Porfimer: Difference between revisions
Steven Bellm (talk | contribs) No edit summary |
m Protected "Porfimer": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite)) |
||
| (18 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag=Steven | |authorTag={{Steven}} | ||
|genericName=Porfimer Sodium | |genericName=Porfimer Sodium | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass= | |drugClass=photosensitizing agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication= | |indication=obstructive [[esophageal cancer]], completely or partially obstructing endobronchial [[NSCLC|non-small-cell lung cancer]] (NSCLC), [[Barrett's esophagus]] | ||
|adverseReactions=[[constipation]], [[dysphagia]], [[esophageal stricture]], [[anemia]], [[backache]], [[insomnia]], [[bronchitis]], [[dyspnea]], obstruction of bronchus, [[pharyngitis]], stenosis of bronchus, [[fever]], pain | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=* [[Barrett's esophagus]], Ablation of high-grade [[dysplasia]] in patients not undergoing surgery: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer; patients may receive an additional course a minimum of 90 days after therapy; MAX number of courses is 3 (each separated by 90 days). | |||
* [[Barrett's esophagus]], Ablation of high-grade dysplasia in patients not undergoing surgery: The manufacturer recommends a light dose of 130 Joules/centimeter; acceptable light intensity for the balloon/diffuser combinations are 200 to 270 milliwatts/centimeter of diffuser. If repeated, use a light dose of 50 Joules/centimeter of fiber optic diffuser length 96 to 120 hours after initial injection. | |||
* [[Carcinoma of esophagus]], Completely or partially obstructed disease in patients ineligible for Nd/YAG laser therapy (palliative): 2 mg/kg IV over 3-5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later. A second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; max. number of courses is 3 (each separated by 30 days). | |||
* [[Carcinoma of esophagus]], Completely or partially obstructed disease in patients ineligible for Nd/YAG laser therapy (palliative): The manufacturer recommends 300 Joules/centimeter (J/cm) of tumor length with the fiber tip set to deliver the light dose using exposure times of 12 minutes and 30 seconds. | |||
* [[Cholangiocarcinoma]] of biliary tract, Unresectable, after double stenting: 2 mg/kg IV administered 48 hours before laser activation was used in a clinical trial; photoactivation was performed at 630 nanometers using a light dose of 180 joules/cm(2). | |||
* [[Non-small cell lung cancer]], Completely or partially obstructing endobronchial disease: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; MAX number of courses is 3 (each separated by 30 days). | |||
* [[Non-small cell lung cancer]], Microinvasive endobronchial disease; in patients ineligible for surgery or radiotherapy: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; MAX number of courses is 3 (each separated by 30 days) | |||
* [[Non-small cell lung cancer]], Microinvasive endobronchial disease; in patients ineligible for surgery or radiotherapy: The manufacturer recommends 200 Joules/centimeter (J/cm) of tumor length for both palliation and treatment; with the fiber tip set to deliver the light dose using exposure times of 8 minutes and 20 seconds. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Porfimer in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Porfimer in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Porfimer in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Porfimer in adult patients. | ||
|fdaLIADPed=Safety and effectiveness in children have not been established. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Porfimer in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Porfimer in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Porfimer in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Porfimer in pediatric patients. | ||
|alcohol=Alcohol- | |contraindications=PHOTOFRIN® is contraindicated in patients with [[porphyria]] or in patients with known [[allergies]] to porphyrins. | ||
[[Photodynamic therapy]] is contraindicated in patients with an existing tracheoesophageal or bronchoesophageal [[fistula]]. | |||
[[Photodynamic therapy]] is contraindicated in patients with tumors eroding into a major blood vessel. | |||
[[Photodynamic therapy]] is not suitable for emergency treatment of patients with [[severe acute respiratory distress]] caused by an obstructing endobronchial lesion because 40 to 50 hours are required between injection with PHOTOFRIN® and laser light treatment. | |||
[[Photodynamic therapy]] is not suitable for patients with esophageal or gastric [[varices]], or patients with [[esophageal ulcers]] >1 cm in diameter. | |||
|warnings=Following injection with PHOTOFRIN® precautions must be taken to avoid exposure of skin and eyes to direct sunlight or bright indoor light (see PRECAUTIONS, GENERAL PRECAUTIONS AND INFORMATION FOR PATIENTS). | |||
===Esophageal Cancer=== | |||
If the [[esophageal tumor]] is eroding into the trachea or bronchial tree, the likelihood of tracheoesophageal or bronchoesophageal [[fistula]] resulting from treatment is sufficiently high that PDT is not recommended. | |||
Patients with [[esophageal varices]] should be treated with extreme caution. Light should not be given directly to the variceal area because of the high risk of bleeding. | |||
===Endobronchial Cancer=== | |||
Patients should be assessed for the possibility that a tumor may be eroding into a pulmonary blood vessel (see CONTRAINDICATIONS). Patients at high risk for fatal massive [[hemoptysis]] (FMH) include those with large, centrally located tumors, those with cavitating tumors or those with extensive tumor extrinsic to the bronchus. | |||
If the [[endobronchial tumor]] invades deeply into the bronchial wall, the possibility exists for fistula formation upon resolution of tumor. | |||
[[Photodynamic therapy]] should be used with extreme caution for [[endobronchial tumors]] in locations where treatment-induced inflammation could obstruct the main airway, e.g., long or circumferential tumors of the trachea, tumors of the carina that involve both mainstem bronchi circumferentially, or circumferential tumors in the mainstem bronchus in patients with prior pneumonectomy. | |||
===High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE)=== | |||
The long-term effect of PDT on HGD in BE is unknown. There is always a risk of cancer or abnormal epithelium that is invisible to the endoscopist beneath the new squamous cell epithelium; these facts emphasize the risk of overlooking cancer in such patients and the need for rigorous continuing surveillance despite the endoscopic appearance of complete squamous cell reepithelialization. It is recommended that endoscopic biopsy surveillance be conducted every three months, until four consecutive negative evaluations for HGD have been recorded; further follow-up may be scheduled every 6 to 12 months, as per judgment of physicians. The follow-up period of the pivotal study at the time of analysis was a minimum of two years (ranging from 2 to 3.6 years). | |||
|clinicalTrials=Systemically induced effects associated with PDT with PHOTOFRIN® consist of photosensitivity and mild constipation. All patients who receive PHOTOFRIN® will be photosensitive and must observe precautions to avoid sunlight and bright indoor light. Photosensitivity reactions occurred in approximately 20% of cancer patients and in 68% of high-grade dysplasia (HGD) in [[Barrett’s esophagus]] (BE) patients treated with PHOTOFRIN®. Typically these reactions were mostly mild to moderate [[erythema]] but they also included swelling, itching, burning sensation, feeling hot, or blisters. In a single study of 24 healthy subjects, some evidence of photosensitivity reactions occurred in all subjects. Other less common skin manifestations were also reported in areas where photosensitivity reactions had occurred, such as increased hair growth, skin discoloration, skin nodules, increased wrinkles and increased skin fragility. These manifestations may be attributable to a pseudoporphyria state (temporary drug-induced cutaneous porphyria). | |||
Most toxicities associated with this therapy are local effects seen in the region of illumination and occasionally in surrounding tissues. The local adverse reactions are characteristic of an inflammatory response induced by the photodynamic effect. | |||
A few cases of fluid imbalance have been reported following the use of PDT with PHOTOFRIN® in patients with overtly disseminated intraperitoneal malignancies. Fluid imbalance is an expected PDT treatment-related event. | |||
A case of [[cataracts]] has been reported in a 51 year-old obese man treated with PHOTOFRIN® PDT for HGD in BE. The patient suffered from a PDT response with development of a deep [[esophageal ulcer]]. Within two months post PDT, the patient noted difficulty with his distant vision. A thorough eye examination revealed a change in the refractive error that later progressed to [[cataracts]] in both eyes. Both of his parents had a history of [[cataracts]] in their 70s. Whether PHOTOFRIN® directly caused or accelerated a familial underlying condition is unknown. | |||
===Esophageal Carcinoma=== | |||
The following adverse events were reported over the entire follow-up period in at least 5% of patients treated with PHOTOFRIN® PDT, who had completely or partially obstructing [[esophageal cancer]]. Table 7 presents data from 88 patients who received the currently marketed formulation. The relationship of many of these adverse events to PDT with PHOTOFRIN® is uncertain. | |||
[[File:PHOTOFRIN- table07.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Location of the tumor was a prognostic factor for three adverse events: upper-third of the esophagus ([[esophageal edema]]), middle-third ([[atrial fibrillation]]), and lower-third, the most vascular region ([[anemia]]). Also, patients with large tumors (>10 cm) were more likely to experience [[anemia]]. Two of 17 patients with complete [[esophageal obstruction]] from tumor experienced esophageal perforations, which were considered to be possibly treatment associated; these perforations occurred during subsequent endoscopies. | |||
Serious and other notable adverse events observed in less than 5% of PDT-treated patients with obstructing [[esophageal cancer]] in the clinical studies include the following; their relationship to therapy is uncertain. In the gastrointestinal system, [[esophageal perforation]], [[gastric ulcer]], [[ileus]], [[jaundice]], and [[peritonitis]] have occurred. Sepsis has been reported occasionally. Cardiovascular events have included [[angina pectoris]], [[bradycardia]], [[myocardial infarction]], [[sick sinus syndrome]], and [[supraventricular tachycardia]]. Respiratory events of [[bronchitis]], [[bronchospasm]], [[laryngotracheal edema]], [[pneumonitis]], [[pulmonary hemorrhage]], [[pulmonary edema]], [[respiratory failure]], and [[stridor]] have occurred. The temporal relationship of some gastrointestinal, cardiovascular and respiratory events to the administration of light was suggestive of mediastinal inflammation in some patients. Vision-related events of abnormal vision, diplopia, eye pain and photophobia have been reported. | |||
===Obstructing Endobronchial Cancer=== | |||
