Pulmonary hypertension pathophysiology: Difference between revisions
No edit summary |
|||
| (25 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Pulmonary hypertension}} | {{Pulmonary hypertension}} | ||
{{CMG}}; '''Assistant Editor(s)-in-Chief:''' [[User:Ralph Matar|Ralph Matar]]; [[User:Rim Halaby|Rim Halaby]] | {{CMG}}; '''Assistant Editor(s)-in-Chief:''' [[User:Ralph Matar|Ralph Matar]]; [[User:Rim Halaby|Rim Halaby]] {{Jose}} | ||
==Overview== | ==Overview== | ||
Pulmonary hypertension (PH) is a pathological condition of the pulmonary vasculature present in several disease states | Pulmonary hypertension (PH) is a pathological condition of the pulmonary vasculature present in several disease states that presents with elevated mean pulmonary artery pressure (PAP) as measured by [[right heart catheterization]] at rest. The factors that are in involved in the pathophysiology of the increase in the mean pulmonary arterial pressure are: increase in pulmonary [[vascular resistance]], increase in the right-side cardiac output and increase in the [[mean pulmonary venous pressure]]. Pulmonary arterial hypertension is characterized by [[endothelial]] dysfunction resulting from an imbalance between [[apoptosis]] and proliferation of pulmonary artery smooth muscle cells favoring the proliferation. The other types of [[pulmonary hypertension]] are caused by hemodynamic changes that result in increased [[pulmonary blood pressure]] or architectural changes to the lung or its [[vasculature]] that result in the same outcome. | ||
==Pathophysiology== | ==Pathophysiology== | ||
The pathophysiology of pulmonary hypertension<ref name="pmid15059929">{{cite journal |vauthors=Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S |title=Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension |journal=Circ. Res. |volume=94 |issue=9 |pages=1263–70 |date=May 2004 |pmid=15059929 |doi=10.1161/01.RES.0000126847.27660.69 |url=}}</ref><ref name="pmid10024307">{{cite journal |vauthors=Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S |title=Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin |journal=Circ. Res. |volume=84 |issue=3 |pages=329–36 |date=February 1999 |pmid=10024307 |doi= |url=}}</ref><ref name="pmid26479975">{{cite journal |vauthors=Huber LC, Bye H, Brock M |title=The pathogenesis of pulmonary hypertension--an update |journal=Swiss Med Wkly |volume=145 |issue= |pages=w14202 |date=2015 |pmid=26479975 |doi=10.4414/smw.2015.14202 |url=}}</ref><ref name="pmid23457160">{{cite journal |vauthors=Peacock A |title=Pulmonary hypertension |journal=Eur Respir Rev |volume=22 |issue=127 |pages=20–5 |date=March 2013 |pmid=23457160 |doi=10.1183/09059180.00006912 |url=}}</ref><ref name="pmid24267295">{{cite journal |vauthors=Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB |title=Pathology of pulmonary hypertension |journal=Clin. Chest Med. |volume=34 |issue=4 |pages=639–50 |date=December 2013 |pmid=24267295 |doi=10.1016/j.ccm.2013.08.009 |url=}}</ref><ref name="pmid24951761">{{cite journal |vauthors=Michelakis ED |title=Pulmonary arterial hypertension: yesterday, today, tomorrow |journal=Circ. Res. |volume=115 |issue=1 |pages=109–14 |date=June 2014 |pmid=24951761 |doi=10.1161/CIRCRESAHA.115.301132 |url=}}</ref> | The pathophysiology of pulmonary hypertension<ref name="pmid15059929">{{cite journal |vauthors=Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S |title=Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension |journal=Circ. Res. |volume=94 |issue=9 |pages=1263–70 |date=May 2004 |pmid=15059929 |doi=10.1161/01.RES.0000126847.27660.69 |url=}}</ref><ref name="pmid10024307">{{cite journal |vauthors=Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S |title=Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin |journal=Circ. Res. |volume=84 |issue=3 |pages=329–36 |date=February 1999 |pmid=10024307 |doi= |url=}}</ref><ref name="pmid26479975">{{cite journal |vauthors=Huber LC, Bye H, Brock M |title=The pathogenesis of pulmonary hypertension--an update |journal=Swiss Med Wkly |volume=145 |issue= |pages=w14202 |date=2015 |pmid=26479975 |doi=10.4414/smw.2015.14202 |url=}}</ref><ref name="pmid23457160">{{cite journal |vauthors=Peacock A |title=Pulmonary hypertension |journal=Eur Respir Rev |volume=22 |issue=127 |pages=20–5 |date=March 2013 |pmid=23457160 |doi=10.1183/09059180.00006912 |url=}}</ref><ref name="pmid24267295">{{cite journal |vauthors=Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB |title=Pathology of pulmonary hypertension |journal=Clin. Chest Med. |volume=34 |issue=4 |pages=639–50 |date=December 2013 |pmid=24267295 |doi=10.1016/j.ccm.2013.08.009 |url=}}</ref><ref name="pmid24951761">{{cite journal |vauthors=Michelakis ED |title=Pulmonary arterial hypertension: yesterday, today, tomorrow |journal=Circ. Res. |volume=115 |issue=1 |pages=109–14 |date=June 2014 |pmid=24951761 |doi=10.1161/CIRCRESAHA.115.301132 |url=}}</ref><ref name="pmid21110189">{{cite journal |vauthors=Douwes JM, Berger RM |title=The maze of vasodilator response criteria |journal=Pediatr Cardiol |volume=32 |issue=2 |pages=245–6 |date=February 2011 |pmid=21110189 |doi=10.1007/s00246-010-9849-8 |url=}}</ref><ref name="pmid25593290">{{cite journal |vauthors=Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F |title=Endothelial-to-mesenchymal transition in pulmonary hypertension |journal=Circulation |volume=131 |issue=11 |pages=1006–18 |date=March 2015 |pmid=25593290 |doi=10.1161/CIRCULATIONAHA.114.008750 |url=}}</ref><ref name="pmid27045138">{{cite journal |vauthors=Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M |title=In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug |journal=Circulation |volume=133 |issue=18 |pages=1783–94 |date=May 2016 |pmid=27045138 |pmc=4856565 |doi=10.1161/CIRCULATIONAHA.115.020617 |url=}}</ref><ref name="pmid11401896">{{cite journal |vauthors=Jobe AH, Bancalari E |title=Bronchopulmonary dysplasia |journal=Am. J. Respir. Crit. Care Med. |volume=163 |issue=7 |pages=1723–9 |date=June 2001 |pmid=11401896 |doi=10.1164/ajrccm.163.7.2011060 |url=}}</ref> | ||
PH is defined as an elevated mean pulmonary artery pressure (PAP) ≥ 25 mmHg as measured by right heart catheterization at rest. The elevation in PAP results from an elevation in the pulmonary vascular resistance caused by a multifactorial pathogenesis involving genetic and environmental factors. | PH is defined as an elevated mean pulmonary artery pressure (PAP) ≥ 25 mmHg as measured by right heart catheterization at rest. The elevation in PAP results from an elevation in the pulmonary vascular resistance caused by a multifactorial pathogenesis involving genetic and environmental factors. | ||
| Line 12: | Line 12: | ||
Pulmonary hypertension has several pathophysiologic mechanisms depending on the underlying etiology. Nevertheless, the following sequence of events is almost always present: | Pulmonary hypertension has several pathophysiologic mechanisms depending on the underlying etiology. Nevertheless, the following sequence of events is almost always present: | ||
*An initiating factor leads to increased resistance in the pulmonary vasculature causing narrowing of the [[vessels]] and impaired [[blood flow]]. | *Vasoconstriction (ocurring early in the disease); | ||
*Thrombosis (during the evolution of the disease); | |||
*As a consequence, the right [[ventricle]] adapts by increasing right ventricular [[systolic]] pressures to preserve the [[cardiac output]] from the right heart. | *Remodeling (then takes place and becomes the most important factor).<ref name="pmid26479975">{{cite journal| author=Huber LC, Bye H, Brock M, Swiss Society of Pulmonary Hypertension| title=The pathogenesis of pulmonary hypertension--an update. | journal=Swiss Med Wkly | year= 2015 | volume= 145 | issue= | pages= w14202 | pmid=26479975 | doi=10.4414/smw.2015.14202 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26479975 }} </ref> | ||
**An initiating factor leads to increased resistance in the pulmonary vasculature causing narrowing of the [[vessels]] and impaired [[blood flow]]. | |||
*Over time, increasing right ventricular [[systolic]] pressures will subsequently result in chronic changes in the [[pulmonary circulation]] and the affected blood vessels progressively become [[Fibrosis|stiffer and thicker]], further increasing the blood pressure within the lungs and impairing blood flow. | **As a consequence, the right [[ventricle]] adapts by increasing right ventricular [[systolic]] pressures to preserve the [[cardiac output]] from the right heart. | ||
**Over time, increasing right ventricular [[systolic]] pressures will subsequently result in chronic changes in the [[pulmonary circulation]] and the affected blood vessels progressively become [[Fibrosis|stiffer and thicker]], further increasing the blood pressure within the lungs and impairing blood flow. | |||
*In addition, the increased workload of the heart causes thickening and enlargement of the [[right ventricle]], making the heart less able to pump blood through the lungs, causing right [[heart failure]]. Factors that might affect the ability of the right ventricle to adapt to an increased pulmonary vascular resistance are: | **In addition, the increased workload of the heart causes thickening and enlargement of the [[right ventricle]], making the heart less able to pump blood through the lungs, causing right [[heart failure]]. Factors that might affect the ability of the right ventricle to adapt to an increased pulmonary vascular resistance are: | ||
** Age of the patient at onset | *** Age of the patient at onset | ||
** Rapidity of onset of pulmonary hypertension | *** Rapidity of onset of pulmonary hypertension | ||
** Coexisting [[hypoxemia]] | *** Coexisting [[hypoxemia]] | ||
==Pulmonary Arterial Hypertension== | ==Pulmonary Arterial Hypertension== | ||
