Traveller vaccination rabies
To read more about rabies, click here.
|
Traveler Vaccination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Usama Talib, BSc, MD [2]
Disease cause
Transmission
Rabies is a zoonotic disease affecting a wide range of domestic and wild mammals, including bats. The virus is present primarily in saliva, and infection of humans usually occurs through the bite of an infected animal, usually a dog, which may not show signs of rabies. Transmission may occasionally occur also through other contact with a rabid animal, for example following a penetrating scratch with bleeding, or through licking of broken skin and mucosa. Laboratory confirmed person-to-person transmission other than through organ transplant has not been reported.[1]
Nature of the disease
Rabies is an acute viral encephalomyelitis, which is almost invariably fatal. The initial signs include a sense of apprehension, headache, fever, malaise and sensory changes around the site of the animal bite. Excitability, hallucinations and abnormal fear of drafts of air (aerophobia) are common, followed in some cases by fear of water (hydrophobia) due to spasms of the swallowing muscles. A few days after onset, the disease progresses to delirium, convulsions and death. A less common form, paralytic rabies, is characterized by paralysis and loss of sensation, weakness and pain.
Geographical distribution
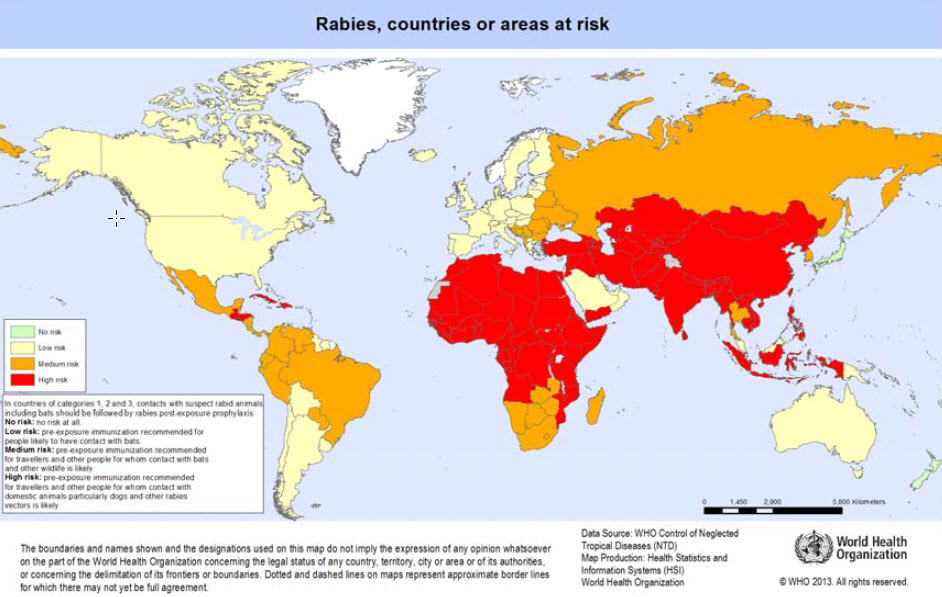
Rabies is present in mammals in most parts of the world (see map). Most of the estimated 55 000 human rabies deaths per year occur in Africa and Asia.
Risk for travellers
- The risk to travellers in areas where rabies occurs (see map) is proportional to the probability of contact with rabid mammals. In most developing countries, the estimated ratio of dogs, both owned and ownerless, to human beings is 1:10 and an average 100 suspected rabid dog bites per 100 000 inhabitants are reported annually. As rabies is a lethal disease, medical advice should be sought immediately at a competent medical centre – ideally, the rabies treatment centre of a major city hospital. First-aid measures should also be started immediately (see Post-exposure prophylaxis, below).
- Travellers should avoid contact with free-roaming animals, especially dogs and cats, and with wild, free-ranging or captive animals. For travellers who participate in caving or spelunking, casual exposure to cave air is not a concern, but cavers should be warned not to handle bats. In most countries of the world, suspected contact with bats should be followed by post-exposure prophylaxis.
- The map shows WHO’s categories of risk, from no risk (rabies-free) countries or areas, to countries or areas of low, medium and high risk. Categorization is based primarily on the animal host species in which the rabies virus is maintained, e.g. bats and/or other wildlife and/or dogs, and on the availability of reliable laboratory-based surveillance data on these reservoir species. Access to proper medical care and the availability of modern rabies vaccines have also been taken into consideration on a country basis. In countries or areas belonging to categories 2–4, pre-exposure immunization against rabies is recommended for travellers with certain characteristics:
- Category 1: no risk.