Table 8 presents adverse events that were reported over the entire follow-up period in at least 5% of patients with obstructing endobronchial cancer treated with PHOTOFRIN® PDT or Nd:YAG. These data are based on the 86 patients who received the currently marketed formulation. Since it seems likely that most adverse events caused by these acute acting therapies would occur within 30 days of treatment, Table 8 presents those events occurring within 30 days of a treatment procedure, as well as those occurring over the entire follow-up period. It should be noted that follow-up was 33% longer for the PDT group than for the Nd:YAG group, thereby introducing a bias against PDT when adverse event rates are compared for the entire follow-up period. The extent of follow-up in the 30-day period following treatment was comparable between groups (only 9% more for PDT). | |||
[[File:PHOTOFRIN- table08.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Transient inflammatory reactions in PDT-treated patients occur in about 10% of patients and manifest as [[fever]], [[bronchitis]], [[chest pain]], and [[dyspnea]]. The incidences of [[bronchitis]] and dyspnea were higher with PDT than with Nd:YAG. Most cases of [[bronchitis]] occurred within 1 week of treatment and all but one were mild or moderate in intensity. The events usually resolved within 10 days with antibiotic therapy. Treatment-related worsening of dyspnea is generally transient and self-limiting. Debridement of the treated area is mandatory to remove exudate and necrotic tissue. Life-threatening respiratory insufficiency likely due to therapy occurred in 3% of PDT-treated patients and 2% of Nd:YAG-treated patients. | |||
There was a trend toward a higher rate of fatal massive hemoptysis (FMH) occurring on the PDT arm (10%) versus the Nd:YAG arm (5%), however, the rate of FMH occurring within 30 days of treatment was the same for PDT and Nd:YAG (4% total events, 3% treatment-associated events). Patients who have received radiation therapy have a higher incidence of FMH after treatment with PDT and after other forms of local therapy than patients who have not received radiation therapy, but analyses suggest that this increased risk may be due to associated prognostic factors such as having a centrally located tumor. The incidence of FMH in patients previously treated with radiotherapy was 21% (6/29) in the PDT group and 10% (3/29) in the Nd:YAG group. In patients with no prior radiotherapy, the overall incidence of FMH was less than 1%. | |||
Other serious or notable adverse events were observed in less than 5% of PDT-treated patients with [[endobronchial cancer]]; their relationship to therapy is uncertain. In the [[respiratory system]], [[pulmonary thrombosis]], [[pulmonary embolism]], and [[lung abscess]] have occurred. [[Cardiac failure]], [[sepsis]], and possible cerebrovascular accident have also been reported in one patient each. | |||
===Superficial Endobronchial Tumors=== | |||
The following adverse events were reported over the entire follow-up period in at least 5% of patients with superficial tumors (microinvasive or carcinoma in situ) who received the currently marketed formulation. | |||
[[File:PHOTOFRIN- table09.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
In patients with superficial [[endobronchial tumors]], 44 of 90 patients (49%) experienced an adverse event, two-thirds of which were related to the respiratory system. The most common reaction to therapy was a mucositis reaction in one-fifth of the patients, which manifested as edema, exudate, and obstruction. The obstruction (mucus plug) is easily removed with suction or forceps. Mucositis can be minimized by avoiding exposure of normal tissue to excessive light (see PRECAUTIONS). Three patients experienced life-threatening dyspnea: one was given a double dose of light, one was treated concurrently in both mainstem bronchi and the other had had prior pneumonectomy and was treated in the sole remaining main airway. Stent placement was required in 3% of the patients due to endobronchial stricture. Fatal massive [[hemoptysis]] occurred within 30 days of treatment in one patient with superficial tumors (1%). | |||
===High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE)=== | |||
Table 10 presents adverse events that were reported, regardless of the relationship to treatment, over the follow-up period in at least 5% of patients with HGD in BE in either controlled or uncontrolled clinical trials. | |||
[[File:PHOTOFRIN- table10.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
In the PHOTOFRIN® PDT + OM group, severe treatment-associated adverse events included chest pain of non-cardiac origin, dysphagia, nausea, vomiting, regurgitation, and heartburn. The severity of these symptoms decreased within 4 to 6 weeks following treatment. | |||
The majority of the photosensitivity reactions occurred within 90 days following PHOTOFRIN® injection and was of mild (69%) or moderate (24%) intensity. Almost all (98%) of the photosensitivity reactions were considered to be associated with treatment. Fourteen (10%) patients reported severe reactions, all of which resolved. The typical reaction was described as skin disorder, sunburn or rash, and affected mostly the face, hands, and neck. Associated symptoms and signs were swelling, pruritis, erythema, blisters, itching, burning sensation, and feeling of heat. | |||
The majority of esophageal stenosis and strictures reported in the PHOTOFRIN® PDT + OM group were of mild (55%) or moderate (37%) intensity, while approximately 8% were of severe intensity. The majority of esophageal strictures were reported during Course 2 of treatment. All esophageal strictures were considered to be associated with treatment. Most esophageal strictures were manageable through dilations (see PRECAUTIONS). | |||
Laboratory Abnormalities | |||
In patients with esophageal cancer, PDT with PHOTOFRIN® may result in anemia due to tumor bleeding. No significant effects were observed for other parameters in patients with endobronchial carcinoma or with HGD in BE. | |||
|postmarketing=The following adverse reactions have been identified during post-approval use of Photofrin with PDT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
Infusion reactions: Infusion reactions including [[urticaria]], [[bradycardia]], [[hypotension]], [[dizziness]], and [[hypertension]]. | |||
|drugInteractions====Other Photosensitizing Agents=== | |||
There have been no formal interaction studies of PHOTOFRIN and any other drugs. However, it is possible that concomitant use of other photosensitizing agents (e.g., tetracyclines, sulfonamides, phenothiazines, sulfonylurea hypoglycemic agents, thiazide diuretics, griseofulvin, and fluoroquinolones) could increase the risk of photosensitivity reaction. | |||
===Concomitant Therapy=== | |||
Photodynamic therapy (PDT) with PHOTOFRIN causes direct intracellular damage by initiating radical chain reactions that damage intracellular membranes and mitochondria. Tissue damage also results from ischemia secondary to vasoconstriction, platelet activation and aggregation and clotting. Research in animals and in cell culture has suggested that many drugs could influence the effects of PDT, possible examples of which are described below. There are no human data that support or rebut these possibilities. | |||
Compounds that quench active oxygen species or scavenge radicals, such as dimethyl sulfoxide, β-carotene, ethanol, formate and mannitol would be expected to decrease PDT activity. Preclinical data also suggest that tissue ischemia, allopurinol, calcium channel blockers and some prostaglandin synthesis inhibitors could interfere with PHOTOFRIN PDT. Drugs that decrease clotting, vasoconstriction or platelet aggregation, e.g., thromboxane A2 inhibitors, could decrease the efficacy of PDT. Glucocorticoid hormones given before or concomitant with PDT may decrease the efficacy of the treatment. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=There are no adequate and well-controlled studies in pregnant women. PHOTOFRIN® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
PHOTOFRIN® given to rat dams during fetal organogenesis intravenously at 8 mg/kg/d (0.64 times the clinical dose on a mg/m2 basis) for 10 days caused no major malformations or developmental changes. This dose caused maternal and fetal toxicity resulting in increased resorptions, decreased litter size, delayed ossification, and reduced fetal weight. PHOTOFRIN® caused no major malformations when given to rabbits intravenously during organogenesis at 4 mg/kg/d (0.65 times the clinical dose on a mg/m2 basis) for 13 days. This dose caused maternal toxicity resulting in increased resorptions, decreased litter size, and reduced fetal body weight. | |||
PHOTOFRIN® given to rats during late pregnancy through lactation intravenously at 4 mg/kg/d (0.32 times the clinical dose on a mg/m2 basis) for at least 42 days caused a reversible decrease in growth of offspring. Parturition was unaffected. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether PHOTOFRIN is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from PHOTOFRIN, a decision should be made whether not to treat or to discontinue breastfeeding, taking into account the importance of the drug to the mother. | |||
|useInPed=* Safety and effectiveness in children have not been established. | |||
|useInGeri=* Approximately 70% of the patients treated with PDT using PHOTOFRIN in clinical trials were over 60 years of age. There was no apparent difference in effectiveness or safety in these patients compared to younger people. Dose modification based upon age is not required. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Intravenous | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|overdose='''PHOTOFRIN Overdose''' | |||
* There is no information on overdosage situations involving PHOTOFRIN. Higher than recommended drug doses of two 2 mg/kg doses given two days apart (10 patients) and three 2 mg/kg doses given within two weeks (one patient), were tolerated without notable adverse reactions. Effects of overdosage on the duration of photosensitivity are unknown. Laser treatment should not be given if an overdose of PHOTOFRIN is administered. In the event of an overdose, patients should protect their eyes and skin from direct sunlight or bright indoor lights for 30 days. At this time, patients should test for residual photosensitivity. PHOTOFRIN is not dialyzable. | |||
'''Overdose of Laser Light Following PHOTOFRIN Injection''' | |||
* Light doses of two to three times the recommended dose have been administered to a few patients with superficial endobronchial tumors. One patient experienced life-threatening dyspnoea and the others had no notable complications. Increased symptoms and damage to normal tissue might be expected following an overdose of light. There is no information on overdose of laser light following PHOTOFRIN injection in patients with esophageal cancer or in patients with high-grade dysplasia in Barrett's esophagus. | |||
|drugBox=[[File:Porfimer sodium drug box.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|mechAction=* Cellular damage caused by photodynamic therapy (PDT) with PHOTOFRIN is a consequence of the propagation of radical reactions. Radical initiation may occur after porfimer sodium absorbs light to form a porphyrin excited state. Spin transfer from porfimer sodium to molecular oxygen may then generate singlet oxygen. Subsequent radical reactions can form superoxide and hydroxyl radicals. Tumor death also occurs through ischemic necrosis secondary to vascular occlusion that appears to be partly mediated by thromboxane A2 release. As opposed to a thermal effect, the laser treatment with porfimer sodium induces a photochemical effect. The necrotic reaction and associated inflammatory responses may evolve over several days. | |||