| Line 28: | Line 28: | ||
* There is also thickened and disordered [[adventitia]] due to excessive amounts of adventitial [[metalloproteinase]]s. | * There is also thickened and disordered [[adventitia]] due to excessive amounts of adventitial [[metalloproteinase]]s. | ||
* Several pathways are implicated in development of vascular remodeling, which is the hallmark of pathologic changes in pulmonary hypertension: | * Several pathways are implicated in development of vascular remodeling, which is the hallmark of pathologic changes in pulmonary hypertension: | ||
1. '''''Thrombosis-mediated remodeling''''' | 1. '''''Vasoconstriction-mediated remodeling''''' | ||
* Imbalance of vasoactive signals is suggested in the pathogenesis of remodeling. | |||
* Reduced levels of vasodilatory mediators, in particular [[PGI2|prostaglandin I<sub>2</sub>]], [[nitric oxide]], and [[cGMP|cyclic guanine monophosphate]], and elevated levels of vasocontricting molecules such as [[endothelin 1]], [[thromboxane]], and [[5-hydroxytryptamine]] contribute to alterations in the vascular tone. | |||
* Down regulation of the [[voltage-gated potassium channel]]s has also been linked with altered pulmonary vascular tone, dysregulation of cellular homeostasis, and induction of proliferative sequelae in vascular [[smooth muscle cell]]s. | |||
* Patients with [[PAH]] seem to have higher concentrations of serum [[endothelin-1]] (a potent vasoconstrictor) than controls.<ref name="pmid26479975">{{cite journal| author=Huber LC, Bye H, Brock M, Swiss Society of Pulmonary Hypertension| title=The pathogenesis of pulmonary hypertension--an update. | journal=Swiss Med Wkly | year= 2015 | volume= 145 | issue= | pages= w14202 | pmid=26479975 | doi=10.4414/smw.2015.14202 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26479975 }} </ref> | |||
* May occur in response to [[hypoxia]]; | |||
* May be related to [[calcium channel]] dysfunction - in fact, there are mutations in the [[ion channels]] (KCNK3) which predispose to [[hereditary PAH]]; | |||
* May be reversible - patients treated early in the disease with [[calcium channel blockers]] seem to have a much better prognosis.<ref name="pmid26479975">{{cite journal| author=Huber LC, Bye H, Brock M, Swiss Society of Pulmonary Hypertension| title=The pathogenesis of pulmonary hypertension--an update. | journal=Swiss Med Wkly | year= 2015 | volume= 145 | issue= | pages= w14202 | pmid=26479975 | doi=10.4414/smw.2015.14202 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26479975 }} </ref> | |||
2. '''''Thrombosis-mediated remodeling''''' | |||
* Many [[coagulopathies]] were described in patients with [[PAH]], such as increased [[von Willebrand factor]] activity, [[protein C]] and [[protein S deficiency]].<ref name="pmid8797416">{{cite journal| author=Welsh CH, Hassell KL, Badesch DB, Kressin DC, Marlar RA| title=Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. | journal=Chest | year= 1996 | volume= 110 | issue= 3 | pages= 710-7 | pmid=8797416 | doi=10.1378/chest.110.3.710 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8797416 }} </ref><ref name="pmid9877507">{{cite journal| author=Hoeper MM, Sosada M, Fabel H| title=Plasma coagulation profiles in patients with severe primary pulmonary hypertension. | journal=Eur Respir J | year= 1998 | volume= 12 | issue= 6 | pages= 1446-9 | pmid=9877507 | doi=10.1183/09031936.98.12061446 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9877507 }} </ref> | |||
* [[Thrombin]] plays a key role in mediating vascular remodeling by activating [[platelet]], upregulating [[angiogenesis]]-related genes (including [[tissue factor|tissue factor [TF]]], [[basic fibroblast growth factor|basic fibroblast growth factor [bFGF]]], and [[matrix metalloproteinase]]-2), and transactivating [[vascular endothelial growth factor|vascular endothelial growth factor (VEGF)]] by inducing the production of [[reactive oxygen species|reactive oxygen species (ROS)]] and the expression of the [[Hypoxia inducible factors|hypoxia inducible factor-1 alpha]]. | * [[Thrombin]] plays a key role in mediating vascular remodeling by activating [[platelet]], upregulating [[angiogenesis]]-related genes (including [[tissue factor|tissue factor [TF]]], [[basic fibroblast growth factor|basic fibroblast growth factor [bFGF]]], and [[matrix metalloproteinase]]-2), and transactivating [[vascular endothelial growth factor|vascular endothelial growth factor (VEGF)]] by inducing the production of [[reactive oxygen species|reactive oxygen species (ROS)]] and the expression of the [[Hypoxia inducible factors|hypoxia inducible factor-1 alpha]]. | ||
* Upregulation of TF in the vasculature leads to initiation of coagulation cascade and migration and proliferation of [[smooth muscle cell]]s upon vascular injury. | * Upregulation of TF in the vasculature leads to initiation of coagulation cascade and migration and proliferation of [[smooth muscle cell]]s upon vascular injury. | ||
* In addition, [[platelet activation]] by [[thrombin]] results in the release of granules containing substances that promote [[mitogenesis]] and [[vasoconstriction]] including [[VEGF]], [[bFGF]], [[platelet-derived growth factor]], and [[serotonin]], which also contribute to increased endothelial cell migration and proliferation. | * In addition, [[platelet activation]] by [[thrombin]] results in the release of granules containing substances that promote [[mitogenesis]] and [[vasoconstriction]] including [[VEGF]], [[bFGF]], [[platelet-derived growth factor]], and [[serotonin]], which also contribute to increased endothelial cell migration and proliferation. | ||
3. '''''Proliferation-mediated remodeling''''' | 3. '''''Proliferation-mediated remodeling''''' | ||
* The pathogenic pathway of lung vasculature remodeling in PH is not fully understood; | |||
* It is regulated by a variety of growth factors, cytokines, mytogens, ion channels, neurotransmitters viruses, transcription factors and receptors, the most studied being the [[bone morphogenetic protein receptor type II]] (BMPR2). | |||
* BMPR2 loss-of-function mutations were found in 70% of patients with hereditary PAH and in 20% of patients with idiopathic PAH;<ref name="pmid25612240">{{cite journal| author=Momose Y, Aimi Y, Hirayama T, Kataoka M, Ono M, Yoshino H | display-authors=etal| title=De novo mutations in the BMPR2 gene in patients with heritable pulmonary arterial hypertension. | journal=Ann Hum Genet | year= 2015 | volume= 79 | issue= 2 | pages= 85-91 | pmid=25612240 | doi=10.1111/ahg.12096 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25612240 }} </ref><ref name="pmid16429403">{{cite journal| author=Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G | display-authors=etal| title=BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. | journal=Hum Mutat | year= 2006 | volume= 27 | issue= 2 | pages= 212-3 | pmid=16429403 | doi=10.1002/humu.9398 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16429403 }} </ref> | |||
* Anecdotal reports have stated that [[imatinib]] has been able to cause significant improvement of PAH patients;<ref name="pmid16192491">{{cite journal| author=Ghofrani HA, Seeger W, Grimminger F| title=Imatinib for the treatment of pulmonary arterial hypertension. | journal=N Engl J Med | year= 2005 | volume= 353 | issue= 13 | pages= 1412-3 | pmid=16192491 | doi=10.1056/NEJMc051946 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16192491 }} </ref> | |||
* [[Serotonin]] exerts both vasoconstrictive and mitogenic effects on [[smooth muscle cell]]s (SMC). | * [[Serotonin]] exerts both vasoconstrictive and mitogenic effects on [[smooth muscle cell]]s (SMC). | ||
* Serotonin m bind to 5-hydroxytryptamine 1A and 2B receptors or may enter SMC via [[SERT|serotonin transporter]] which induces generation of [[reactive oxygen species|ROS]], [[rho kinase]], and [[MAPK|mitogen-activated protein kinases]]. | * Serotonin m bind to 5-hydroxytryptamine 1A and 2B receptors or may enter SMC via [[SERT|serotonin transporter]] which induces generation of [[reactive oxygen species|ROS]], [[rho kinase]], and [[MAPK|mitogen-activated protein kinases]]. | ||
| Line 48: | Line 57: | ||
Shown below is an image depicting remodeling in pulmonary arterial hypertension. | Shown below is an image depicting remodeling in pulmonary arterial hypertension. | ||
[[File:Pathophysiology of pulmonary hypertension.png|661x661px]] | |||
===Genetic Mutations=== | ===Genetic Mutations=== | ||
====TGF-β Receptor Pathway==== | ====TGF-β Receptor Pathway==== | ||
* | * The bone morphogenetic protein receptor II (BMPR2) gene encodes a [[serine]]/[[threonine]] [[kinase]] that functions as a receptor for bone morphogenetic proteins which forms a heterodimer cytoplasmic complex of two type II and two type I receptors. The downstream signaling involves activation of SMAD transcription factor which mediates anti-proliferation in smooth muscle cells. | ||
* Activin-like kinase 1 gene mutations are detected in patients with [[hereditary hemorrhagic telangiectasia]] and pulmonary arterial hypertension. Mutation would cause growth-promoting alterations. | * Activin-like kinase 1 gene mutations are detected in patients with [[hereditary hemorrhagic telangiectasia]] and pulmonary arterial hypertension. Mutation would cause growth-promoting alterations. | ||
| Line 59: | Line 68: | ||
