is accompanied by regenerative nodule formation in the hepatic parenchyma.
- Advancement of cirrhosis may lead to parenchymal dysfunction and development of portal hypertension.
- Portal HTN results from the combination of the following:
- Structural disturbances associated with advanced liver disease account for 70% of total hepatic vascular resistance.
- Functional abnormalities such as endothelial dysfunction and increased hepatic vascular tone account for 30% of total hepatic vascular resistance.
Pathogenesis of Cirrhosis due to Alcohol:
- More than 66 percent of all American adults consume alcohol.
- Cirrhosis due to alcohol accounts for approximately forty percent of mortality rates due to cirrhosis.
- Mechanisms of alcohol-induced damage include:
- Impaired protein synthesis, secretion, glycosylation
- Ethanol intake leads to elevated accumulation of intracellular triglycerides by:
- Lipoprotein secretion
- Decreased fatty acid oxidation
- Increased fatty acid uptake
- Alcohol is converted by Alcohol dehydrogenase to acetaldehyde.
- Due to the high reactivity of acetaldehyde, it forms acetaldehyde-protein adducts which cause damage to cells by:
- Trafficking of hepatic proteins
- Interrupting microtubule formation
- Interfering with enzyme activities
- Damage of hepatocytes leads to the formation of reactive oxygen species that activate Kupffer cells.[1]
- Kupffer cell activation leads to the production of profibrogenic cytokines that stimulates stellate cells.
- Stellate cell activation leads to the production of extracellular matrix and collagen.
- Portal triads develop connections with central veins due to connective tissue formation in pericentral and periportal zones, leading to the formation of regenerative nodules.
- Shrinkage of the liver occurs over years due to repeated insults that lead to:
- Loss of hepatocytes
- Increased production and deposition of collagen
Pathology
- There are four stages of Cirrhosis as it progresses:
- Chronic nonsuppurative destructive cholangitis - inflammation and necrosis of portal tracts with lymphocyte infiltration leading to the destruction of the bile ducts.
- Development of biliary stasis and fibrosis
- Periportal fibrosis progresses to bridging fibrosis
- Increased proliferation of smaller bile ductules leading to regenerative nodule formation.
Video codes
Normal video
{{#ev:youtube|x6e9Pk6inYI}}
Video in table
Floating video
| Title |
| https://https://www.youtube.com/watch?v=ypYI_lmLD7g%7C350}} |
Redirect
- REDIRECTEsophageal web
synonym website
https://mq.b2i.sg/snow-owl/#!terminology/snomed/10743008
Image

Image to the right
 |
Image and text to the right
<figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline> </figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
Gallery
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]
References
- ↑ 1.0 1.1 1.2 Arthur MJ (2002). "Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C". Gastroenterology. 122 (5): 1525–8. PMID 11984538.
- ↑ Arthur MJ, Iredale JP (1994). "Hepatic lipocytes, TIMP-1 and liver fibrosis". J R Coll Physicians Lond. 28 (3): 200–8. PMID 7932316.
- ↑ Friedman SL (1993). "Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies". N. Engl. J. Med. 328 (25): 1828–35. doi:10.1056/NEJM199306243282508. PMID 8502273.
- ↑ Iredale JP (1996). "Matrix turnover in fibrogenesis". Hepatogastroenterology. 43 (7): 56–71. PMID 8682489.
- ↑ Gressner AM (1994). "Perisinusoidal lipocytes and fibrogenesis". Gut. 35 (10): 1331–3. PMC 1374996. PMID 7959178.
- ↑ Iredale JP (2007). "Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ". J. Clin. Invest. 117 (3): 539–48. doi:10.1172/JCI30542. PMC 1804370. PMID 17332881.
- ↑ Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G (1995). "Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension". Hepatology. 21 (5): 1238–47. PMID 7737629.
- ↑ Fernández M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J (2009). "Angiogenesis in liver disease". J. Hepatol. 50 (3): 604–20. doi:10.1016/j.jhep.2008.12.011. PMID 19157625.
- ↑ García-Pagán JC, Gracia-Sancho J, Bosch J (2012). "Functional aspects on the pathophysiology of portal hypertension in cirrhosis". J. Hepatol. 57 (2): 458–61. doi:10.1016/j.jhep.2012.03.007. PMID 22504334.
- ↑ Schuppan D, Afdhal NH (2008). "Liver cirrhosis". Lancet. 371 (9615): 838–51. doi:10.1016/S0140-6736(08)60383-9. PMC 2271178. PMID 18328931.
- ↑ Desmet VJ, Roskams T (2004). "Cirrhosis reversal: a duel between dogma and myth". J. Hepatol. 40 (5): 860–7. doi:10.1016/j.jhep.2004.03.007. PMID 15094237.
- ↑ Wanless IR, Nakashima E, Sherman M (2000). "Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis". Arch. Pathol. Lab. Med. 124 (11): 1599–607. doi:10.1043/0003-9985(2000)124<1599:ROHC>2.0.CO;2. PMID 11079009.
- ↑ 13.0 13.1 13.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
REFERENCES

![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[13]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)