|structure=* PHOTOFRIN (porfimer sodium) for Injection is a photosensitizing agent used in the photodynamic therapy (PDT) of tumors and of high-grade dysplasia (HGD) in Barrett’s esophagus (BE). Following reconstitution of the freeze-dried product with 5% Dextrose Injection (USP) or 0.9% Sodium Chloride Injection (USP), it is injected intravenously. This is followed 40–50 hours later by illumination of the tumor or the esophageal segment with HGD in BE with laser light (630 nm wavelength). PHOTOFRIN is not a single chemical entity; it is a mixture of oligomers formed by ether and ester linkages of up to eight porphyrin units. It is a dark red to reddish brown cake or powder. Each vial of PHOTOFRIN contains 75 mg of porfimer sodium as a sterile freeze-dried cake or powder. Hydrochloric Acid and/or Sodium Hydroxide may be added during manufacture to adjust the pH to within 7.2-7.9. There are no preservatives or other additives. The structural formula below is representative of the components present in PHOTOFRIN. | |||
[[File:Porfimer sodium structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|PD=* The cytotoxic and antitumor actions of PHOTOFRIN are light and oxygen dependent. PDT with PHOTOFRIN is a two-stage process. The first stage is the intravenous injection of PHOTOFRIN. Clearance from a variety of tissues occurs over 40-72 hours, but tumors, skin, and organs of the reticuloendothelial system (including liver and spleen) retain PHOTOFRIN for a longer period. Illumination with 630 nm wavelength laser light constitutes the second stage of therapy. Tumor selectivity in treatment occurs through a combination of selective retention of PHOTOFRIN and selective delivery of light. | |||
|PK=* The pharmacokinetics of PHOTOFRIN were studied in 18 cancer patients who received two doses of PHOTOFRIN, 2 mg/kg each, administered 30 to 45 days appart as slow IV injection over 3 to 5 mintues. The mean Cmax values were comparable after the first and second administrations (43.1 ± 10.5 mcg/mL and 41.3 ± 8.7 mcg/mL, respectively). However, the mean AUC0-inf of porfimer was about 34% higher after the second administration than that after the first administration (3937 ± 1034 mcg.h/mL and 2937 ± 627 mcg.hour/mL, respectively), indicating some accumulation upon repeated administration. The elimination half-life of porfimer increased from 410 to 725 hours after the first and second administrations, respectively. | |||
* PHOTOFRIN was approximately 90% protein bound in human serum, studied in vitro. The binding was independent of concentration over the concentration range of 20–100 mcg/mL. | |||
''Effect of Gender'': | |||
* The effect of gender was determined in 18 patients (8 males and 10 females) who received two administrations of PHOTOFRIN 2 mg/kg within 30-45 days apart as slow IV injection over 3 to 5 minutes. The mean Cmax value and AUC values were comparable between males and females following either the first of the second administrations. | |||
''Effect of Hepatic Renal Impairment'': | |||
* The effect of hepatic and renal impairment has not been studied. | |||
|nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | |||
* No long-term studies have been conducted to evaluate the carcinogenic potential of porfimer sodium. | |||
* In the presence of light, in vitro, porfimer sodium PDT did not cause mutations in the Ames test, nor did it cause chromosome aberrations or mutations (HGPRT locus) in Chinese hamster ovary (CHO) cells. Porfimer sodium PDT caused <2-fold, but significant, increases in sister chromatid exchange in CHO cells irradiated with visible light and a 3-fold increase in Chinese hamster lung fibroblasts irradiated with near UV light. Porfimer sodium PDT caused an increase in thymidine kinase mutants and DNA-protein cross-links in mouse L5178Y cells, but not mouse LYR83 cells. Porfimer sodium PDT caused a light-dose dependant increase in DNA-strand breaks in malignant human cervical carcinoma cells, but not in normal cells. In the absence of light, porfimer sodium was negative in a Chinese hamster ovarian cells (CHO/HGPRT) mutation test. In vivo, porfimer sodium did not cause chromosomal aberrations in the mouse micronucleus test. | |||
* Porfimer sodium given to male and female rats intravenously, at 4 mg/kg/d (0.32 times the clinical dose on a mg/m2 basis) before conception and through Day 7 of pregnancy caused no impairment of fertility. In this study, long-term dosing with porfimer sodium caused discoloration of testes and ovaries and hypertrophy of the testes. Porfimer sodium also caused decreased body weight in the parent rats. | |||
|clinicalStudies=* Clinical studies of photodynamic therapy (PDT) with PHOTOFRIN were conducted in patients with obstructing esophageal and endobronchial non-small-cell lung cancers, in patients with early-stage radiologically occult endobronchial cancer, and in patients with high-grade dysplasia (HGD) in Barrett's esophagus (BE). In all clinical studies, the method of PDT administration was essentially identical. A course of therapy consisted of one injection of PHOTOFRIN (2 mg/kg administered as a slow intravenous injection over 3–5 minutes) followed by up to two non-thermal applications of 630 nm laser light. Light doses of 300 J/cm of diffuser length were used in esophageal cancer. Light doses of 200 J/cm of diffuser length were used in endobronchial cancer for both palliation of obstructing cancer and treatment of superficial lesions. For the ablation of HGD in BE, the light dose administered was 130 J/cm of diffuser length using a centering balloon for the first application and 50 J/cm of diffuser length without a centering balloon for the second application. In all cases, the first application of light occurred 40–50 hours after PHOTOFRIN injection. | |||
* For treatment of esophageal cancer debridement of residua via endoscopy is optional 96–120 hours after injection, after which any residual tumor could be retreated with a second laser light application at the same light dose used for the initial treatment. Additional courses of PDT with PHOTOFRIN were allowed after one month, up to a maximum of three courses. | |||
* For treatment of endobronchial cancer, debridement of residua was performed via bronchoscopy 96–120 hours after injection, after which any residual tumor could be retreated with a second laser light application at the same light dose used for the initial treatment. Additional courses of PDT with PHOTOFRIN-were allowed after one month, up to a maximum of three courses. | |||
* For ablation of HGD in BE, a second laser light application of 50 J/cm of diffuser length without a centering balloon could be given 96-120 hours after the PHOTOFRIN injection for untreated areas ("skip" areas). Additional courses of PDT with PHOTOFRIN were allowed after three months, up to a maximum of three courses. | |||
'''Esophageal Cancer''' | |||
* PDT with PHOTOFRIN was utilized in a multicenter, single-arm study in 17 patients with completely obstructing esophageal carcinoma. Assessments were made at 1 week and 1 month after the last treatment procedure. As shown in TABLE 10, after a single course of therapy, 94% of patients obtained an objective tumor response and 76% of patients experienced some palliation of their dysphagia. On average, before treatment these patients had difficulty swallowing liquids, even saliva. After one course of therapy, there was a statistically significant improvement in mean dysphagia grade (1.5 units, p <0.05) and 13 of 17 patients could swallow liquids without difficulty 1 week and/or 1 month after treatment. Based on all courses, three patients achieved a complete tumor response (CR). In two of these patients, the CR was documented only at Week 1 as they had no further assessments. The third patient achieved a CR after a second course of therapy, which was supported by negative histopathology and maintained for the entire follow-up of 6 months. | |||
* Of the 17 treated patients, 11 (65%) received clinically important benefit from PDT. Clinically important benefit was defined hierarchically as a complete tumor response (3 patients), achievement of normal swallowing (2 patients went from Grade 5 dysphagia to Grade 1), or achievement of a marked improvement of two or more grades of dysphagia with minimal adverse reactions (6 patients). The median duration of benefit in these patients was 69 days. Duration of benefit was calculated only for the period with documented evidence of improvement. All of these patients were still in response at their last assessment and, therefore, the estimate of 69 days is conservative. The median survival for these 11 patients was 115 days. | |||
'''Endobronchial Cancer''' | |||
* Two randomized multicenter Phase III studies were conducted to compare the safety and efficacy of PHOTOFRIN PDT versus Nd:YAG laser therapy for reduction of obstruction and palliation of symptomatic patients with partially or completely obstructing endobronchial non-small-cell lung cancer. Assessments were made at 1 week and at monthly intervals after treatment. TABLE 11 shows the results from all randomized patients in the two studies combined. Objective tumor response rates (CR + PR), which demonstrate reduction of obstruction, were 59% for PDT and 58% for Nd:YAG at Week 1. The response rate at 1 month or later was 60% for PDT and 41% for Nd:YAG. | |||
* Patient symptoms were evaluated using a 5- or 6-grade pulmonary symptom severity rating scale for dyspnoea, cough, and hemoptysis. Patients with moderate to severe symptoms are those most in need of palliation. Improvements of 2 or more grades are considered to be clinically significant. TABLE 12 shows the percentages of patients with moderate to severe symptoms at baseline who demonstrated a 2-grade improvement at any time during the interval evaluated. | |||
[[File:Porfimer sodium table10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
[[File:Porfimer sodium table11.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
[[File:Porfimer sodium table12.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* In a separate retrospective analysis, patients were individually evaluated to identify those patients whose benefit to risk ratio was most favorable, i.e., those who obtained clinically important benefit with minimal adverse reactions. Clinically important benefit was defined as one of the following: | |||
:*A substantial improvement in pulmonary symptoms at Month 1 or later (dyspnoea ≥2 grades, hemoptysis ≥3 grades, cough ≥3 grades or increase in FEV1 ≥40%); | |||
A moderate improvement in symptoms at Month 2 or later (dyspnoea 1 grade, cough 2 grades, hemoptysis 2 grades or increase in FEV1 ≥20%); or | |||
A durable objective tumor response (CR or PR maintained to Month 2 or longer). | |||
Thirty-six (36) of the 99 PDT-treated patients (36%) and 23 of the 99 Nd:YAG-treated patients (23%) received clinically important benefit with only minimal or moderate toxicities of short duration. Thirty-four of 99 PDT-treated patients demonstrated improvements in 2 or more efficacy endpoints (dyspnoea, cough, hemoptysis, sputum, atelectasis, pulmonary function tests of FEV1 or FVC, Karnofsky Performance Score or tumor response) and 29 patients had improvements in 3 or more. | |||
:*The median duration of documented benefit in the 36 patients was 63 days. In these patients with late-stage obstructing lung cancer, median survival was 174 days in PDT-treated patients and 161 days in Nd:YAG-treated patients. | |||