* 5- Hydroxytryptamine (serotonin) transporter mutations have also been associated with the proliferation of [[smooth muscle cell]]s of pulmonary arteries.<ref name="pmid11602621">{{cite journal| author=Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F et al.| title=Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. | journal=J Clin Invest | year= 2001 | volume= 108 | issue= 8 | pages= 1141-50 | pmid=11602621 | doi=10.1172/JCI12805 | pmc=PMC209526 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11602621 }} </ref> | * 5- Hydroxytryptamine (serotonin) transporter mutations have also been associated with the proliferation of [[smooth muscle cell]]s of pulmonary arteries.<ref name="pmid11602621">{{cite journal| author=Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F et al.| title=Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. | journal=J Clin Invest | year= 2001 | volume= 108 | issue= 8 | pages= 1141-50 | pmid=11602621 | doi=10.1172/JCI12805 | pmc=PMC209526 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11602621 }} </ref> | ||
==Genetics== | |||
'''Familial PAH''' often results from a [[mutation]] in the bone morphogenic protein receptor-2 (BMPR2) and is inherited as an [[autosomal dominant]] disease with incomplete [[penetrance]] and anticipation.<ref name="Rich-1987">{{Cite journal | last1 = Rich | first1 = S. | last2 = Dantzker | first2 = DR. | last3 = Ayres | first3 = SM. | last4 = Bergofsky | first4 = EH. | last5 = Brundage | first5 = BH. | last6 = Detre | first6 = KM. | last7 = Fishman | first7 = AP. | last8 = Goldring | first8 = RM. | last9 = Groves | first9 = BM. | title = Primary pulmonary hypertension. A national prospective study. | journal = Ann Intern Med | volume = 107 | issue = 2 | pages = 216-23 | month = Aug | year = 1987 | doi = | PMID = 3605900 }}</ref> | |||
==Associated conditions== | |||
*PAH is also associated with:<ref>ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension</ref> | |||
#[[Congenital heart disease]] (30% of untreated cases) | |||
#[[Connective tissue diseases]] (12% of patients with [[scleroderma]] and up to 21% of patients with [[rheumatoid arthritis]]) | |||
#[[HIV]] (0.5%) | |||
#[[Portal hypertension]] (2-6%) | |||
#[[Sickle cell disease]] (20 to 40%) | |||
#[[Systemic lupus erythematosus]] (4 to 14%) | |||
#[[Hemoglobinopathies]] | |||
#[[Myeloproliferative disorders]] | |||
#Drugs and toxins | |||
==Histopathology== | ==Histopathology== | ||
PH is a pathological condition present in different disease states that share similar clinical manifestation and some common histopathological features. Shown below is a table that summarizes the classification of PH based on histopathology findings.<ref name="pmid15194175">{{cite journal| author=Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM et al.| title=Pathologic assessment of vasculopathies in pulmonary hypertension. | journal=J Am Coll Cardiol | year= 2004 | volume= 43 | issue= 12 Suppl S | pages= 25S-32S | pmid=15194175 | doi=10.1016/j.jacc.2004.02.033 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15194175 }} </ref> | PH is a pathological condition present in different disease states that share similar clinical manifestation and some common histopathological features. Shown below is a table that summarizes the classification of PH based on histopathology findings.<ref name="pmid15194175">{{cite journal| author=Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM et al.| title=Pathologic assessment of vasculopathies in pulmonary hypertension. | journal=J Am Coll Cardiol | year= 2004 | volume= 43 | issue= 12 Suppl S | pages= 25S-32S | pmid=15194175 | doi=10.1016/j.jacc.2004.02.033 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15194175 }} </ref> | ||
| Line 64: | Line 86: | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align="left" |'''Class''' || style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align="left" |'''Histopathological findings'''<ref name="pmid15194175">{{cite journal| author=Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM et al.| title=Pathologic assessment of vasculopathies in pulmonary hypertension. | journal=J Am Coll Cardiol | year= 2004 | volume= 43 | issue= 12 Suppl S | pages= 25S-32S | pmid=15194175 | doi=10.1016/j.jacc.2004.02.033 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15194175 }} </ref> | | style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align="left" |'''Class''' || style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align="left" |'''Histopathological findings'''<ref name="pmid15194175">{{cite journal| author=Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM et al.| title=Pathologic assessment of vasculopathies in pulmonary hypertension. | journal=J Am Coll Cardiol | year= 2004 | volume= 43 | issue= 12 Suppl S | pages= 25S-32S | pmid=15194175 | doi=10.1016/j.jacc.2004.02.033 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15194175 }} </ref> | ||
| | ! style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align="left" |Images | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary arteriopathy''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Constrictive lesions in pulmonary arteries''''' <br> | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary arteriopathy''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Constrictive lesions in pulmonary arteries''''' <br> | ||
| Line 74: | Line 96: | ||
* Dilatation lesions | * Dilatation lesions | ||
* [[Arteritis]] <br> | * [[Arteritis]] <br> | ||
|[[File:Pulmonary hypertension arteriopathy intimal and medial thickening.jpg|thumb]] | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |[[File:Pulmonary hypertension arteriopathy intimal and medial thickening.jpg|thumb]][[File:Pulmonary hypertension arteriopathy adventitial thickening.jpg|thumb]][[File:Pulmonary hypertension arteriopathy intimal and adventitial thickening.jpg|thumb]] | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary arteriopathy with venous-venular changes''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | ''Changes similar to pulmonary arteriopathy'' <br> PLUS<br> ''Changes in venules and veins'' | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary arteriopathy with venous-venular changes''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | ''Changes similar to pulmonary arteriopathy'' <br> PLUS<br> ''Changes in venules and veins'' | ||
| | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |[[File:Pulmonary hypertension arteriopathy medial muscle layer thickening.jpg|thumb]][[File:Pulmonary hypertension Plexiform lesion 2.jpg|thumb]] | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width:50%" align="left" |'''Pulmonary occlusive venopathy''' <br> (with or without arteriopathy) || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Changes in venules and veins''''' <br> | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5; width:50%" align="left" |'''Pulmonary occlusive venopathy''' <br> (with or without arteriopathy) || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Changes in venules and veins''''' <br> | ||
| Line 91: | Line 113: | ||
* [[Fibrosis]] | * [[Fibrosis]] | ||
* [[Hemosiderosis]] | * [[Hemosiderosis]] | ||
| | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary microvasculopathy''' <br> (with or without arteriopathy and/on venopathy)|| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Changes in the capillaries''''' | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Pulmonary microvasculopathy''' <br> (with or without arteriopathy and/on venopathy)|| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''''Changes in the capillaries''''' | ||
| Line 99: | Line 121: | ||
* [[Fibrosis]] | * [[Fibrosis]] | ||
* [[Hemosiderosis]] | * [[Hemosiderosis]] | ||
| | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |[[File:Pulmonary hypertension Pulmonary capillary hemangiomatosis.jpg|thumb]][[File:Pulmonary hypertension pulmonary capillary hemangiomatosis.jpg|thumb]] | ||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Unclassified''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | ''Non specific changes'' | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" |'''Unclassified''' || style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | ''Non specific changes'' | ||
| | | style="padding: 0 5px; font-size: 100%; background: #F5F5F5;" align="left" | | ||
|} | |} | ||
| Line 160: | Line 182: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:Medicine]] | |||
[[Category:Cardiology]] | [[Category:Cardiology]] | ||
[[Category:Pulmonology]] | [[Category:Pulmonology]] | ||
[[Category: | [[Category:Emergency medicine]] | ||
[[Category: | [[Category:Up-To-Date]] | ||
Latest revision as of 16:56, 9 June 2021
|
Pulmonary Hypertension Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Pulmonary hypertension pathophysiology On the Web |
|
American Roentgen Ray Society Images of Pulmonary hypertension pathophysiology |
|
Risk calculators and risk factors for Pulmonary hypertension pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor(s)-in-Chief: Ralph Matar; Rim Halaby José Eduardo Riceto Loyola Junior, M.D.[2]
Overview
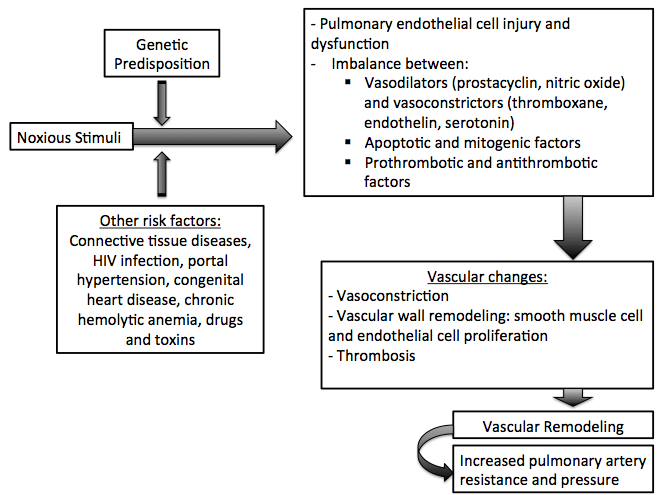
Pulmonary hypertension (PH) is a pathological condition of the pulmonary vasculature present in several disease states that presents with elevated mean pulmonary artery pressure (PAP) as measured by right heart catheterization at rest. The factors that are in involved in the pathophysiology of the increase in the mean pulmonary arterial pressure are: increase in pulmonary vascular resistance, increase in the right-side cardiac output and increase in the mean pulmonary venous pressure. Pulmonary arterial hypertension is characterized by endothelial dysfunction resulting from an imbalance between apoptosis and proliferation of pulmonary artery smooth muscle cells favoring the proliferation. The other types of pulmonary hypertension are caused by hemodynamic changes that result in increased pulmonary blood pressure or architectural changes to the lung or its vasculature that result in the same outcome.
