Rituximab sandbox
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: FATAL INFUSION REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
Fatal infusion reactions within 24 hours of Rituxan infusion; approximately 80% of fatal reactions occurred with first infusion. Monitor patients and discontinue Rituxan infusion for severe reactions (5.1).
Severe mucocutaneous reactions, some with fatal outcomes (5.2). Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death (5.3). Progressive multifocal leukoencephalopathy (PML) resulting in death (5.4). |
Overview
Rituximab sandbox is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of Non–Hodgkin's Lymphoma (NHL), Chronic Lymphocytic Leukemia (CLL), Rheumatoid Arthritis (RA), Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA),. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Hypotension, Peripheral edema, Night sweats, Pruritus, Rash, Abdominal pain, Diarrhea, Nausea, Vomiting, Anemia, Arthralgia, Backache, Myalgia, Asthenia, Dizziness, Headache, Sensory neuropathy, Increasing frequency of cough, Rhinitis, Fever, Infectious disease, Pain, Shivering.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Non-Hodgkin's Lymphoma (NHL)
- Dosing information
- Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
- Administer once weekly for 4 or 8 doses.
- Retreatment for Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
- Administer once weekly for 4 doses.
- Previously Untreated, Follicular, CD20-Positive, B-Cell NHL
- Administer on Day 1 of each cycle of chemotherapy, for up to 8 doses. In patients with complete or partial response, initiate Rituxan maintenance eight weeks following completion of Rituxan in combination with chemotherapy. Administer Rituxan as a single-agent every 8 weeks for 12 doses.
- Non-progressing, Low-Grade, CD20-Positive, B-cell NHL, after first-line CVP chemotherapy
- Following completion of 6–8 cycles of CVP chemotherapy, administer once weekly for 4 doses at 6-month intervals to a maximum of 16 doses.
- Diffuse Large B-Cell NHL
- Administer on Day 1 of each cycle of chemotherapy for up to 8 infusions.
Chronic Lymphocytic Leukemia (CLL)
- Dosing information
- Recommended dosage:
- 375 mg/m2 the day prior to the initiation of FC chemotherapy
- 500 mg/m2 on Day 1 of cycles 2–6 (every 28 days).
Recommended Dose as a Component of Zevalin®
- Dosing information
- Infuse rituximab 250 mg/m2 within 4 hours prior to the administration of Indium-111-(In-111-) Zevalin and within 4 hours prior to the administration of Yttrium-90- (Y-90-) Zevalin.
- Administer Rituxan and In-111-Zevalin 7–9 days prior to Rituxan and Y-90- Zevalin.
- Refer to the Zevalin package insert for full prescribing information regarding the Zevalin therapeutic regimen.
Recommended Dose for Rheumatoid Arthritis (RA)
- Dosing information
- Administer Rituxan as two-1000 mg intravenous infusions separated by 2 weeks.
- Glucocorticoids administered as methylprednisolone 100 mg intravenous or its equivalent 30 minutes prior to each infusion are recommended to reduce the incidence and severity of infusion reactions.
- Subsequent courses should be administered every 24 weeks or based on clinical evaluation, but not sooner than every 16 weeks.
- Rituxan is given in combination with methotrexate.
Recommended Dose for Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
- Dosing information
- Administer Rituxan as a 375 mg/m2 intravenous infusion once weekly for 4 weeks.
- Glucocorticoids administered as methylprednisolone 1000 mg intravenously per day for 1 to 3 days followed by oral prednisone 1 mg/kg/day (not to exceed 80 mg/day and tapered per clinical need) are recommended to treat severe vasculitis symptoms. This regimen should begin within 14 days prior to or with the initiation of Rituxan and may continue during and after the 4 week course of Rituximab treatment.
- Safety and efficacy of treatment with subsequent courses of Rituxan have not been established
Recommended Concomitant Medications
- Dosing information
- Premedicate before each infusion with acetaminophen and an antihistamine. For patients administered Rituxan according to the 90-minute infusion rate, the glucocorticoid component of their chemotherapy regimen should be administered prior to infusion
- For RA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion.