- Category 2: low risk.
- In these countries travellers involved in activities that might bring them into direct contact with bats (for example, wildlife professionals, researchers, veterinarians and adventure travellers visiting areas where bats are commonly found) should receive pre-exposure prophylaxis.
- Category 3: medium risk.
- In these countries, travellers involved in any activities that might bring them into direct contact with bats and other wild animals (especially carnivores), for example, wildlife professionals, researchers, veterinarians and travellers visiting areas where bats and wildlife are commonly found, should receive pre-exposure prophylaxis.
- Category 4: high risk.
- In these countries, travellers spending considerable periods of time in rural areas and involved in activities such as running, bicycling, camping or hiking should receive pre-exposure prophylaxis. Prophylaxis is also recommended for people with significant occupational risks, such as veterinarians, and expatriates living in areas with a significant risk of exposure to domestic animals, particularly dogs, and wild carnivores. Children should be immunized, as they are at higher risk through playing with animals, particularly dogs and cats; they may receive more severe bites and are less likely to report contact with animals suspected of having rabies
Vaccine
Vaccination against rabies is used in two distinct situations:
- To protect those who are at risk of exposure to rabies, in other words pre-exposure vaccination.
- To prevent the development of clinical rabies after exposure has occurred, usually following the bite of an animal suspected of having rabies, in other words post-exposure prophylaxis.
The vaccines used for pre-exposure and post-exposure vaccination are the same, but the immunization schedule differs. Rabies immunoglobulin is used only for post-exposure prophylaxis. Modern vaccines of cell-culture or embryonated-egg origin are safer and more effective than the older vaccines, which were produced in mouse brain tissue. These modern rabies vaccines are now available in major urban centers of most countries of the developing world. Rabies immunoglobulin, on the other hand, is in short supply worldwide and may not be available, even in major urban centres, in many countries where canine rabies is prevalent.
Pre-exposure vaccination
- Pre-exposure immunization is recommended for all individuals living in or traveling to areas where rabies is highly enzootic, and for those exposed to rabies by nature of their occupation, including laboratory staff, veterinarians, animal handlers and wildlife officers. However, according to age-stratified studies of incidence, those at greatest risk are children living in rabies-enzootic regions of the developing world. Pre-exposure vaccination is therefore advisable for children living in or visiting areas with a high risk of rabies. Pre-exposure vaccination is also recommended for individuals travelling to isolated areas or to areas where immediate access to appropriate medical care is limited or to countries where modern rabies vaccines are in short supply and locally available rabies vaccines might be unsafe and/or ineffective.
- Pre-exposure rabies vaccination consists of three full intramuscular (IM) doses of cell-culture- or embryonated-egg-based vaccine given on days 0, 7 and 21 (or day 28, if more convenient); a few days’ variation in the timing is not important. For adults and children aged ≥2 years, the vaccine should always be administered in the deltoid area of the arm; for children aged <2 years, the anterolateral area of the thigh is recommended. Rabies vaccine should never be administered in the gluteal area: administration in this manner results in lower neutralizing antibody titres.
- To reduce the cost of cell-derived vaccines for pre-exposure rabies vaccination, intradermal vaccination in doses of 0.1 ml on days 0, 7 and either 21 or 28 may be considered. This method of administration is an acceptable alternative to the standard intramuscular administration, but it is technically more demanding and requires appropriately trained staff and qualified medical supervision. Concurrent use of chloroquine can reduce the antibody response to intradermal application of cell-culture rabies vaccines. People who are currently receiving malaria prophylaxis or who are unable to complete the entire three-dose pre-exposure series before starting malarial prophylaxis should therefore receive pre-exposure vaccination by the intramuscular route.
- Periodic booster injections are not recommended for general travellers. However, in the event of exposure through the bite or scratch of an animal known or suspected to be rabid, individuals who have previously received a complete series of pre- or post-exposure rabies vaccine (with cell-culture or embryonated-egg-derived vaccine) should receive two booster doses of vaccine. Ideally, the first dose should be administered on the day of exposure and the second 3 days later. This should be combined with thorough wound treatment (see Post-exposure prophylaxis, below). Rabies immunoglobulin is not required for patients who have previously received a complete vaccination series.