Pathophysiology
The pathophysiology of pulmonary hypertension[1][2][3][4][5][6][7][8][9][10]
PH is defined as an elevated mean pulmonary artery pressure (PAP) ≥ 25 mmHg as measured by right heart catheterization at rest. The elevation in PAP results from an elevation in the pulmonary vascular resistance caused by a multifactorial pathogenesis involving genetic and environmental factors.
Pulmonary hypertension has several pathophysiologic mechanisms depending on the underlying etiology. Nevertheless, the following sequence of events is almost always present:
- Vasoconstriction (ocurring early in the disease);
- Thrombosis (during the evolution of the disease);
- Remodeling (then takes place and becomes the most important factor).[3]
- An initiating factor leads to increased resistance in the pulmonary vasculature causing narrowing of the vessels and impaired blood flow.
- As a consequence, the right ventricle adapts by increasing right ventricular systolic pressures to preserve the cardiac output from the right heart.
- Over time, increasing right ventricular systolic pressures will subsequently result in chronic changes in the pulmonary circulation and the affected blood vessels progressively become stiffer and thicker, further increasing the blood pressure within the lungs and impairing blood flow.
- In addition, the increased workload of the heart causes thickening and enlargement of the right ventricle, making the heart less able to pump blood through the lungs, causing right heart failure. Factors that might affect the ability of the right ventricle to adapt to an increased pulmonary vascular resistance are:
- Age of the patient at onset
- Rapidity of onset of pulmonary hypertension
- Coexisting hypoxemia
Pulmonary Arterial Hypertension
Molecular and Cellular Changes
- Pulmonary arterial hypertension is characterized by endothelial dysfunction resulting from an imbalance between apoptosis and proliferation of pulmonary artery smooth muscle cells favoring the proliferation.
- There is also thickened and disordered adventitia due to excessive amounts of adventitial metalloproteinases.
- Several pathways are implicated in development of vascular remodeling, which is the hallmark of pathologic changes in pulmonary hypertension:
1. Vasoconstriction-mediated remodeling
- Imbalance of vasoactive signals is suggested in the pathogenesis of remodeling.
- Reduced levels of vasodilatory mediators, in particular prostaglandin I2, nitric oxide, and cyclic guanine monophosphate, and elevated levels of vasocontricting molecules such as endothelin 1, thromboxane, and 5-hydroxytryptamine contribute to alterations in the vascular tone.
- Down regulation of the voltage-gated potassium channels has also been linked with altered pulmonary vascular tone, dysregulation of cellular homeostasis, and induction of proliferative sequelae in vascular smooth muscle cells.
- Patients with PAH seem to have higher concentrations of serum endothelin-1 (a potent vasoconstrictor) than controls.[3]
- May occur in response to hypoxia;
- May be related to calcium channel dysfunction - in fact, there are mutations in the ion channels (KCNK3) which predispose to hereditary PAH;
- May be reversible - patients treated early in the disease with calcium channel blockers seem to have a much better prognosis.[3]
2. Thrombosis-mediated remodeling
- Many coagulopathies were described in patients with PAH, such as increased von Willebrand factor activity, protein C and protein S deficiency.[11][12]
- Thrombin plays a key role in mediating vascular remodeling by activating platelet, upregulating angiogenesis-related genes (including tissue factor [TF], basic fibroblast growth factor [bFGF], and matrix metalloproteinase-2), and transactivating vascular endothelial growth factor (VEGF) by inducing the production of reactive oxygen species (ROS) and the expression of the hypoxia inducible factor-1 alpha.
- Upregulation of TF in the vasculature leads to initiation of coagulation cascade and migration and proliferation of smooth muscle cells upon vascular injury.
- In addition, platelet activation by thrombin results in the release of granules containing substances that promote mitogenesis and vasoconstriction including VEGF, bFGF, platelet-derived growth factor, and serotonin, which also contribute to increased endothelial cell migration and proliferation.
3. Proliferation-mediated remodeling
- The pathogenic pathway of lung vasculature remodeling in PH is not fully understood;
- It is regulated by a variety of growth factors, cytokines, mytogens, ion channels, neurotransmitters viruses, transcription factors and receptors, the most studied being the bone morphogenetic protein receptor type II (BMPR2).
- BMPR2 loss-of-function mutations were found in 70% of patients with hereditary PAH and in 20% of patients with idiopathic PAH;[13][14]
- Anecdotal reports have stated that imatinib has been able to cause significant improvement of PAH patients;[15]
- Serotonin exerts both vasoconstrictive and mitogenic effects on smooth muscle cells (SMC).
- Serotonin m bind to 5-hydroxytryptamine 1A and 2B receptors or may enter SMC via serotonin transporter which induces generation of ROS, rho kinase, and mitogen-activated protein kinases.
- This in turn leads to the expression of growth factors and proliferation. Bone morphogenetic protein (BMP) is also involved in the SMC proliferation.
- Upon binding of BMP to its heterodimerized receptors, a group of cytoplasmic proteins known as receptor-mediated Smads are phosphorylated and translocated to the nucleus where they upregulate genes related to anti-proliferation.
- Serotonin is shown to antagonize the BMP/Smad pathway thus facilitating the proliferation of smooth muscle cells.
4. Inflammation-mediated remodeling
- Influx of inflammatory effector cells is stimulated by the release of chemokines such as CCL5 and CX3CL1.
- On the other hand, endothelial cell and smooth muscle cell dysfunction contributes to the release of vasomotor and growth factors, activation of transcription factors, influx of calcium, and dysfunction of mitochondria.
- The net effect is a shift of balance in favor of proliferation and suppressed apoptosis, leading to remodeling of the pulmonary vasculature.
Shown below is an image depicting remodeling in pulmonary arterial hypertension.
Genetic Mutations
TGF-β Receptor Pathway
- The bone morphogenetic protein receptor II (BMPR2) gene encodes a serine/threonine kinase that functions as a receptor for bone morphogenetic proteins which forms a heterodimer cytoplasmic complex of two type II and two type I receptors. The downstream signaling involves activation of SMAD transcription factor which mediates anti-proliferation in smooth muscle cells.
- Activin-like kinase 1 gene mutations are detected in patients with hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension. Mutation would cause growth-promoting alterations.