:*The efficacy of PHOTOFRIN PDT was also evaluated in the treatment of microinvasive endobronchial tumors in 62 inoperable patients in three noncomparative studies. Microinvasive lung cancer is defined histologically as disease, which invades beyond the basement membrane but not through or into the cartilage. For 11 of the 62 patients, it was clearly documented that surgery and radiotherapy were not indicated. These 11 patients were all inoperable for medical or technical reasons. Radiotherapy was not indicated due to prior high-dose radiotherapy (7 patients), poor pulmonary function (2 patients), multifocal multilobar disease (1 patient), and poor medical condition (1 patient). As shown in TABLE 13, the complete tumor response rate, biopsy-proven at least 3 months after treatment, was 50%, median time to tumor recurrence was more than 2.7 years, median survival was 2.9 years and disease-specific survival was 4.1 years. | |||
[[File:Porfimer sodium table13.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
'''High-Grade Dysplasia in Barrett's Esophagus''' | |||
* The safety and efficacy of PDT with PHOTOFRIN in ablation of HGD in patients with BE was assessed in one controlled randomized clinical study and two supportive studies. | |||
'''Controlled Randomized Study''' | |||
* A multicenter, pathology blinded, randomized, controlled study was conducted in North America and Europe to assess the efficacy of PDT with PHOTOFRIN for Injection plus omeprazole (PHOTOFRIN PDT + OM) in producing complete ablation of HGD in patients with BE compared to control patients receiving omeprazole alone (OM Only). A total of 485 patients with the diagnosis of HGD were screened for the study; 208 (43%) were randomized to treatment, 237 (49%) were excluded because the diagnosis of HGD was not confirmed and 40 (8%) did not meet other screening criteria or declined to participate in the study. The high patient exclusion rate re-enforces the recommendation by the American College of Gastroenterology that the diagnosis of HGD in BE should be confirmed by an expert GI pathologist. Patients were centrally randomized in a 2:1 proportion to receive PHOTOFRIN PDT + OM (138 patients) or OM Only (70 patients). All patients underwent rigorous systematic quarterly endoscopic biopsy surveillance. Four-quadrant jumbo biopsies at every 2 cm of the entire Barrett’s mucosa were obtained at each follow-up visit (every three months or six months if four consecutive quarterly follow-up endoscopic biopsy results were negative for HGD). All histological assessments were carried out at a central pathology laboratory and read by pathologists blinded to the treatment administered. | |||
* A total of 208 patients who had biopsy-proven HGD in BE were enrolled in the initial 2-year phase of the study. Of those, 199 patients were considered evaluable: 130 of 138 (94%) patients randomized to the PHOTOFRIN PDT + OM group and 69 of 70 (99%) randomized to the OM Only group had no esophageal invasive cancer, suspicion of esophageal invasive cancer, lymph node involvement, or metastases, and had received at least one PHOTOFRIN PDT course or one week of OM treatment, respectively. A disproportionate percentage of patients were discontinued from the OM Only group during the initial 2-year phase leaving 81 (59%) patients in the PHOTOFRIN PDT + OM group and 21 (30%) patients in the OM Only group at the end of the 2-year phase. Consequently, a total of 102 patients who completed the initial 2-year phase were eligible for continuation into the long-term phase until completion of 5 years; of those, 48 (59%) patients from the PHOTOFRIN PDT + OM group and 13 (62%) patients from the OM Only group consented to pursue the long-term phase until completion of 5 years. The mean age was 66 years (38 to 89 years) in the PHOTOFRIN PDT + OM group, and 67 (36 to 88 years) in the OM Only group. The patients in both treatment groups were predominantly male (85%), Caucasian (99%), and former smokers (64%). These characteristics are typical of patients with HGD in BE. Patients randomized to the PHOTOFRIN PDT + OM treatment received up to three courses of treatment separated by at least 90 days. Each course consisted of intravenous administration of 2.0 mg/kg of PHOTOFRIN followed 40-50 hours later by a 630 nm laser light dose of 130 J/cm of diffuser length delivered using a centering balloon. A second laser light dose of 50 J/cm of diffuser length could be administered without a centering balloon 96-120 hours after the injection of PHOTOFRIN for treatment of "skip" areas. Since centering balloons are up to 7 cm in length, patients with more extensive HGD were treated with two or three courses. Both the PHOTOFRIN PDT treatment group and the control group received 20 mg of omeprazole BID to decrease reflux esophagitis. The mean duration of the follow-up period was 34 months (0-67 months) for the PHOTOFRIN PDT + OM group and 25 months (0-65 months) for the OM Only group. | |||
* The primary efficacy endpoint was the Complete Response rate (CR3 or better) at any one of the endoscopic assessment time points. The CR3 or better response was defined as the complete ablation of HGD and referred to as a composite of the following three response levels. | |||
:*CR1 – Complete replacement of all Barrett’s metaplasia and dysplasia with normal squamous cell epithelium; | |||
:*CR2 – Ablation of all histological grades of dysplasia, including patients with indefinite grade of dysplasia, but some areas of Barrett’s epithelium still remain; and | |||
:*CR3 – Ablation of all areas of HGD but with some areas of low-grade dysplasia with or without areas which are indefinite for dysplasia, or areas of Barrett’s metaplastic epithelium. | |||
* Additional efficacy endpoints included: | |||
:*Quality of Complete Response, which consisted of CR1 and CR2 or better. | |||
:*Duration of CR; | |||
:*Time to Progression to Cancer. | |||
* TABLE 14 presents the overall clinical response for both treatment groups in the intent-to-treat (ITT) population whose response was CR3 or better at any one of the evaluation time points. Overall, PHOTOFRIN PDT + OM was effective in eliminating HGD in patients with BE. The proportion of responders was significantly higher in the PHOTOFRIN PDT + OM group than in the OM Only group (77% vs. 39%, respectively; p<0.0001). | |||
* The quality of response in the PHOTOFRIN PDT + OM group was significantly better than that measured in the OM Only group at all response levels (p<0.0001). Seventy-two (52%) patients in the PHOTOFRIN PDT + OM group achieved a CR1 response as compared to only five (7%) patients in the OM Only group. Eighty-one (59%) patients in the PHOTOFRIN PDT + OM group achieved a CR2 or better response as compared to ten (14%) patients in the OM Only group. | |||
[[File:Porfimer sodium table14.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
'''NOTE''': Six patients in the PHOTOFRIN PDT + OM group and three patients in the OM Only group without post-baseline biopsy data are considered as non-responders. | |||
* At the end of the long-term phase, the median response duration was 44.6 months (95% CI: 15.0-not reached, months) in the PHOTOFRIN PDT + OM group compared to 3.2 months (95% CI: 3.0-3.4, months) in the OM Only group. | |||
* At the end of the initial 2 year phase, the time to progression to cancer was significantly longer in the PHOTOFRIN PDT + OM group compared to the OM Only group (HR=0.36 (95% CI: 0.19-0.69), a hazard ratio less than 1 favors the PHOTOFRIN PDT + OM group). The proportion of patients' progression to cancer was lower in the PHOTOFRIN PDT + OM group than in the OM Only group: 13% (18 of 138 patients) vs. 28% (20 of 70 patients). | |||
* Complete response was influenced by the following factors: treatment with PHOTOFRIN PDT + OM (vs. OM Only), single focus of HGD (vs. multiple foci), and prior omeprazole intake of at least 3 months (yes vs. no). Complete response was not influenced by the duration of HGD, length of BE, nodular conditions, gender, age, smoking history, and study center’s size. | |||
''Supportive Studies'' | |||
* Two uncontrolled, supportive studies were conducted that were physician-sponsored, single center Phase II trials. Both studies included patients that had low-grade dysplasia (LGD), HGD and early adenocarcinoma. All HGD in BE patients were treated with PHOTOFRIN PDT and omeprazole. | |||
* The first study enrolled 99 patients (44 with HGD); the purpose of this study was to determine the required light dose to produce effective results. The second study enrolled 86 patients (42 with HGD), who were randomized to receive either PHOTOFRIN PDT with prednisone or PHOTOFRIN PDT without prednisone to determine whether steroid treatment would reduce the incidence and severity of esophageal strictures. | |||
* A CR3 or better response was demonstrated in 93% of 44 patients with HGD in the first study and in 95% of 42 patients with HGD in the second study after a minimum follow-up of 12 months. A CR2 or better response was achieved in 82% of patients in the first study and in 91% of patients in the second study. A CR1 response occurred in 57% of patients in the first study and in 60% of the second study. Progression to cancer during the above follow-up period occurred in 18% of patients in the first study and in 7% of patients in the second study. No reduction in the incidence or severity of esophageal strictures was found in the prednisone group in the second study. | |||
|howSupplied=* PHOTOFRIN (porfimer sodium) for Injection is supplied as a freeze-dried cake or powder as follows: | |||
NDC 76128-155-75, 75 mg vial | |||
|storage=* PHOTOFRIN freeze-dried cake or powder should be stored at Controlled Room Temperature 20-25°C (68-77°F) [see USP]. | |||
|fdaPatientInfo='''Photosensitivity''' | |||
* Patients should be warned to avoid exposure of skin and eyes to direct sunlight or bright indoor light for at least 30 days following injection with PHOTOFRIN. | |||
* Patients should be informed that photosensitivity might last for more than 90 days if patients suffer from liver impairment. | |||
* Patients should be instructed to wear protective clothing and dark sunglasses when outdoors, which have an average white light transmittance of < 4%. | |||
* Patients should be encouraged to expose their skin to ambient indoor light to facilitate elimination of PHOTOFRIN from their skin. | |||
'''Common Adverse Reactions''' | |||
* Patients should be informed that treatment with photodynamic therapy might lead to adverse reactions which include ocular sensitivity, chest pain, respiratory distress or esophageal strictures. In such cases, patients should call their physicians. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=* PHOTOFRIN | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | }} | ||
{{PillImage | |||
|fileName=Porfimer sodium ingredients and appearance.png | |||
}} | |||
{{LabelImage | |||
|fileName=Porfimer image.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Porfimer image1.jpg | |||
}} | |||
{{LabelImage | |||
|fileName= | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Latest revision as of 16:58, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Steven Bellm, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Porfimer is a photosensitizing agent that is FDA approved for the treatment of obstructive esophageal cancer, completely or partially obstructing endobronchial non-small-cell lung cancer (NSCLC), Barrett's esophagus. Common adverse reactions include constipation, dysphagia, esophageal stricture, anemia, backache, insomnia, bronchitis, dyspnea, obstruction of bronchus, pharyngitis, stenosis of bronchus, fever, pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Barrett's esophagus, Ablation of high-grade dysplasia in patients not undergoing surgery: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer; patients may receive an additional course a minimum of 90 days after therapy; MAX number of courses is 3 (each separated by 90 days).