- For GPA and MPA patients, glucocorticoids are given in combination with Rituxan
- Pneumocystis jiroveci pneumonia (PCP) and anti-herpetic viral prophylaxis is recommended for patients with CLL during treatment and for up to 12 months following treatment as appropriate.
- PCP prophylaxis is also recommended for patients with GPA and MPA during treatment and for at least 6 months following the last Rituxan infusion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rituximab sandbox in adult patients.
Non–Guideline-Supported Use
Acquired factor VIII deficiency disease
- Dosing information
- 375 mg/m(2) given once weekly14996701 12384448 17212724
Autoimmune hemolytic anemia
- Dosing information
- 375 mg/m(2) IV weekly for 4 consecutive weeks16321854, 15070665
B-cell lymphoma
- Dosing information
- 375 mg/m(2) 14739217, 24986783
Evans syndrome
- Dosing information
- 375 mg/m(2) weekly for 2 doses12679656
Graft-versus-host disease
- Dosing information
- 375 mg/m(2) IV weekly for 4 consecutive weeks20663943, 16551963
Hairy cell leukemia
- Dosing information
- 375 mg/m(2) per week10970137
Hodgkin's disease
- Dosing information
- 375 mg/m(2) IV once weekly for 4 weeks 12586628, 12509381
Idiopathic thrombocytopenic purpura
- Dosing information
- 375 mg/m(2) once a week for 4 weeks 23293082
Mantle cell lymphoma
- Dosing information
- 375 mg/m(2) on day 0) every 3 weeks for 6 cycles15668467
- 375 mg/m(2) IV was administered on the first day of cycles 4 and 5 and on the first and ninth day of cycle 6 18625886
Pemphigus vulgaris
- Dosing information
- 375 mg/m(2) IV once weekly for a 4-week cycle17687130
Post-transplant lymphoproliferative disorder
- Dosing information
- 375 mg/m(2) IV once weekly for 4 weeks22173060
- 375 mg/m(2) weekly for 4 weeks every 6 months 15888049
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Rituximab sandbox FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rituximab sandbox in pediatric patients.
Non–Guideline-Supported Use
Autoimmune hemolytic anemia
- Dosing information
- 375 mg/m(2)11705566, 19208098
Post-transplant lymphoproliferative disorder
- Dosing information
- 375 mg/m(2) IV once weekly for 4 to 8 cycles19344338
Contraindications
None.
Warnings
|
WARNING: FATAL INFUSION REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete Boxed Warning.
Fatal infusion reactions within 24 hours of Rituxan infusion; approximately 80% of fatal reactions occurred with first infusion. Monitor patients and discontinue Rituxan infusion for severe reactions (5.1).
Severe mucocutaneous reactions, some with fatal outcomes (5.2). Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death (5.3). Progressive multifocal leukoencephalopathy (PML) resulting in death (5.4). |
Infusion Reactions
Rituxan can cause severe, including fatal, infusion reactions. Severe reactions typically occurred during the first infusion with time to onset of 30–120 minutes. Rituxan-induced infusion reactions and sequelae include urticaria, hypotension, angioedema, hypoxia, bronchospasm, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, cardiogenic shock, anaphylactoid events, or death. Premedicate patients with an antihistamine and acetaminophen prior to dosing. For RA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion. Institute medical management (e.g. glucocorticoids, epinephrine, bronchodilators, or oxygen) for infusion reactions as needed. Depending on the severity of the infusion reaction and the required interventions, temporarily or permanently discontinue Rituxan. Resume infusion at a minimum 50% reduction in rate after symptoms have resolved. Closely monitor the following patients: those with pre-existing cardiac or pulmonary conditions, those who experienced prior cardiopulmonary adverse reactions, and those with high numbers of circulating malignant cells ( ≥ 25,000/mm3).
Severe Mucocutaneous Reactions
Mucocutaneous reactions, some with fatal outcome, can occur in patients treated with Rituxan. These reactions include paraneoplastic pemphigus, Stevens-Johnson syndrome, lichenoid dermatitis, vesiculobullous dermatitis, and toxic epidermal necrolysis. The onset of these reactions has been variable and includes reports with onset on the first day of Rituxan exposure. Discontinue Rituxan in patients who experience a severe mucocutaneous reaction. The safety of readministration of Rituxan to patients with severe mucocutaneous reactions has not been determined. [See Boxed Warning, Adverse Reactions (6, 6.1)].