Serotoninergic Pathway
- 5- Hydroxytryptamine (serotonin) transporter mutations have also been associated with the proliferation of smooth muscle cells of pulmonary arteries.[16]
Genetics
Familial PAH often results from a mutation in the bone morphogenic protein receptor-2 (BMPR2) and is inherited as an autosomal dominant disease with incomplete penetrance and anticipation.[17]
Associated conditions
- PAH is also associated with:[18]
- Congenital heart disease (30% of untreated cases)
- Connective tissue diseases (12% of patients with scleroderma and up to 21% of patients with rheumatoid arthritis)
- HIV (0.5%)
- Portal hypertension (2-6%)
- Sickle cell disease (20 to 40%)
- Systemic lupus erythematosus (4 to 14%)
- Hemoglobinopathies
- Myeloproliferative disorders
- Drugs and toxins
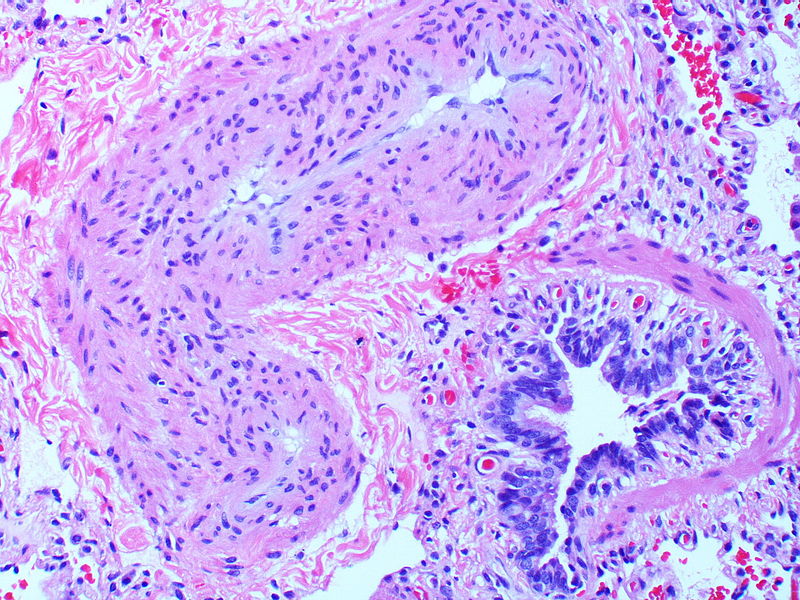
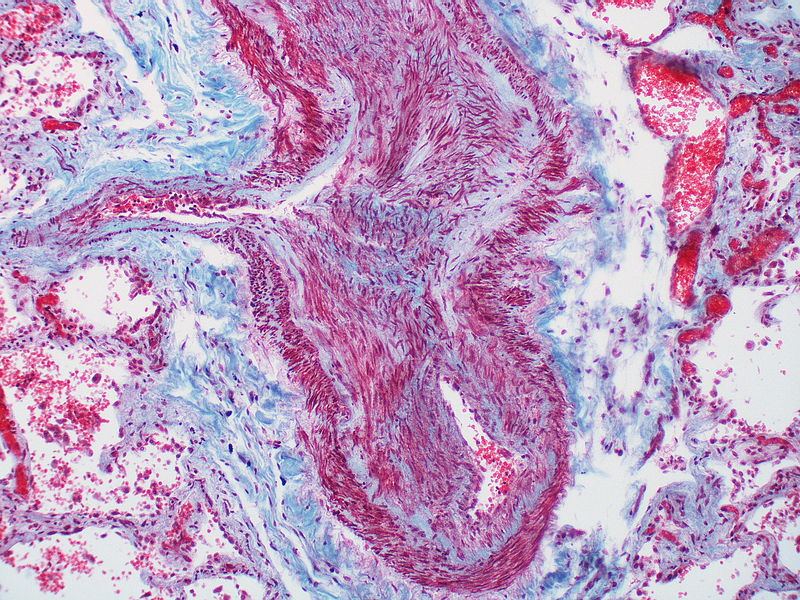