- Barrett's esophagus, Ablation of high-grade dysplasia in patients not undergoing surgery: The manufacturer recommends a light dose of 130 Joules/centimeter; acceptable light intensity for the balloon/diffuser combinations are 200 to 270 milliwatts/centimeter of diffuser. If repeated, use a light dose of 50 Joules/centimeter of fiber optic diffuser length 96 to 120 hours after initial injection.
- Carcinoma of esophagus, Completely or partially obstructed disease in patients ineligible for Nd/YAG laser therapy (palliative): 2 mg/kg IV over 3-5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later. A second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; max. number of courses is 3 (each separated by 30 days).
- Carcinoma of esophagus, Completely or partially obstructed disease in patients ineligible for Nd/YAG laser therapy (palliative): The manufacturer recommends 300 Joules/centimeter (J/cm) of tumor length with the fiber tip set to deliver the light dose using exposure times of 12 minutes and 30 seconds.
- Cholangiocarcinoma of biliary tract, Unresectable, after double stenting: 2 mg/kg IV administered 48 hours before laser activation was used in a clinical trial; photoactivation was performed at 630 nanometers using a light dose of 180 joules/cm(2).
- Non-small cell lung cancer, Completely or partially obstructing endobronchial disease: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; MAX number of courses is 3 (each separated by 30 days).
- Non-small cell lung cancer, Microinvasive endobronchial disease; in patients ineligible for surgery or radiotherapy: 2 mg/kg IV over 3 to 5 minutes followed by local application of laser light (630 nanometers wavelength) to the tumor 40 to 50 hours later; a second laser light application may be given 96 to 120 hours after administration of porfimer, preceded by careful debridement of residual tumor; patients may receive an additional course a minimum of 30 days after therapy; MAX number of courses is 3 (each separated by 30 days)
- Non-small cell lung cancer, Microinvasive endobronchial disease; in patients ineligible for surgery or radiotherapy: The manufacturer recommends 200 Joules/centimeter (J/cm) of tumor length for both palliation and treatment; with the fiber tip set to deliver the light dose using exposure times of 8 minutes and 20 seconds.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Porfimer in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Porfimer in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Porfimer in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Porfimer in pediatric patients.
Contraindications
PHOTOFRIN® is contraindicated in patients with porphyria or in patients with known allergies to porphyrins.
Photodynamic therapy is contraindicated in patients with an existing tracheoesophageal or bronchoesophageal fistula.
Photodynamic therapy is contraindicated in patients with tumors eroding into a major blood vessel.
Photodynamic therapy is not suitable for emergency treatment of patients with severe acute respiratory distress caused by an obstructing endobronchial lesion because 40 to 50 hours are required between injection with PHOTOFRIN® and laser light treatment.
Photodynamic therapy is not suitable for patients with esophageal or gastric varices, or patients with esophageal ulcers >1 cm in diameter.
Warnings
Following injection with PHOTOFRIN® precautions must be taken to avoid exposure of skin and eyes to direct sunlight or bright indoor light (see PRECAUTIONS, GENERAL PRECAUTIONS AND INFORMATION FOR PATIENTS).
Esophageal Cancer
If the esophageal tumor is eroding into the trachea or bronchial tree, the likelihood of tracheoesophageal or bronchoesophageal fistula resulting from treatment is sufficiently high that PDT is not recommended.
Patients with esophageal varices should be treated with extreme caution. Light should not be given directly to the variceal area because of the high risk of bleeding.
Endobronchial Cancer
Patients should be assessed for the possibility that a tumor may be eroding into a pulmonary blood vessel (see CONTRAINDICATIONS). Patients at high risk for fatal massive hemoptysis (FMH) include those with large, centrally located tumors, those with cavitating tumors or those with extensive tumor extrinsic to the bronchus.
If the endobronchial tumor invades deeply into the bronchial wall, the possibility exists for fistula formation upon resolution of tumor.
Photodynamic therapy should be used with extreme caution for endobronchial tumors in locations where treatment-induced inflammation could obstruct the main airway, e.g., long or circumferential tumors of the trachea, tumors of the carina that involve both mainstem bronchi circumferentially, or circumferential tumors in the mainstem bronchus in patients with prior pneumonectomy.
High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE)
The long-term effect of PDT on HGD in BE is unknown. There is always a risk of cancer or abnormal epithelium that is invisible to the endoscopist beneath the new squamous cell epithelium; these facts emphasize the risk of overlooking cancer in such patients and the need for rigorous continuing surveillance despite the endoscopic appearance of complete squamous cell reepithelialization. It is recommended that endoscopic biopsy surveillance be conducted every three months, until four consecutive negative evaluations for HGD have been recorded; further follow-up may be scheduled every 6 to 12 months, as per judgment of physicians. The follow-up period of the pivotal study at the time of analysis was a minimum of two years (ranging from 2 to 3.6 years).
Adverse Reactions
Clinical Trials Experience
Systemically induced effects associated with PDT with PHOTOFRIN® consist of photosensitivity and mild constipation. All patients who receive PHOTOFRIN® will be photosensitive and must observe precautions to avoid sunlight and bright indoor light. Photosensitivity reactions occurred in approximately 20% of cancer patients and in 68% of high-grade dysplasia (HGD) in Barrett’s esophagus (BE) patients treated with PHOTOFRIN®. Typically these reactions were mostly mild to moderate erythema but they also included swelling, itching, burning sensation, feeling hot, or blisters. In a single study of 24 healthy subjects, some evidence of photosensitivity reactions occurred in all subjects. Other less common skin manifestations were also reported in areas where photosensitivity reactions had occurred, such as increased hair growth, skin discoloration, skin nodules, increased wrinkles and increased skin fragility. These manifestations may be attributable to a pseudoporphyria state (temporary drug-induced cutaneous porphyria).
Most toxicities associated with this therapy are local effects seen in the region of illumination and occasionally in surrounding tissues. The local adverse reactions are characteristic of an inflammatory response induced by the photodynamic effect.
A few cases of fluid imbalance have been reported following the use of PDT with PHOTOFRIN® in patients with overtly disseminated intraperitoneal malignancies. Fluid imbalance is an expected PDT treatment-related event.
A case of cataracts has been reported in a 51 year-old obese man treated with PHOTOFRIN® PDT for HGD in BE. The patient suffered from a PDT response with development of a deep esophageal ulcer. Within two months post PDT, the patient noted difficulty with his distant vision. A thorough eye examination revealed a change in the refractive error that later progressed to cataracts in both eyes. Both of his parents had a history of cataracts in their 70s. Whether PHOTOFRIN® directly caused or accelerated a familial underlying condition is unknown.
Esophageal Carcinoma
The following adverse events were reported over the entire follow-up period in at least 5% of patients treated with PHOTOFRIN® PDT, who had completely or partially obstructing esophageal cancer. Table 7 presents data from 88 patients who received the currently marketed formulation. The relationship of many of these adverse events to PDT with PHOTOFRIN® is uncertain.

Location of the tumor was a prognostic factor for three adverse events: upper-third of the esophagus (esophageal edema), middle-third (atrial fibrillation), and lower-third, the most vascular region (anemia). Also, patients with large tumors (>10 cm) were more likely to experience anemia. Two of 17 patients with complete esophageal obstruction from tumor experienced esophageal perforations, which were considered to be possibly treatment associated; these perforations occurred during subsequent endoscopies.
Serious and other notable adverse events observed in less than 5% of PDT-treated patients with obstructing esophageal cancer in the clinical studies include the following; their relationship to therapy is uncertain. In the gastrointestinal system, esophageal perforation, gastric ulcer, ileus, jaundice, and peritonitis have occurred. Sepsis has been reported occasionally. Cardiovascular events have included angina pectoris, bradycardia, myocardial infarction, sick sinus syndrome, and supraventricular tachycardia. Respiratory events of bronchitis, bronchospasm, laryngotracheal edema, pneumonitis, pulmonary hemorrhage, pulmonary edema, respiratory failure, and stridor have occurred. The temporal relationship of some gastrointestinal, cardiovascular and respiratory events to the administration of light was suggestive of mediastinal inflammation in some patients. Vision-related events of abnormal vision, diplopia, eye pain and photophobia have been reported.
Obstructing Endobronchial Cancer
Table 8 presents adverse events that were reported over the entire follow-up period in at least 5% of patients with obstructing endobronchial cancer treated with PHOTOFRIN® PDT or Nd:YAG. These data are based on the 86 patients who received the currently marketed formulation. Since it seems likely that most adverse events caused by these acute acting therapies would occur within 30 days of treatment, Table 8 presents those events occurring within 30 days of a treatment procedure, as well as those occurring over the entire follow-up period. It should be noted that follow-up was 33% longer for the PDT group than for the Nd:YAG group, thereby introducing a bias against PDT when adverse event rates are compared for the entire follow-up period. The extent of follow-up in the 30-day period following treatment was comparable between groups (only 9% more for PDT).