Hepatitis B Virus Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, can occur in patients treated with drugs classified as CD20-directed cytolytic antibodies, including Rituxan. Cases have been reported in patients who are hepatitis B surface antigen (HBsAg) positive and also in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation also has occurred in patients who appear to have resolved hepatitis B infection (i.e., HBsAg negative, anti-HBc positive and hepatitis B surface antibody [ anti-HBs ] positive). HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, i.e., increase in transaminase levels. In severe cases increase in bilirubin levels, liver failure, and death can occur. Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with Rituxan. For patients who show evidence of prior hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult with physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy before and/or during Rituxan treatment. Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following Rituxan therapy. HBV reactivation has been reported up to 24 months following completion of Rituxan therapy. In patients who develop reactivation of HBV while on Rituxan, immediately discontinue Rituxan and any concomitant chemotherapy, and institute appropriate treatment. Insufficient data exist regarding the safety of resuming Rituxan in patients who develop HBV reactivation. Resumption of Rituxan in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing hepatitis B.
Progressive Multifocal Leukoencephalopathy (PML)
JC virus infection resulting in PML and death can occur in Rituxan-treated patients with hematologic malignancies or with autoimmune diseases. The majority of patients with hematologic malignancies diagnosed with PML received Rituxan in combination with chemotherapy or as part of a hematopoietic stem cell transplant. The patients with autoimmune diseases had prior or concurrent immunosuppressive therapy. Most cases of PML were diagnosed within 12 months of their last infusion of Rituxan. Consider the diagnosis of PML in any patient presenting with new-onset neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture. Discontinue Rituxan and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML. [See Boxed Warning, Adverse Reactions (6)].
Tumor Lysis Syndrome (TLS)
Acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, or hyperphosphatemia from tumor lysis, some fatal, can occur within 12–24 hours after the first infusion of Rituxan in patients with NHL. A high number of circulating malignant cells ( ≥ 25,000/mm3) or high tumor burden, confers a greater risk of TLS. Administer aggressive intravenous hydration and anti-hyperuricemic therapy in patients at high risk for TLS. Correct electrolyte abnormalities, monitor renal function and fluid balance, and administer supportive care, including dialysis as indicated.
Infections
Serious, including fatal, bacterial, fungal, and new or reactivated viral infections can occur during and following the completion of Rituxan-based therapy. Infections have been reported in some patients with prolonged hypogammaglobulinemia (defined as hypogammaglobulinemia >11 months after rituximab exposure). New or reactivated viral infections included cytomegalovirus, herpes simplex virus, parvovirus B19, varicella zoster virus, West Nile virus, and hepatitis B and C. Discontinue Rituxan for serious infections and institute appropriate anti-infective therapy.
Cardiovascular
Discontinue infusions for serious or life-threatening cardiac arrhythmias. Perform cardiac monitoring during and after all infusions of Rituxan for patients who develop clinically significant arrhythmias, or who have a history of arrhythmia or angina.
Renal
Severe, including fatal, renal toxicity can occur after Rituxan administration in patients with NHL. Renal toxicity has occurred in patients who experience tumor lysis syndrome and in patients with NHL administered concomitant cisplatin therapy during clinical trials. The combination of cisplatin and Rituxan is not an approved treatment regimen. Monitor closely for signs of renal failure and discontinue Rituxan in patients with a rising serum creatinine or oliguria.
Bowel Obstruction and Perforation
Abdominal pain, bowel obstruction and perforation, in some cases leading to death, can occur in patients receiving Rituxan in combination with chemotherapy. In postmarketing reports, the mean time to documented gastrointestinal perforation was 6 (range 1–77) days in patients with NHL. Evaluate if symptoms of obstruction such as abdominal pain or repeated vomiting occur. [See Adverse Reactions (6)].