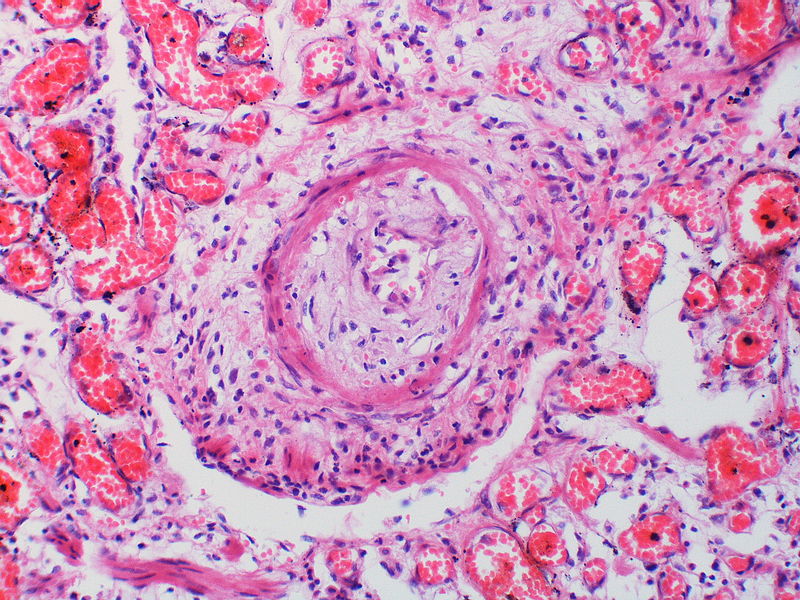
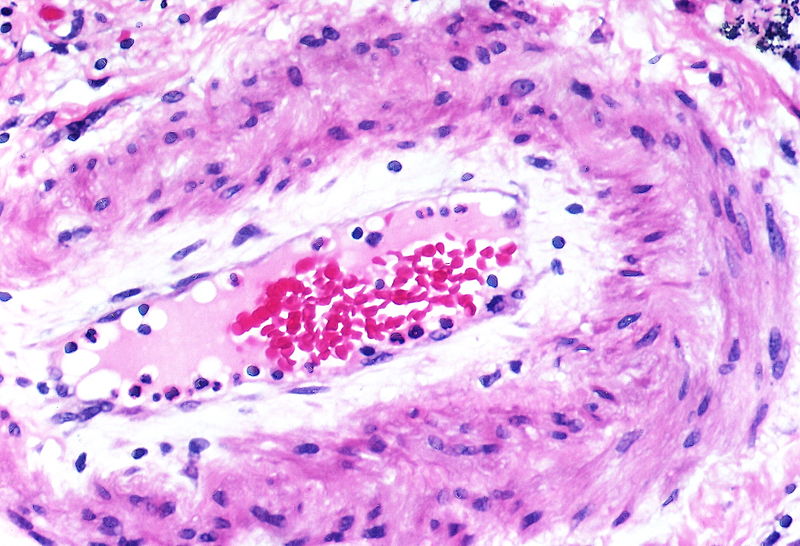
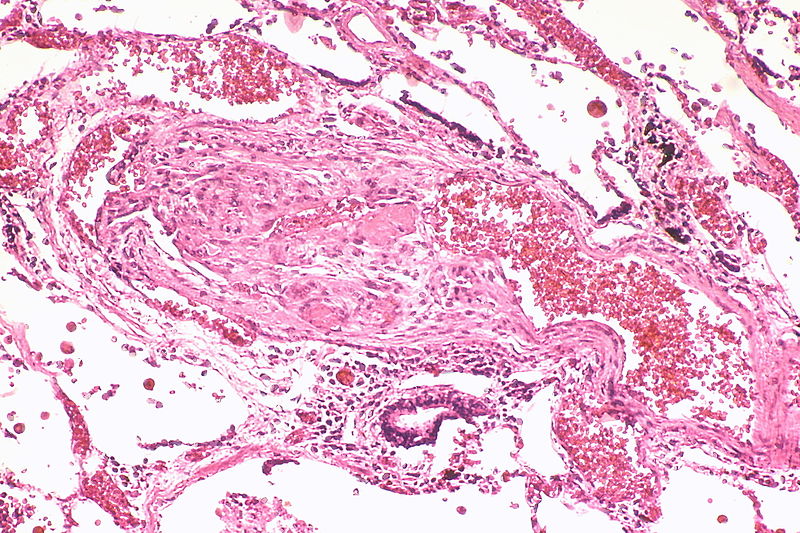
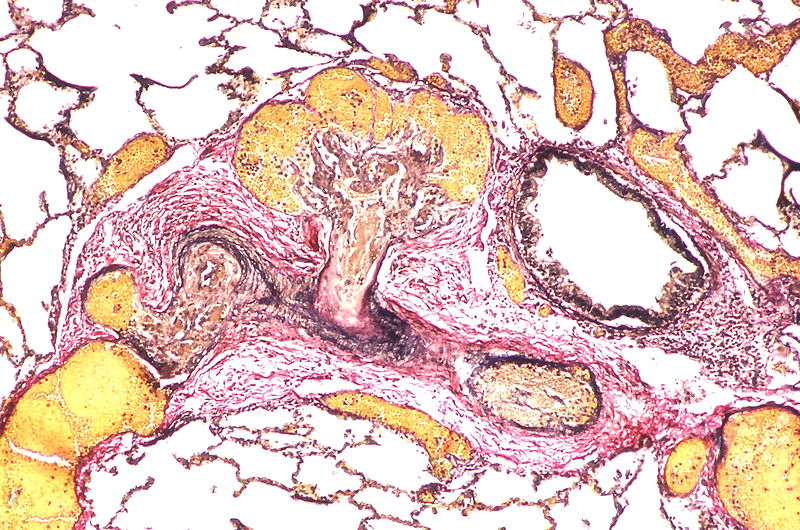
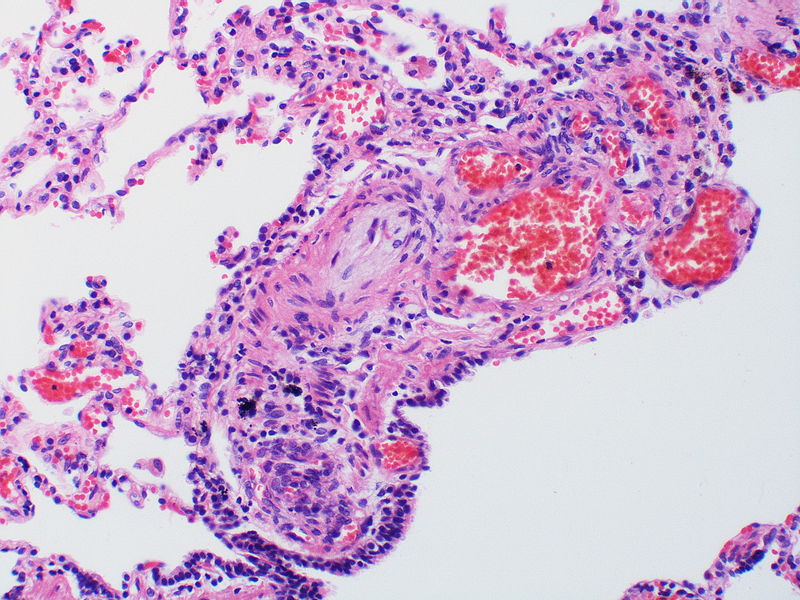
Histopathology
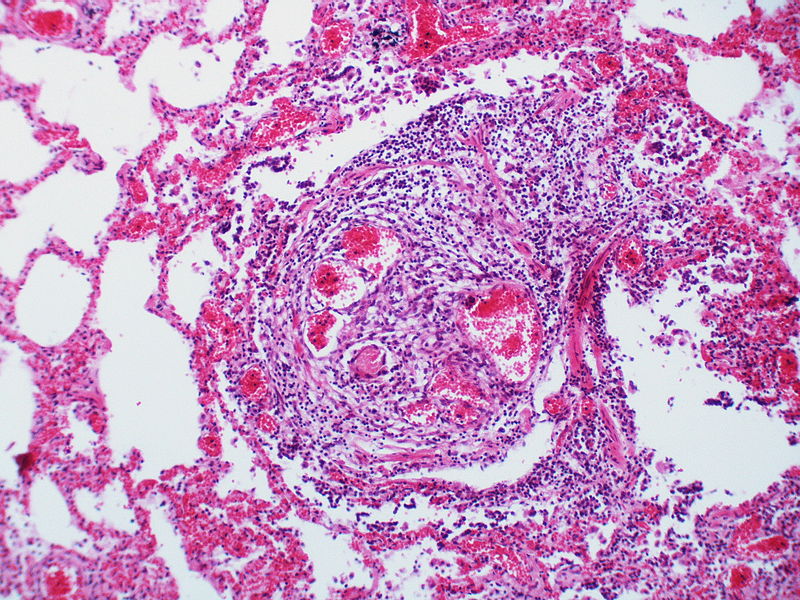
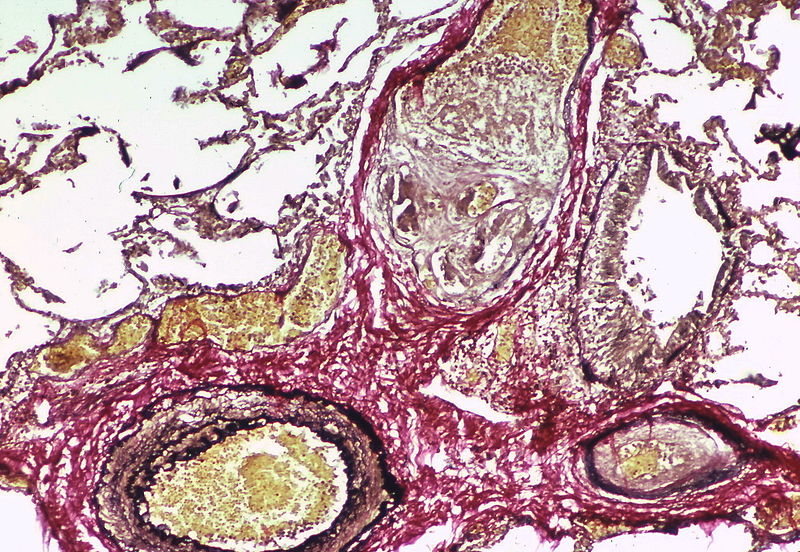
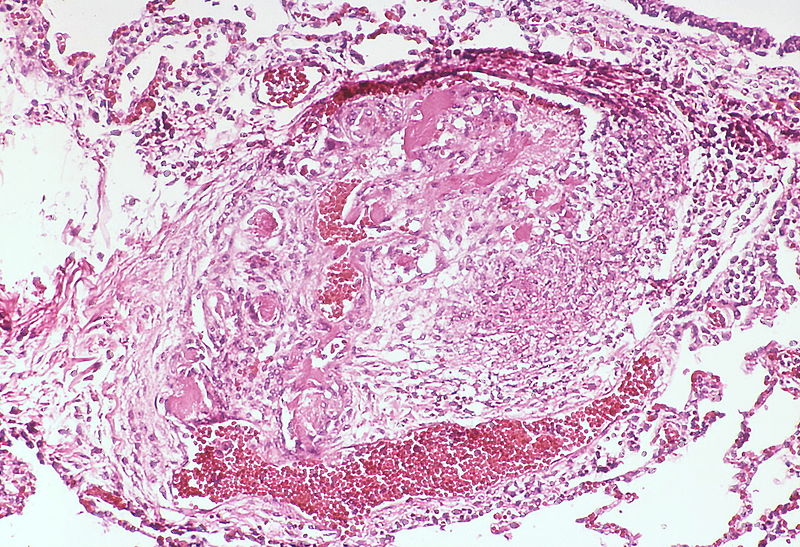
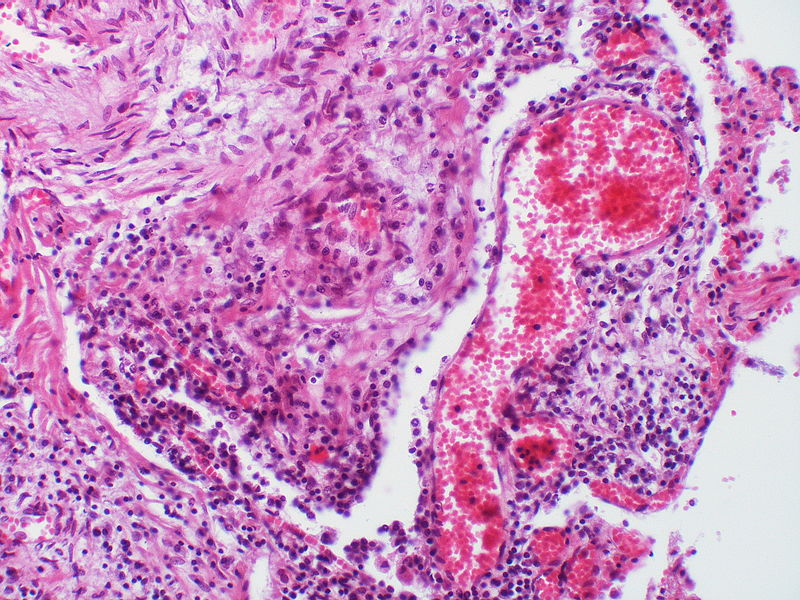
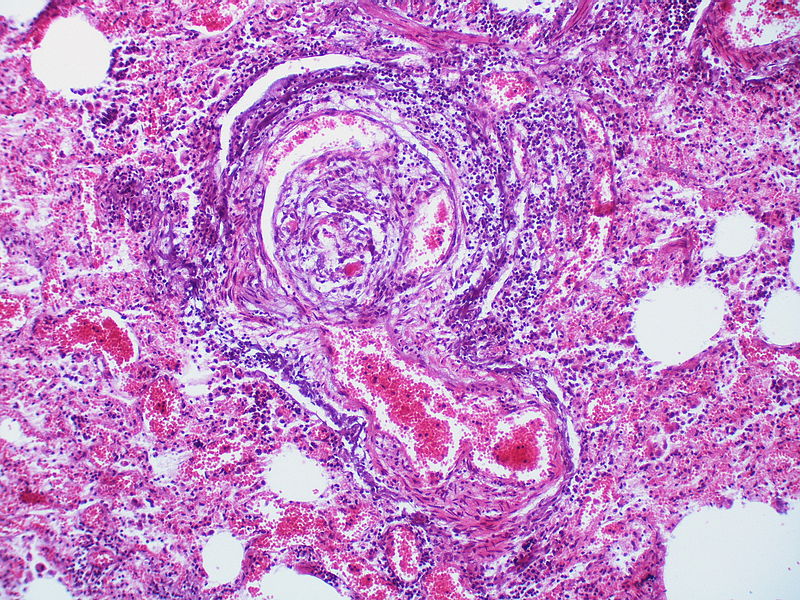
PH is a pathological condition present in different disease states that share similar clinical manifestation and some common histopathological features. Shown below is a table that summarizes the classification of PH based on histopathology findings.[19]
| Class | Histopathological findings[19] | Images |
|---|---|---|
| Pulmonary arteriopathy | Constrictive lesions in pulmonary arteries
Complex lesions in pulmonary arteries
