Traveller vaccination yellow fever
|
Traveler Vaccination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Usama Talib, BSc, MD [2]
Disease cause
Transmission
Yellow fever occurs in urban and rural areas of Africa and Central and South America. In jungle and forest areas, monkeys are the main reservoir of infection, which is spread by mosquitoes from monkey to monkey and, occasionally, to human beings. In urban settings mosquitoes transmit the virus from person to person, and introduction of infection into densely populated urban areas can lead to large epidemics of yellow fever. In Africa, an intermediate pattern of transmission is common in humid savannah regions where mosquitoes infect both monkeys and human beings, causing localized outbreaks.
Nature of the disease
Although most infections are asymptomatic, some lead to an acute illness characterized by two phases. Initially, there is fever, muscular pain, headache, chills, anorexia, nausea and vomiting, often with bradycardia. About 15% of patients progress to a second phase after a few days, with resurgence of fever, development of jaundice, abdominal pain, vomiting and haemorrhagic manifestations; up to half these patients die 10–14 days after the onset of illness.
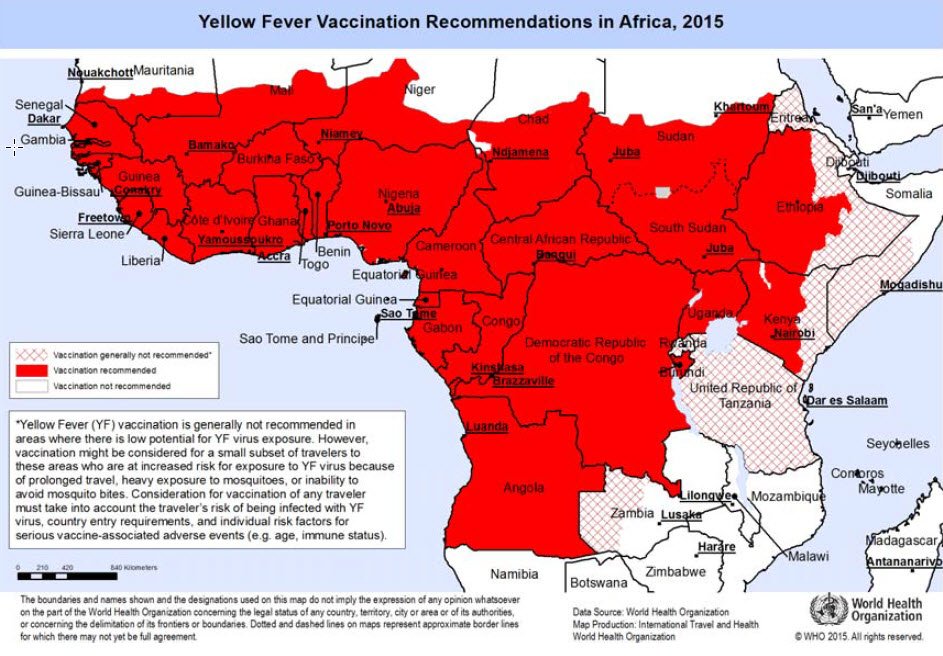
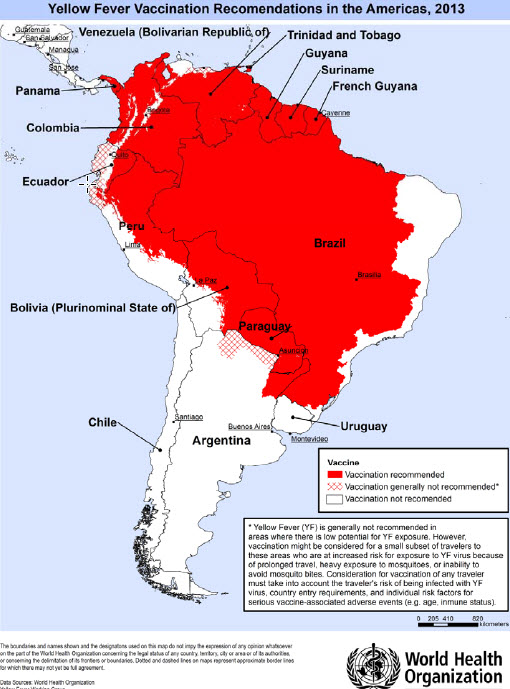
Geographical distribution
In tropical areas of Africa and Central and South America (see maps) yellow fever virus can be transmitted at altitudes up to 2300 metres (in Africa, possibly higher). The number of countries or areas where yellow fever virus is present far exceeds those officially reported. Some countries may have no reported cases simply because of a high level of vaccine coverage against yellow fever, or because of poor surveillance.