Immunization
The safety of immunization with live viral vaccines following Rituxan therapy has not been studied and vaccination with live virus vaccines is not recommended. For RA patients, physicians should follow current immunization guidelines and administer non-live vaccines at least 4 weeks prior to a course of Rituxan. The effect of Rituxan on immune responses was assessed in a randomized, controlled study in patients with RA treated with Rituxan and methotrexate (MTX) compared to patients treated with MTX alone. A response to pneumococcal vaccination (a T-cell independent antigen) as measured by an increase in antibody titers to at least 6 of 12 serotypes was lower in patients treated with Rituxan plus MTX as compared to patients treated with MTX alone (19% vs. 61%). A lower proportion of patients in the Rituxan plus MTX group developed detectable levels of anti-keyhole limpet hemocyanin antibodies (a novel protein antigen) after vaccination compared to patients on MTX alone (47% vs. 93%). A positive response to tetanus toxoid vaccine (a T-cell dependent antigen with existing immunity) was similar in patients treated with Rituxan plus MTX compared to patients on MTX alone (39% vs. 42%). The proportion of patients maintaining a positive Candida skin test (to evaluate delayed type hypersensitivity) was also similar (77% of patients on Rituxan plus MTX vs. 70% of patients on MTX alone). Most patients in the Rituxan-treated group had B-cell counts below the lower limit of normal at the time of immunization. The clinical implications of these findings are not known.
Laboratory Monitoring
In patients with lymphoid malignancies, during treatment with Rituxan monotherapy, obtain complete blood counts (CBC) and platelet counts prior to each Rituxan course. During treatment with Rituxan and chemotherapy, obtain CBC and platelet counts at weekly to monthly intervals and more frequently in patients who develop cytopenias . In patients with RA, GPA or MPA, obtain CBC and platelet counts at two to four month intervals during Rituxan therapy. The duration of cytopenias caused by Rituxan can extend months beyond the treatment period.
Concomitant Use with Biologic Agents and DMARDS other than Methotrexate in RA, GPA and MPA
Limited data are available on the safety of the use of biologic agents or DMARDs other than methotrexate in RA patients exhibiting peripheral B-cell depletion following treatment with rituximab. Observe patients closely for signs of infection if biologic agents and/or DMARDs are used concomitantly. Use of concomitant immunosuppressants other than corticosteroids has not been studied in GPA or MPA patients exhibiting peripheral B-cell depletion following treatment with Rituxan.
Use in RA Patients Who Have Not Had Prior Inadequate Response to Tumor Necrosis Factor (TNF) Antagonists
While the efficacy of Rituxan was supported in four controlled trials in patients with RA with prior inadequate responses to non-biologic DMARDs, and in a controlled trial in MTX-naïve patients, a favorable risk-benefit relationship has not been established in these populations. The use of Rituxan in patients with RA who have not had prior inadequate response to one or more TNF antagonists is not recommended [see Clinical Studies (14.6)].
Retreatment in Patients with Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Limited data are available on the safety and efficacy of subsequent courses of Rituxan in patients with GPA and MPA. The safety and efficacy of retreatment with Rituxan have not been established
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Infusion reactions
- Mucocutaneous reactions
- Hepatitis B reactivation with fulminant hepatitis
- Progressive multifocal leukoencephalopathy
- Tumor lysis syndrome
- Infections
- Cardiac arrhythmias
- Renal toxicity
- Bowel obstruction and perforation
The most common adverse reactions of Rituxan (incidence ≥ 25%) observed in clinical trials of patients with NHL were infusion reactions, fever, lymphopenia, chills, infection, and asthenia. The most common adverse reactions of Rituxan (incidence ≥ 25%) observed in clinical trials of patients with CLL were: infusion reactions and neutropenia.
Clinical Trials Experience in Lymphoid Malignancies
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The data described below reflect exposure to Rituxan in 2783 patients, with exposures ranging from a single infusion up to 2 years. Rituxan was studied in both single-arm and controlled trials (n=356 and n=2427). The population included 1180 patients with low grade or follicular lymphoma, 927 patients with DLBCL, and 676 patients with CLL. Most NHL patients received Rituxan as an infusion of 375 mg/m2 per infusion, given as a single agent weekly for up to 8 doses, in combination with chemotherapy for up to 8 doses, or following chemotherapy for up to 16 doses. CLL patients received Rituxan 375 mg/m2 as an initial infusion followed by 500 mg/m2 for up to 5 doses, in combination with fludarabine and cyclophosphamide. Seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of Rituxan-based therapy.