Transient inflammatory reactions in PDT-treated patients occur in about 10% of patients and manifest as fever, bronchitis, chest pain, and dyspnea. The incidences of bronchitis and dyspnea were higher with PDT than with Nd:YAG. Most cases of bronchitis occurred within 1 week of treatment and all but one were mild or moderate in intensity. The events usually resolved within 10 days with antibiotic therapy. Treatment-related worsening of dyspnea is generally transient and self-limiting. Debridement of the treated area is mandatory to remove exudate and necrotic tissue. Life-threatening respiratory insufficiency likely due to therapy occurred in 3% of PDT-treated patients and 2% of Nd:YAG-treated patients.
There was a trend toward a higher rate of fatal massive hemoptysis (FMH) occurring on the PDT arm (10%) versus the Nd:YAG arm (5%), however, the rate of FMH occurring within 30 days of treatment was the same for PDT and Nd:YAG (4% total events, 3% treatment-associated events). Patients who have received radiation therapy have a higher incidence of FMH after treatment with PDT and after other forms of local therapy than patients who have not received radiation therapy, but analyses suggest that this increased risk may be due to associated prognostic factors such as having a centrally located tumor. The incidence of FMH in patients previously treated with radiotherapy was 21% (6/29) in the PDT group and 10% (3/29) in the Nd:YAG group. In patients with no prior radiotherapy, the overall incidence of FMH was less than 1%.
Other serious or notable adverse events were observed in less than 5% of PDT-treated patients with endobronchial cancer; their relationship to therapy is uncertain. In the respiratory system, pulmonary thrombosis, pulmonary embolism, and lung abscess have occurred. Cardiac failure, sepsis, and possible cerebrovascular accident have also been reported in one patient each.
Superficial Endobronchial Tumors
The following adverse events were reported over the entire follow-up period in at least 5% of patients with superficial tumors (microinvasive or carcinoma in situ) who received the currently marketed formulation.

In patients with superficial endobronchial tumors, 44 of 90 patients (49%) experienced an adverse event, two-thirds of which were related to the respiratory system. The most common reaction to therapy was a mucositis reaction in one-fifth of the patients, which manifested as edema, exudate, and obstruction. The obstruction (mucus plug) is easily removed with suction or forceps. Mucositis can be minimized by avoiding exposure of normal tissue to excessive light (see PRECAUTIONS). Three patients experienced life-threatening dyspnea: one was given a double dose of light, one was treated concurrently in both mainstem bronchi and the other had had prior pneumonectomy and was treated in the sole remaining main airway. Stent placement was required in 3% of the patients due to endobronchial stricture. Fatal massive hemoptysis occurred within 30 days of treatment in one patient with superficial tumors (1%).
High-Grade Dysplasia (HGD) in Barrett’s Esophagus (BE)
Table 10 presents adverse events that were reported, regardless of the relationship to treatment, over the follow-up period in at least 5% of patients with HGD in BE in either controlled or uncontrolled clinical trials.

In the PHOTOFRIN® PDT + OM group, severe treatment-associated adverse events included chest pain of non-cardiac origin, dysphagia, nausea, vomiting, regurgitation, and heartburn. The severity of these symptoms decreased within 4 to 6 weeks following treatment.
The majority of the photosensitivity reactions occurred within 90 days following PHOTOFRIN® injection and was of mild (69%) or moderate (24%) intensity. Almost all (98%) of the photosensitivity reactions were considered to be associated with treatment. Fourteen (10%) patients reported severe reactions, all of which resolved. The typical reaction was described as skin disorder, sunburn or rash, and affected mostly the face, hands, and neck. Associated symptoms and signs were swelling, pruritis, erythema, blisters, itching, burning sensation, and feeling of heat.
The majority of esophageal stenosis and strictures reported in the PHOTOFRIN® PDT + OM group were of mild (55%) or moderate (37%) intensity, while approximately 8% were of severe intensity. The majority of esophageal strictures were reported during Course 2 of treatment. All esophageal strictures were considered to be associated with treatment. Most esophageal strictures were manageable through dilations (see PRECAUTIONS).
Laboratory Abnormalities
In patients with esophageal cancer, PDT with PHOTOFRIN® may result in anemia due to tumor bleeding. No significant effects were observed for other parameters in patients with endobronchial carcinoma or with HGD in BE.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Photofrin with PDT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infusion reactions: Infusion reactions including urticaria, bradycardia, hypotension, dizziness, and hypertension.
Drug Interactions
Other Photosensitizing Agents
There have been no formal interaction studies of PHOTOFRIN and any other drugs. However, it is possible that concomitant use of other photosensitizing agents (e.g., tetracyclines, sulfonamides, phenothiazines, sulfonylurea hypoglycemic agents, thiazide diuretics, griseofulvin, and fluoroquinolones) could increase the risk of photosensitivity reaction.
Concomitant Therapy
Photodynamic therapy (PDT) with PHOTOFRIN causes direct intracellular damage by initiating radical chain reactions that damage intracellular membranes and mitochondria. Tissue damage also results from ischemia secondary to vasoconstriction, platelet activation and aggregation and clotting. Research in animals and in cell culture has suggested that many drugs could influence the effects of PDT, possible examples of which are described below. There are no human data that support or rebut these possibilities.
Compounds that quench active oxygen species or scavenge radicals, such as dimethyl sulfoxide, β-carotene, ethanol, formate and mannitol would be expected to decrease PDT activity. Preclinical data also suggest that tissue ischemia, allopurinol, calcium channel blockers and some prostaglandin synthesis inhibitors could interfere with PHOTOFRIN PDT. Drugs that decrease clotting, vasoconstriction or platelet aggregation, e.g., thromboxane A2 inhibitors, could decrease the efficacy of PDT. Glucocorticoid hormones given before or concomitant with PDT may decrease the efficacy of the treatment.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies in pregnant women. PHOTOFRIN® should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
PHOTOFRIN® given to rat dams during fetal organogenesis intravenously at 8 mg/kg/d (0.64 times the clinical dose on a mg/m2 basis) for 10 days caused no major malformations or developmental changes. This dose caused maternal and fetal toxicity resulting in increased resorptions, decreased litter size, delayed ossification, and reduced fetal weight. PHOTOFRIN® caused no major malformations when given to rabbits intravenously during organogenesis at 4 mg/kg/d (0.65 times the clinical dose on a mg/m2 basis) for 13 days. This dose caused maternal toxicity resulting in increased resorptions, decreased litter size, and reduced fetal body weight.
PHOTOFRIN® given to rats during late pregnancy through lactation intravenously at 4 mg/kg/d (0.32 times the clinical dose on a mg/m2 basis) for at least 42 days caused a reversible decrease in growth of offspring. Parturition was unaffected.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Porfimer in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Porfimer during labor and delivery.
Nursing Mothers
- It is not known whether PHOTOFRIN is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from PHOTOFRIN, a decision should be made whether not to treat or to discontinue breastfeeding, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in children have not been established.
Geriatic Use
- Approximately 70% of the patients treated with PDT using PHOTOFRIN in clinical trials were over 60 years of age. There was no apparent difference in effectiveness or safety in these patients compared to younger people. Dose modification based upon age is not required.
Gender
There is no FDA guidance on the use of Porfimer with respect to specific gender populations.
Race
There is no FDA guidance on the use of Porfimer with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Porfimer in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Porfimer in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Porfimer in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Porfimer in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Porfimer in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Porfimer in the drug label.
Overdosage
PHOTOFRIN Overdose
- There is no information on overdosage situations involving PHOTOFRIN. Higher than recommended drug doses of two 2 mg/kg doses given two days apart (10 patients) and three 2 mg/kg doses given within two weeks (one patient), were tolerated without notable adverse reactions. Effects of overdosage on the duration of photosensitivity are unknown. Laser treatment should not be given if an overdose of PHOTOFRIN is administered. In the event of an overdose, patients should protect their eyes and skin from direct sunlight or bright indoor lights for 30 days. At this time, patients should test for residual photosensitivity. PHOTOFRIN is not dialyzable.
Overdose of Laser Light Following PHOTOFRIN Injection
- Light doses of two to three times the recommended dose have been administered to a few patients with superficial endobronchial tumors. One patient experienced life-threatening dyspnoea and the others had no notable complications. Increased symptoms and damage to normal tissue might be expected following an overdose of light. There is no information on overdose of laser light following PHOTOFRIN injection in patients with esophageal cancer or in patients with high-grade dysplasia in Barrett's esophagus.
Pharmacology
Mechanism of Action
- Cellular damage caused by photodynamic therapy (PDT) with PHOTOFRIN is a consequence of the propagation of radical reactions. Radical initiation may occur after porfimer sodium absorbs light to form a porphyrin excited state. Spin transfer from porfimer sodium to molecular oxygen may then generate singlet oxygen. Subsequent radical reactions can form superoxide and hydroxyl radicals. Tumor death also occurs through ischemic necrosis secondary to vascular occlusion that appears to be partly mediated by thromboxane A2 release. As opposed to a thermal effect, the laser treatment with porfimer sodium induces a photochemical effect. The necrotic reaction and associated inflammatory responses may evolve over several days.