Risk for travellers
Besides areas of high yellow fever endemicity, transmission of yellow fever virus may take place also in areas of low endemicity if the traveller’s itinerary implies heavy exposure to mosquitoes, for example during prolonged travel in rural areas. A valid certificate of vaccination against yellow fever is required for visitors to and from yellow fever endemic areas.
General precautions
Avoid mosquito bites; the highest risk for transmission of yellow fever virus is during the day and early evening.
Vaccine
Yellow fever vaccine is highly effective (approaching 100%). A single dose of yellow fever vaccine is sufficient to confer sustained life-long protective immunity against yellow fever disease; a booster dose is not necessary. Yellow fever vaccine may be administered simultaneously with other vaccines. As a general rule, any live vaccine may be given either simultaneously or at an interval of 4 weeks. Oral polio vaccine may be given at any time in relation to yellow fever vaccination. Vaccine should be offered to all unvaccinated travellers aged >9 months, travelling to and from at-risk areas, unless they belong to the group of individuals for whom yellow fever vaccination is contraindicated. Vaccination is recommended, if indicated, for pregnant or breastfeeding women travelling to endemic areas when such travel cannot be avoided or postponed. Yellow fever vaccine may be offered to asymptomatic HIV-infected persons with CD4+ T-cell counts ≥200 cells/mm³. Although there are limited data on safety and immunogenicity of yellow fever vaccine when used in HIV-infected children, yellow fever vaccine may be administered to all clinically well children. HIV testing is not a prerequisite for vaccination.
Adverse reactions
In clinical trials, non-serious adverse events, such as headache, myalgia, low-grade fever, discomfort at the injection site, pruritus, urticaria and rash were reported by 25% of vaccines. Very rare, but serious adverse events following vaccination with yellow fever vaccine fall into three categories, as follows:
- Immediate severe hypersensitivity or anaphylactic reactions. Anaphylactic reactions have been estimated to occur in 0.8 per 100,000 vaccinations, most commonly in people with allergies to eggs or gelatine.
- Yellow fever vaccine-associated neurologic disease, a group of neurological conditions due to either direct viral invasion of the central nervous system by the vaccine virus resulting in meningitis or encephalitis, or to an autoimmune reaction resulting in conditions such as Guillain-Barré syndrome or acute disseminated encephalomyelitis.
- Yellow fever vaccine-associated viscerotropic disease, which is caused by replication and dissemination of the vaccine virus in a manner similar to the natural virus. People with this condition typically develop multi-organ system dysfunction or failure and >60% of cases have been fatal. The risk of adverse effects is higher in persons aged ≥60 years, but the overall risk remains low.
Contraindications and precautions
The vaccine is contraindicated in children aged <6 months and is not recommended for those aged 6–8 months, except during epidemics when the risk of infection with yellow fever virus may be very high. Other contraindications for yellow fever vaccination are severe hypersensitivity to egg antigens and severe immunodeficiency. Based on currently available data, caution is recommended in vaccinating persons ≥60 years of age against yellow fever. A risk-benefit assessment for yellow fever vaccination should be performed for any person ≥60 years of age who has not been vaccinated and for whom the vaccine is normally recommended. Required Yellow fever vaccination is required for travellers to certain countries and is recommended for all travellers visiting areas subject to endemic and epidemic disease. The unlimited validity of the certificate of vaccination will enter into force legally in June 2016.
Summary of vaccine data
| Considerations for travellers for Yellow fever vaccination | |
|---|---|
| Type of vaccine |
|
| Number of doses |
|
| Boosters |
|
| Contraindications |
|
| Adverse reactions |
|
| Before departure |
|
| Indication |
|
| Special precautions |
|