|
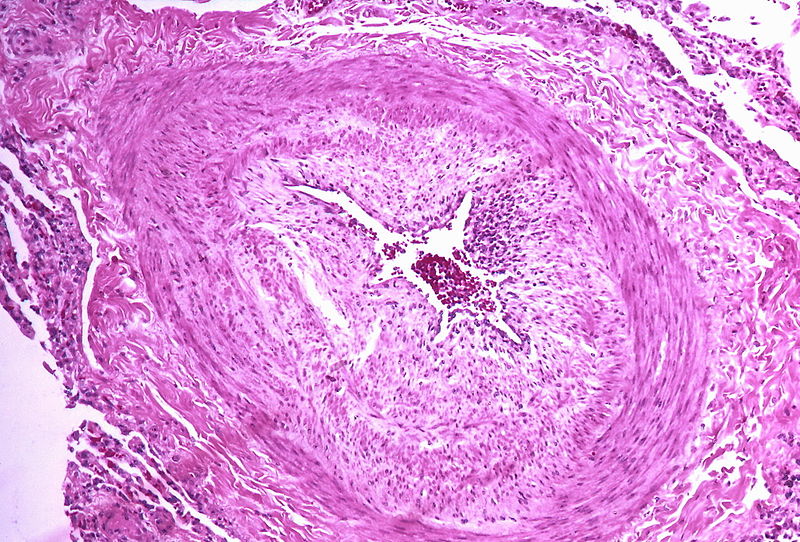 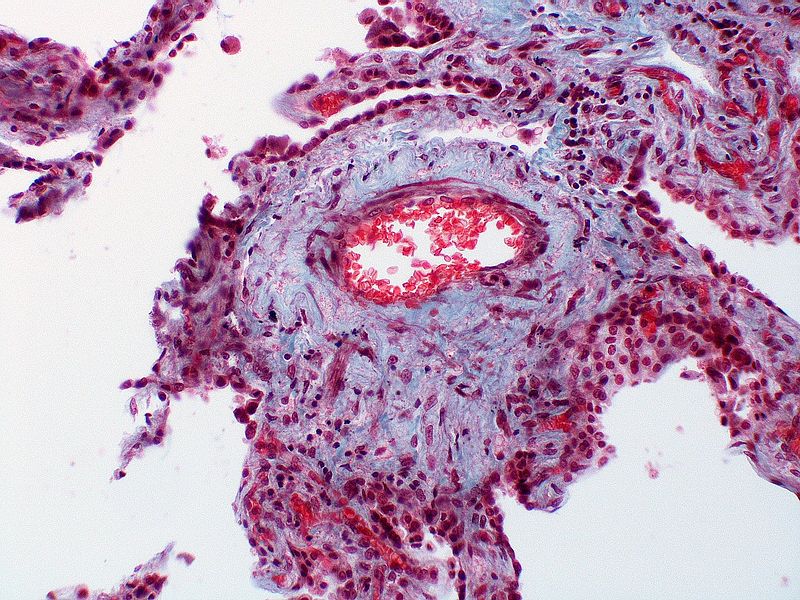 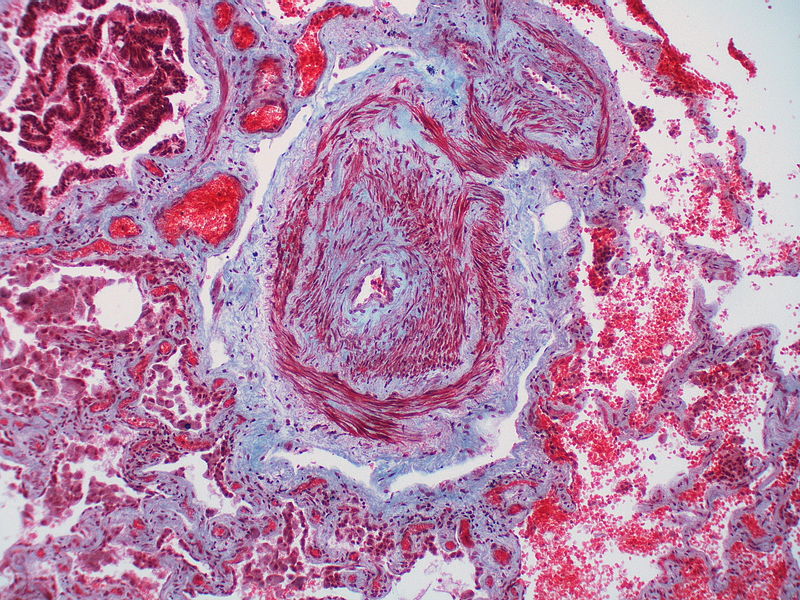 |
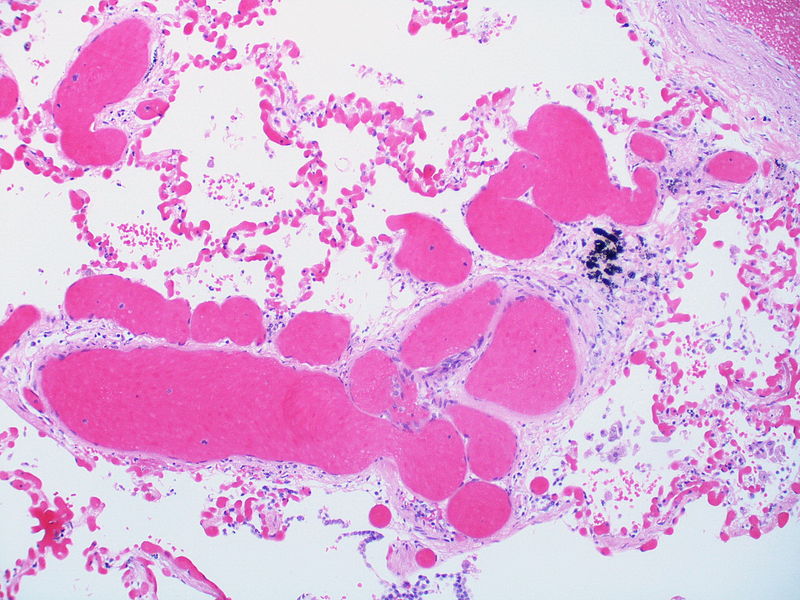
| Pulmonary arteriopathy with venous-venular changes | Changes similar to pulmonary arteriopathy PLUS Changes in venules and veins |
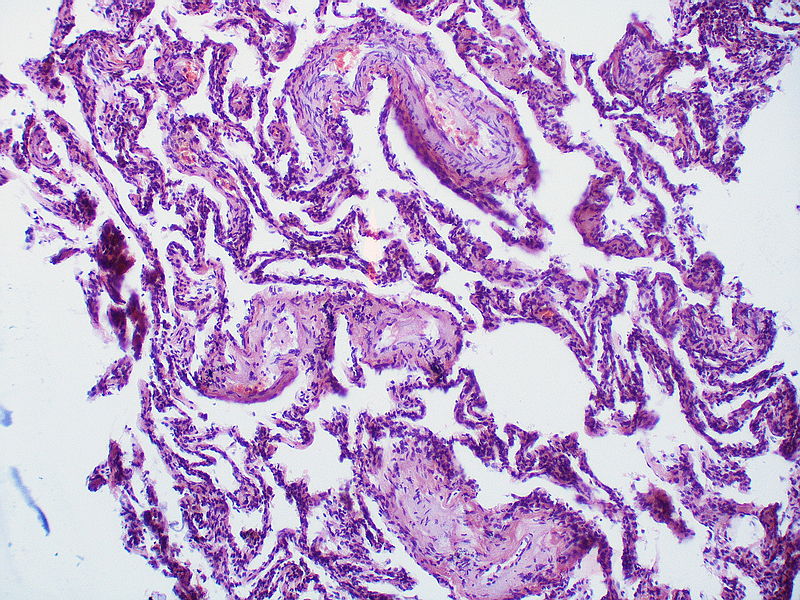  |
| Pulmonary occlusive venopathy (with or without arteriopathy) |
Changes in venules and veins
Changes in the capillaries
Changes in the interstitium |
|
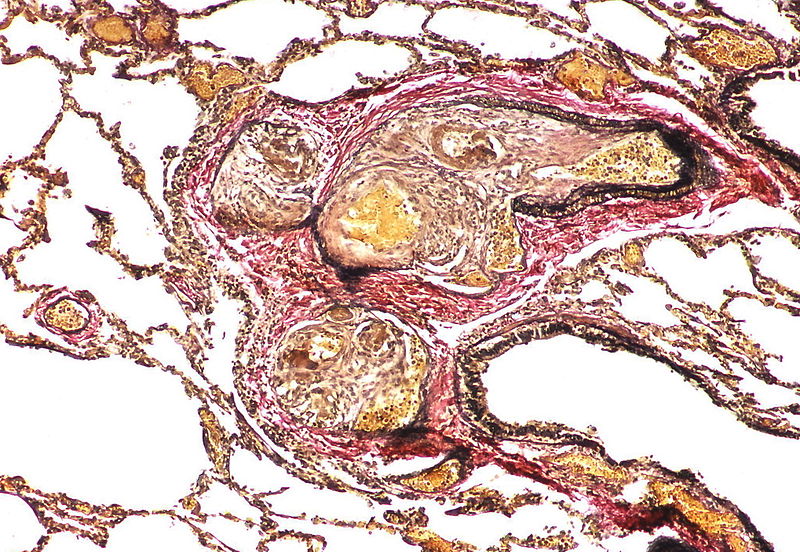
| Pulmonary microvasculopathy (with or without arteriopathy and/on venopathy) |
Changes in the capillaries
Changes in the interstitium |
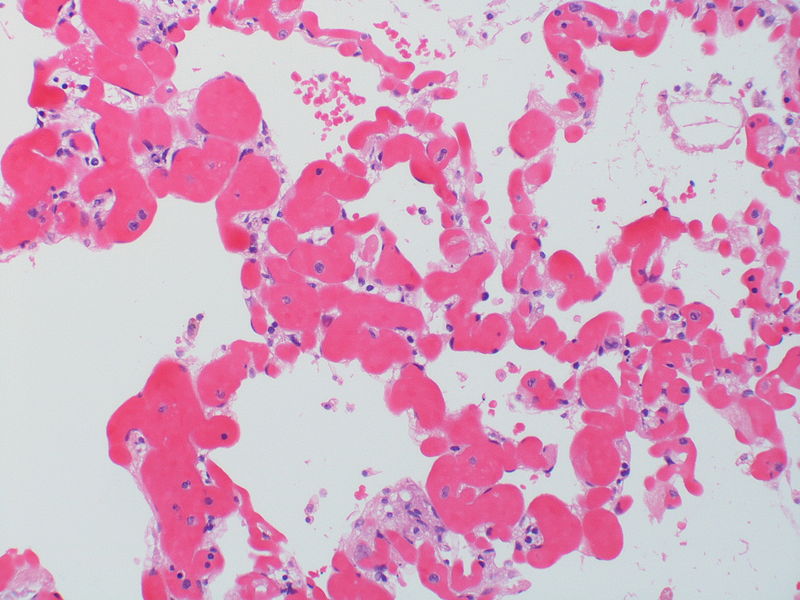  |
| Unclassified | Non specific changes |
Gallery
The images below are courtesy of Dr. Yale Rosen (Source:Wikimedia Commons).