Structure
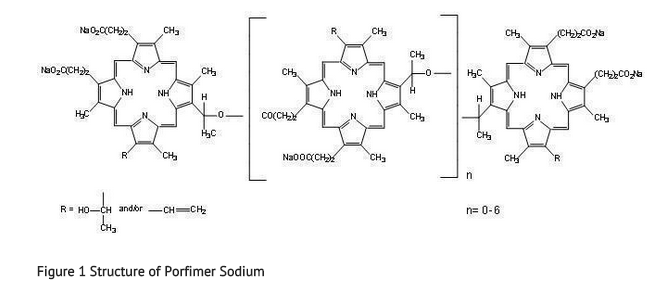
- PHOTOFRIN (porfimer sodium) for Injection is a photosensitizing agent used in the photodynamic therapy (PDT) of tumors and of high-grade dysplasia (HGD) in Barrett’s esophagus (BE). Following reconstitution of the freeze-dried product with 5% Dextrose Injection (USP) or 0.9% Sodium Chloride Injection (USP), it is injected intravenously. This is followed 40–50 hours later by illumination of the tumor or the esophageal segment with HGD in BE with laser light (630 nm wavelength). PHOTOFRIN is not a single chemical entity; it is a mixture of oligomers formed by ether and ester linkages of up to eight porphyrin units. It is a dark red to reddish brown cake or powder. Each vial of PHOTOFRIN contains 75 mg of porfimer sodium as a sterile freeze-dried cake or powder. Hydrochloric Acid and/or Sodium Hydroxide may be added during manufacture to adjust the pH to within 7.2-7.9. There are no preservatives or other additives. The structural formula below is representative of the components present in PHOTOFRIN.
Pharmacodynamics
- The cytotoxic and antitumor actions of PHOTOFRIN are light and oxygen dependent. PDT with PHOTOFRIN is a two-stage process. The first stage is the intravenous injection of PHOTOFRIN. Clearance from a variety of tissues occurs over 40-72 hours, but tumors, skin, and organs of the reticuloendothelial system (including liver and spleen) retain PHOTOFRIN for a longer period. Illumination with 630 nm wavelength laser light constitutes the second stage of therapy. Tumor selectivity in treatment occurs through a combination of selective retention of PHOTOFRIN and selective delivery of light.
Pharmacokinetics
- The pharmacokinetics of PHOTOFRIN were studied in 18 cancer patients who received two doses of PHOTOFRIN, 2 mg/kg each, administered 30 to 45 days appart as slow IV injection over 3 to 5 mintues. The mean Cmax values were comparable after the first and second administrations (43.1 ± 10.5 mcg/mL and 41.3 ± 8.7 mcg/mL, respectively). However, the mean AUC0-inf of porfimer was about 34% higher after the second administration than that after the first administration (3937 ± 1034 mcg.h/mL and 2937 ± 627 mcg.hour/mL, respectively), indicating some accumulation upon repeated administration. The elimination half-life of porfimer increased from 410 to 725 hours after the first and second administrations, respectively.
- PHOTOFRIN was approximately 90% protein bound in human serum, studied in vitro. The binding was independent of concentration over the concentration range of 20–100 mcg/mL.
Effect of Gender:
- The effect of gender was determined in 18 patients (8 males and 10 females) who received two administrations of PHOTOFRIN 2 mg/kg within 30-45 days apart as slow IV injection over 3 to 5 minutes. The mean Cmax value and AUC values were comparable between males and females following either the first of the second administrations.
Effect of Hepatic Renal Impairment:
- The effect of hepatic and renal impairment has not been studied.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term studies have been conducted to evaluate the carcinogenic potential of porfimer sodium.
- In the presence of light, in vitro, porfimer sodium PDT did not cause mutations in the Ames test, nor did it cause chromosome aberrations or mutations (HGPRT locus) in Chinese hamster ovary (CHO) cells. Porfimer sodium PDT caused <2-fold, but significant, increases in sister chromatid exchange in CHO cells irradiated with visible light and a 3-fold increase in Chinese hamster lung fibroblasts irradiated with near UV light. Porfimer sodium PDT caused an increase in thymidine kinase mutants and DNA-protein cross-links in mouse L5178Y cells, but not mouse LYR83 cells. Porfimer sodium PDT caused a light-dose dependant increase in DNA-strand breaks in malignant human cervical carcinoma cells, but not in normal cells. In the absence of light, porfimer sodium was negative in a Chinese hamster ovarian cells (CHO/HGPRT) mutation test. In vivo, porfimer sodium did not cause chromosomal aberrations in the mouse micronucleus test.
- Porfimer sodium given to male and female rats intravenously, at 4 mg/kg/d (0.32 times the clinical dose on a mg/m2 basis) before conception and through Day 7 of pregnancy caused no impairment of fertility. In this study, long-term dosing with porfimer sodium caused discoloration of testes and ovaries and hypertrophy of the testes. Porfimer sodium also caused decreased body weight in the parent rats.
Clinical Studies
- Clinical studies of photodynamic therapy (PDT) with PHOTOFRIN were conducted in patients with obstructing esophageal and endobronchial non-small-cell lung cancers, in patients with early-stage radiologically occult endobronchial cancer, and in patients with high-grade dysplasia (HGD) in Barrett's esophagus (BE). In all clinical studies, the method of PDT administration was essentially identical. A course of therapy consisted of one injection of PHOTOFRIN (2 mg/kg administered as a slow intravenous injection over 3–5 minutes) followed by up to two non-thermal applications of 630 nm laser light. Light doses of 300 J/cm of diffuser length were used in esophageal cancer. Light doses of 200 J/cm of diffuser length were used in endobronchial cancer for both palliation of obstructing cancer and treatment of superficial lesions. For the ablation of HGD in BE, the light dose administered was 130 J/cm of diffuser length using a centering balloon for the first application and 50 J/cm of diffuser length without a centering balloon for the second application. In all cases, the first application of light occurred 40–50 hours after PHOTOFRIN injection.
- For treatment of esophageal cancer debridement of residua via endoscopy is optional 96–120 hours after injection, after which any residual tumor could be retreated with a second laser light application at the same light dose used for the initial treatment. Additional courses of PDT with PHOTOFRIN were allowed after one month, up to a maximum of three courses.
- For treatment of endobronchial cancer, debridement of residua was performed via bronchoscopy 96–120 hours after injection, after which any residual tumor could be retreated with a second laser light application at the same light dose used for the initial treatment. Additional courses of PDT with PHOTOFRIN-were allowed after one month, up to a maximum of three courses.
- For ablation of HGD in BE, a second laser light application of 50 J/cm of diffuser length without a centering balloon could be given 96-120 hours after the PHOTOFRIN injection for untreated areas ("skip" areas). Additional courses of PDT with PHOTOFRIN were allowed after three months, up to a maximum of three courses.
Esophageal Cancer
- PDT with PHOTOFRIN was utilized in a multicenter, single-arm study in 17 patients with completely obstructing esophageal carcinoma. Assessments were made at 1 week and 1 month after the last treatment procedure. As shown in TABLE 10, after a single course of therapy, 94% of patients obtained an objective tumor response and 76% of patients experienced some palliation of their dysphagia. On average, before treatment these patients had difficulty swallowing liquids, even saliva. After one course of therapy, there was a statistically significant improvement in mean dysphagia grade (1.5 units, p <0.05) and 13 of 17 patients could swallow liquids without difficulty 1 week and/or 1 month after treatment. Based on all courses, three patients achieved a complete tumor response (CR). In two of these patients, the CR was documented only at Week 1 as they had no further assessments. The third patient achieved a CR after a second course of therapy, which was supported by negative histopathology and maintained for the entire follow-up of 6 months.
- Of the 17 treated patients, 11 (65%) received clinically important benefit from PDT. Clinically important benefit was defined hierarchically as a complete tumor response (3 patients), achievement of normal swallowing (2 patients went from Grade 5 dysphagia to Grade 1), or achievement of a marked improvement of two or more grades of dysphagia with minimal adverse reactions (6 patients). The median duration of benefit in these patients was 69 days. Duration of benefit was calculated only for the period with documented evidence of improvement. All of these patients were still in response at their last assessment and, therefore, the estimate of 69 days is conservative. The median survival for these 11 patients was 115 days.
Endobronchial Cancer
- Two randomized multicenter Phase III studies were conducted to compare the safety and efficacy of PHOTOFRIN PDT versus Nd:YAG laser therapy for reduction of obstruction and palliation of symptomatic patients with partially or completely obstructing endobronchial non-small-cell lung cancer. Assessments were made at 1 week and at monthly intervals after treatment. TABLE 11 shows the results from all randomized patients in the two studies combined. Objective tumor response rates (CR + PR), which demonstrate reduction of obstruction, were 59% for PDT and 58% for Nd:YAG at Week 1. The response rate at 1 month or later was 60% for PDT and 41% for Nd:YAG.
- Patient symptoms were evaluated using a 5- or 6-grade pulmonary symptom severity rating scale for dyspnoea, cough, and hemoptysis. Patients with moderate to severe symptoms are those most in need of palliation. Improvements of 2 or more grades are considered to be clinically significant. TABLE 12 shows the percentages of patients with moderate to severe symptoms at baseline who demonstrated a 2-grade improvement at any time during the interval evaluated.



- In a separate retrospective analysis, patients were individually evaluated to identify those patients whose benefit to risk ratio was most favorable, i.e., those who obtained clinically important benefit with minimal adverse reactions. Clinically important benefit was defined as one of the following:
- A substantial improvement in pulmonary symptoms at Month 1 or later (dyspnoea ≥2 grades, hemoptysis ≥3 grades, cough ≥3 grades or increase in FEV1 ≥40%);
A moderate improvement in symptoms at Month 2 or later (dyspnoea 1 grade, cough 2 grades, hemoptysis 2 grades or increase in FEV1 ≥20%); or A durable objective tumor response (CR or PR maintained to Month 2 or longer). Thirty-six (36) of the 99 PDT-treated patients (36%) and 23 of the 99 Nd:YAG-treated patients (23%) received clinically important benefit with only minimal or moderate toxicities of short duration. Thirty-four of 99 PDT-treated patients demonstrated improvements in 2 or more efficacy endpoints (dyspnoea, cough, hemoptysis, sputum, atelectasis, pulmonary function tests of FEV1 or FVC, Karnofsky Performance Score or tumor response) and 29 patients had improvements in 3 or more.
- The median duration of documented benefit in the 36 patients was 63 days. In these patients with late-stage obstructing lung cancer, median survival was 174 days in PDT-treated patients and 161 days in Nd:YAG-treated patients.