Precautions and contraindications
Modern rabies vaccines are well tolerated. The frequency of minor adverse reactions (local pain, erythema, swelling and pruritus) varies widely from one report to another. Occasional systemic reactions (malaise, generalized aches and headaches) have been noted after intramuscular or intradermal injections.
Post-exposure prophylaxis
In countries or areas at risk of rabies, the circumstances of an animal bite or other contact with an animal suspected to be rabid may require post-exposure prophylaxis. In such situations, medical advice should be obtained immediately. Strict adherence to the WHO-recommended guidelines for optimal post-exposure rabies prophylaxis virtually guarantees protection from the disease. The administration of vaccine, and of immunoglobulin if required, must be conducted by, or under the direct supervision of, a physician.
| Category | Type of contact with a suspected or confirmed rabid domestic or wild(a) animal or animal unavailable for testing | Type of exposure | Recommended post-exposure prophylaxis |
|---|---|---|---|
| I |
|
None |
|
| II |
|
Minor |
|
| III |
|
Severe |
|
a)Exposure to rodents, rabbits and hares seldom, if ever, requires specific anti-rabies post-exposure prophylaxis.
b)If an apparently healthy dog or cat in or from a low-risk country or area is placed under observation, the situation may warrant delaying initiation of treatment.
c)This observation period applies only to dogs and cats. Except in the case of threatened or endangered species, other domestic and wild animals suspected to be rabid should be humanely killed and their tissues examined for the presence of rabies virus antigen using appropriate laboratory techniques.
d)Post-exposure prophylaxis should be considered for individuals who have been in close contact with bats, particularly following bites or scratches or exposure to mucous membranes.
- Wound treatment : Thorough washing of the wound with soap/detergent and water, followed by the application of ethanol or an aqueous solution of iodine or povidone.
- Passive immunization : Human rabies immunoglobulin or equine rabies immunoglobulin should be used for category III exposures as well as for some category II exposures (see Table 6.2). Passive immunization should be administered just before or shortly after administration of the first dose of vaccine given in the post-exposure prophylaxis regimen. If it is not immediately available, passive immunization can be administered up until the seventh day after initiation of the primary series of post-exposure prophylaxis (with cell-culture or embryonated-egg rabies vaccine).
- Dosage and administration: The dose for human rabies immunoglobulin is 20 IU/kg body weight and for equine rabies immunoglobulin and F(ab)2 products 40 IU/kg body weight. The full dose of rabies immunoglobulin, or as much as is anatomically feasible, should be administered into and around the wound site. Any remainder should be injected intramuscularly at a site distant from the site of active vaccine administration. Multiple needle injections into the wound should be avoided. If the correct dose of rabies immunoglobulin is too small to infiltrate all wounds, as might be true of a severely bitten individual, it can be diluted in physiological buffered saline to ensure greater wound coverage.
- Active immunization: Cell-culture- or embryonated-egg-based rabies vaccines should always be used for post-exposure prophylaxis. They can be administered either intramuscularly or intradermally. Intramuscular regimens: Both a five-dose and a four-dose IM regimen are recommended for post-exposure vaccination; the fivedose regimen is the more commonly used.
- The five-dose regimen is administered on days 0, 3, 7, 14 and 28 into the deltoid muscle.
- The four-dose regimen is administered as two doses on day 0 (one dose in the right and one in the left arm (deltoid muscles), and then one dose on each of days 7 and 21 into the deltoid muscle.
- An alternative post-exposure regimen for healthy, fully immunocompetent exposed people who receive wound care plus high-quality rabies immunoglobulin plus WHO-prequalified rabies vaccines consists of four doses administered intramuscular on days 0, 3, 7 and 14.
- Intradermal regimens: Intradermal administration of cell-culture- and embryonated-egg-based rabies vaccines has been successfully used in many developing countries that cannot afford the five- or four-dose i.m. schedules.
- The two-site intradermal method: one intradermal injection at two sites on days 0, 3, 7 and 28.
- The volume per intradermal injection should be 0.1 ml with both purified Vero cell rabies vaccine and purified chick embryo rabies vaccine.
Summary of vaccine data
| Considerations for travellers for Rabies vaccination | |
|---|---|
| Type of vaccine |
|
| Number of doses |
|
| Boosters |
|
| Contraindications |
|
| Adverse reactions |
|
| Before departure |
|
| Indication |
|
| Special precautions |
|
References
- ↑ "www.who.int" (PDF).