-
Marked intimal and medial thickening
-
Medial muscle layer thickening involving multiple pulmonary arteries.
-
This arteriole exhibits fibrinoid necrosis.
-
Pronounced thickening of the medial muscle layer.
-
Marked intimal and medial thickening.
-
This artery exhibits marked intimal thickening The red-staining cells in the intima are probably myofibroblasts. Masson trichrome stain.
-
Marked adventitial thickening. Masson trichrome stain.
-
This artery exhibits marked intimal thickening as well as adventitial thickening. The red-staining cells in the intima are probably myofibroblasts. Masson trichrome stain.
-
Pulmonary hypertension associated with pulmonary hemangiomatosis. A muscular pulmonary artery surrounded by angiomatous vessels exhibits marked intimal and adventitial thickening.
-
There is thickening of the medial muscle layer as well as mild intimal thickening.
-
Alveolar capillary proliferation as well as the proliferation of larger blood vessels, probably venules.
-
This artery exhibits vasculitis characterized by focal necrosis of the wall accompanied by inflammation.
-
A large dilatation lesion is present on the right side of this image adjacent to an angiomatoid lesion.
-
Two angiomatoid lesions are present. A large dilatation lesion is a present adjacent to the angiomatoid lesion that is located just above center.
-
There is angiomatoid (plexiform) lesion.
-
The appearance suggests multiple lesions but a single lesion cannot be excluded. Note the destruction of the arterial wall at the site of the angiomatoid lesion. Destructive arterial changes are frequently associated with angiomatoid lesions. There is an elastic stain.
-
This angiomatoid lesion is accompanied by inflammation which is frequently associated with these lesions.
-
There is a proliferation of alveolar wall capillaries which are markedly dilated.
-
A dilatation lesion is seen in the left lower quadrant adjacent to the angiomatoid lesion. There is an elastic stain.
-
This angiomatoid lesion contains multiple thrombi within the vascular spaces. A large dilatation lesion is a present adjacent to the bottom of the angiomatoid lesion.
-
A dilatation lesion is present adjacent to the right side of the angiomatoid lesion.
-
There is a disappearance of the arterial wall at the site of the angiomatoid lesions. Arterial wall destruction is a frequent finding in association with angiomatoid lesions. A dilatation lesion encircles the angiomatoid lesion
References
- ↑ Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S (May 2004). "Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension". Circ. Res. 94 (9): 1263–70. doi:10.1161/01.RES.0000126847.27660.69. PMID 15059929.
- ↑ Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S (February 1999). "Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin". Circ. Res. 84 (3): 329–36. PMID 10024307.
- ↑ 3.0 3.1 3.2 3.3 Huber LC, Bye H, Brock M (2015). "The pathogenesis of pulmonary hypertension--an update". Swiss Med Wkly. 145: w14202. doi:10.4414/smw.2015.14202. PMID 26479975.
- ↑ Peacock A (March 2013). "Pulmonary hypertension". Eur Respir Rev. 22 (127): 20–5. doi:10.1183/09059180.00006912. PMID 23457160.
- ↑ Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB (December 2013). "Pathology of pulmonary hypertension". Clin. Chest Med. 34 (4): 639–50. doi:10.1016/j.ccm.2013.08.009. PMID 24267295.
- ↑ Michelakis ED (June 2014). "Pulmonary arterial hypertension: yesterday, today, tomorrow". Circ. Res. 115 (1): 109–14. doi:10.1161/CIRCRESAHA.115.301132. PMID 24951761.
- ↑ Douwes JM, Berger RM (February 2011). "The maze of vasodilator response criteria". Pediatr Cardiol. 32 (2): 245–6. doi:10.1007/s00246-010-9849-8. PMID 21110189.
- ↑ Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F (March 2015). "Endothelial-to-mesenchymal transition in pulmonary hypertension". Circulation. 131 (11): 1006–18. doi:10.1161/CIRCULATIONAHA.114.008750. PMID 25593290.
- ↑ Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M (May 2016). "In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug". Circulation. 133 (18): 1783–94. doi:10.1161/CIRCULATIONAHA.115.020617. PMC 4856565. PMID 27045138.
- ↑ Jobe AH, Bancalari E (June 2001). "Bronchopulmonary dysplasia". Am. J. Respir. Crit. Care Med. 163 (7): 1723–9. doi:10.1164/ajrccm.163.7.2011060. PMID 11401896.
- ↑ Welsh CH, Hassell KL, Badesch DB, Kressin DC, Marlar RA (1996). "Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension". Chest. 110 (3): 710–7. doi:10.1378/chest.110.3.710. PMID 8797416.
- ↑ Hoeper MM, Sosada M, Fabel H (1998). "Plasma coagulation profiles in patients with severe primary pulmonary hypertension". Eur Respir J. 12 (6): 1446–9. doi:10.1183/09031936.98.12061446. PMID 9877507.
- ↑ Momose Y, Aimi Y, Hirayama T, Kataoka M, Ono M, Yoshino H; et al. (2015). "De novo mutations in the BMPR2 gene in patients with heritable pulmonary arterial hypertension". Ann Hum Genet. 79 (2): 85–91. doi:10.1111/ahg.12096. PMID 25612240.
- ↑ Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G; et al. (2006). "BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension". Hum Mutat. 27 (2): 212–3. doi:10.1002/humu.9398. PMID 16429403.
- ↑ Ghofrani HA, Seeger W, Grimminger F (2005). "Imatinib for the treatment of pulmonary arterial hypertension". N Engl J Med. 353 (13): 1412–3. doi:10.1056/NEJMc051946. PMID 16192491.
- ↑ Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F; et al. (2001). "Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension". J Clin Invest. 108 (8): 1141–50. doi:10.1172/JCI12805. PMC 209526. PMID 11602621.
- ↑ Rich, S.; Dantzker, DR.; Ayres, SM.; Bergofsky, EH.; Brundage, BH.; Detre, KM.; Fishman, AP.; Goldring, RM.; Groves, BM. (1987). "Primary pulmonary hypertension. A national prospective study". Ann Intern Med. 107 (2): 216–23. PMID 3605900. Unknown parameter
|month=ignored (help) - ↑ ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension
- ↑ 19.0 19.1 Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM; et al. (2004). "Pathologic assessment of vasculopathies in pulmonary hypertension". J Am Coll Cardiol. 43 (12 Suppl S): 25S–32S. doi:10.1016/j.jacc.2004.02.033. PMID 15194175.