- The efficacy of PHOTOFRIN PDT was also evaluated in the treatment of microinvasive endobronchial tumors in 62 inoperable patients in three noncomparative studies. Microinvasive lung cancer is defined histologically as disease, which invades beyond the basement membrane but not through or into the cartilage. For 11 of the 62 patients, it was clearly documented that surgery and radiotherapy were not indicated. These 11 patients were all inoperable for medical or technical reasons. Radiotherapy was not indicated due to prior high-dose radiotherapy (7 patients), poor pulmonary function (2 patients), multifocal multilobar disease (1 patient), and poor medical condition (1 patient). As shown in TABLE 13, the complete tumor response rate, biopsy-proven at least 3 months after treatment, was 50%, median time to tumor recurrence was more than 2.7 years, median survival was 2.9 years and disease-specific survival was 4.1 years.

High-Grade Dysplasia in Barrett's Esophagus
- The safety and efficacy of PDT with PHOTOFRIN in ablation of HGD in patients with BE was assessed in one controlled randomized clinical study and two supportive studies.
Controlled Randomized Study
- A multicenter, pathology blinded, randomized, controlled study was conducted in North America and Europe to assess the efficacy of PDT with PHOTOFRIN for Injection plus omeprazole (PHOTOFRIN PDT + OM) in producing complete ablation of HGD in patients with BE compared to control patients receiving omeprazole alone (OM Only). A total of 485 patients with the diagnosis of HGD were screened for the study; 208 (43%) were randomized to treatment, 237 (49%) were excluded because the diagnosis of HGD was not confirmed and 40 (8%) did not meet other screening criteria or declined to participate in the study. The high patient exclusion rate re-enforces the recommendation by the American College of Gastroenterology that the diagnosis of HGD in BE should be confirmed by an expert GI pathologist. Patients were centrally randomized in a 2:1 proportion to receive PHOTOFRIN PDT + OM (138 patients) or OM Only (70 patients). All patients underwent rigorous systematic quarterly endoscopic biopsy surveillance. Four-quadrant jumbo biopsies at every 2 cm of the entire Barrett’s mucosa were obtained at each follow-up visit (every three months or six months if four consecutive quarterly follow-up endoscopic biopsy results were negative for HGD). All histological assessments were carried out at a central pathology laboratory and read by pathologists blinded to the treatment administered.
- A total of 208 patients who had biopsy-proven HGD in BE were enrolled in the initial 2-year phase of the study. Of those, 199 patients were considered evaluable: 130 of 138 (94%) patients randomized to the PHOTOFRIN PDT + OM group and 69 of 70 (99%) randomized to the OM Only group had no esophageal invasive cancer, suspicion of esophageal invasive cancer, lymph node involvement, or metastases, and had received at least one PHOTOFRIN PDT course or one week of OM treatment, respectively. A disproportionate percentage of patients were discontinued from the OM Only group during the initial 2-year phase leaving 81 (59%) patients in the PHOTOFRIN PDT + OM group and 21 (30%) patients in the OM Only group at the end of the 2-year phase. Consequently, a total of 102 patients who completed the initial 2-year phase were eligible for continuation into the long-term phase until completion of 5 years; of those, 48 (59%) patients from the PHOTOFRIN PDT + OM group and 13 (62%) patients from the OM Only group consented to pursue the long-term phase until completion of 5 years. The mean age was 66 years (38 to 89 years) in the PHOTOFRIN PDT + OM group, and 67 (36 to 88 years) in the OM Only group. The patients in both treatment groups were predominantly male (85%), Caucasian (99%), and former smokers (64%). These characteristics are typical of patients with HGD in BE. Patients randomized to the PHOTOFRIN PDT + OM treatment received up to three courses of treatment separated by at least 90 days. Each course consisted of intravenous administration of 2.0 mg/kg of PHOTOFRIN followed 40-50 hours later by a 630 nm laser light dose of 130 J/cm of diffuser length delivered using a centering balloon. A second laser light dose of 50 J/cm of diffuser length could be administered without a centering balloon 96-120 hours after the injection of PHOTOFRIN for treatment of "skip" areas. Since centering balloons are up to 7 cm in length, patients with more extensive HGD were treated with two or three courses. Both the PHOTOFRIN PDT treatment group and the control group received 20 mg of omeprazole BID to decrease reflux esophagitis. The mean duration of the follow-up period was 34 months (0-67 months) for the PHOTOFRIN PDT + OM group and 25 months (0-65 months) for the OM Only group.
- The primary efficacy endpoint was the Complete Response rate (CR3 or better) at any one of the endoscopic assessment time points. The CR3 or better response was defined as the complete ablation of HGD and referred to as a composite of the following three response levels.
- CR1 – Complete replacement of all Barrett’s metaplasia and dysplasia with normal squamous cell epithelium;
- CR2 – Ablation of all histological grades of dysplasia, including patients with indefinite grade of dysplasia, but some areas of Barrett’s epithelium still remain; and
- CR3 – Ablation of all areas of HGD but with some areas of low-grade dysplasia with or without areas which are indefinite for dysplasia, or areas of Barrett’s metaplastic epithelium.
- Additional efficacy endpoints included:
- Quality of Complete Response, which consisted of CR1 and CR2 or better.
- Duration of CR;
- Time to Progression to Cancer.
- TABLE 14 presents the overall clinical response for both treatment groups in the intent-to-treat (ITT) population whose response was CR3 or better at any one of the evaluation time points. Overall, PHOTOFRIN PDT + OM was effective in eliminating HGD in patients with BE. The proportion of responders was significantly higher in the PHOTOFRIN PDT + OM group than in the OM Only group (77% vs. 39%, respectively; p<0.0001).
- The quality of response in the PHOTOFRIN PDT + OM group was significantly better than that measured in the OM Only group at all response levels (p<0.0001). Seventy-two (52%) patients in the PHOTOFRIN PDT + OM group achieved a CR1 response as compared to only five (7%) patients in the OM Only group. Eighty-one (59%) patients in the PHOTOFRIN PDT + OM group achieved a CR2 or better response as compared to ten (14%) patients in the OM Only group.

NOTE: Six patients in the PHOTOFRIN PDT + OM group and three patients in the OM Only group without post-baseline biopsy data are considered as non-responders.
- At the end of the long-term phase, the median response duration was 44.6 months (95% CI: 15.0-not reached, months) in the PHOTOFRIN PDT + OM group compared to 3.2 months (95% CI: 3.0-3.4, months) in the OM Only group.
- At the end of the initial 2 year phase, the time to progression to cancer was significantly longer in the PHOTOFRIN PDT + OM group compared to the OM Only group (HR=0.36 (95% CI: 0.19-0.69), a hazard ratio less than 1 favors the PHOTOFRIN PDT + OM group). The proportion of patients' progression to cancer was lower in the PHOTOFRIN PDT + OM group than in the OM Only group: 13% (18 of 138 patients) vs. 28% (20 of 70 patients).
- Complete response was influenced by the following factors: treatment with PHOTOFRIN PDT + OM (vs. OM Only), single focus of HGD (vs. multiple foci), and prior omeprazole intake of at least 3 months (yes vs. no). Complete response was not influenced by the duration of HGD, length of BE, nodular conditions, gender, age, smoking history, and study center’s size.
Supportive Studies
- Two uncontrolled, supportive studies were conducted that were physician-sponsored, single center Phase II trials. Both studies included patients that had low-grade dysplasia (LGD), HGD and early adenocarcinoma. All HGD in BE patients were treated with PHOTOFRIN PDT and omeprazole.
- The first study enrolled 99 patients (44 with HGD); the purpose of this study was to determine the required light dose to produce effective results. The second study enrolled 86 patients (42 with HGD), who were randomized to receive either PHOTOFRIN PDT with prednisone or PHOTOFRIN PDT without prednisone to determine whether steroid treatment would reduce the incidence and severity of esophageal strictures.
- A CR3 or better response was demonstrated in 93% of 44 patients with HGD in the first study and in 95% of 42 patients with HGD in the second study after a minimum follow-up of 12 months. A CR2 or better response was achieved in 82% of patients in the first study and in 91% of patients in the second study. A CR1 response occurred in 57% of patients in the first study and in 60% of the second study. Progression to cancer during the above follow-up period occurred in 18% of patients in the first study and in 7% of patients in the second study. No reduction in the incidence or severity of esophageal strictures was found in the prednisone group in the second study.
How Supplied
- PHOTOFRIN (porfimer sodium) for Injection is supplied as a freeze-dried cake or powder as follows:
NDC 76128-155-75, 75 mg vial
Storage
- PHOTOFRIN freeze-dried cake or powder should be stored at Controlled Room Temperature 20-25°C (68-77°F) [see USP].
Images
Drug Images
{{#ask: Page Name::Porfimer |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Porfimer |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Photosensitivity
- Patients should be warned to avoid exposure of skin and eyes to direct sunlight or bright indoor light for at least 30 days following injection with PHOTOFRIN.
- Patients should be informed that photosensitivity might last for more than 90 days if patients suffer from liver impairment.
- Patients should be instructed to wear protective clothing and dark sunglasses when outdoors, which have an average white light transmittance of < 4%.
- Patients should be encouraged to expose their skin to ambient indoor light to facilitate elimination of PHOTOFRIN from their skin.
Common Adverse Reactions
- Patients should be informed that treatment with photodynamic therapy might lead to adverse reactions which include ocular sensitivity, chest pain, respiratory distress or esophageal strictures. In such cases, patients should call their physicians.
Precautions with Alcohol
- Alcohol-Porfimer interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PHOTOFRIN
Look-Alike Drug Names
There is limited information regarding Porfimer Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Porfimer
|Pill Name=Porfimer sodium ingredients and appearance.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Porfimer |Label Name=Porfimer image.jpg
}}
{{#subobject:
|Label Page=Porfimer |Label Name=Porfimer image1.jpg
}}
{{#subobject:
|Label Page=Porfimer |Label Name=
}}