Infusion Reactions
In the majority of patients with NHL, infusion reactions consisting of fever, chills/rigors, nausea, pruritus, angioedema, hypotension, headache, bronchospasm, urticaria, rash, vomiting, myalgia, dizziness, or hypertension occurred during the first Rituxan infusion. Infusion reactions typically occurred within 30 to 120 minutes of beginning the first infusion and resolved with slowing or interruption of the Rituxan infusion and with supportive care (diphenhydramine, acetaminophen, and intravenous saline). The incidence of infusion reactions was highest during the first infusion (77%) and decreased with each subsequent infusion. [See Boxed Warning, Warnings and Precautions (5.1)]. In patients with previously untreated follicular NHL or previously untreated DLBCL, who did not experience a Grade 3 or 4 infusion-related reaction in Cycle 1 and received a 90-minute infusion of Rituxan at Cycle 2, the incidence of Grade 3-4 infusion-related reactions on the day of, or day after the infusion was 1.1% (95% CI [0.3%, 2.8%]). For Cycles 2-8, the incidence of Grade 3-4 infusion reactions on the day of or day after the 90-minute infusion, was 2.8% (95% CI [1.3%, 5.0%]). [See Warnings and Precautions (5.1), Clinical Studies (14.4)].
Infections
Serious infections (NCI CTCAE Grade 3 or 4), including sepsis, occurred in less than 5% of patients with NHL in the single-arm studies. The overall incidence of infections was 31% (bacterial 19%, viral 10%, unknown 6%, and fungal 1%). [See Warnings and Precautions (5.4), (5.5), (5.6)]. In randomized, controlled studies where Rituxan was administered following chemotherapy for the treatment of follicular or low-grade NHL, the rate of infection was higher among patients who received Rituxan. In diffuse large B-cell lymphoma patients, viral infections occurred more frequently in those who received Rituxan.
Cytopenias and hypogammaglobulinemia
In patients with NHL receiving rituximab monotherapy, NCI-CTC Grade 3 and 4 cytopenias were reported in 48% of patients. These included lymphopenia (40%), neutropenia (6%), leukopenia (4%), anemia (3%), and thrombocytopenia (2%). The median duration of lymphopenia was 14 days (range, 1–588 days) and of neutropenia was 13 days (range, 2–116 days). A single occurrence of transient aplastic anemia (pure red cell aplasia) and two occurrences of hemolytic anemia following Rituxan therapy occurred during the single-arm studies. In studies of monotherapy, Rituxan-induced B-cell depletion occurred in 70% to 80% of patients with NHL. Decreased IgM and IgG serum levels occurred in 14% of these patients. In CLL trials, the frequency of prolonged neutropenia and late-onset neutropenia was higher in patients treated with R-FC compared to patients treated with FC. Prolonged neutropenia is defined as Grade 3-4 neutropenia that has not resolved between 24 and 42 days after the last dose of study treatment. Late-onset neutropenia is defined as Grade 3-4 neutropenia starting at least 42 days after the last treatment dose. In patients with previously untreated CLL, the frequency of prolonged neutropenia was 8.5% for patients who received R-FC (n=402) and 5.8% for patients who received FC (n=398). In patients who did not have prolonged neutropenia, the frequency of late-onset neutropenia was 14.8% of 209 patients who received R-FC and 4.3% of 230 patients who received FC. For patients with previously treated CLL, the frequency of prolonged neutropenia was 24.8% for patients who received R-FC (n=274) and 19.1% for patients who received FC (n=274). In patients who did not have prolonged neutropenia, the frequency of late-onset neutropenia was 38.7% in 160 patients who received R-FC and 13.6% of 147 patients who received FC.
Relapsed or Refractory, Low-Grade NHL
Adverse reactions in Table 1 occurred in 356 patients with relapsed or refractory, low-grade or follicular, CD20-positive, B-cell NHL treated in single-arm studies of Rituxan administered as a single agent [See Clinical Studies (14.1)]. Most patients received Rituxan 375 mg/m2 weekly for 4 doses.

In these single-arm Rituxan studies, bronchiolitis obliterans occurred during and up to 6 months after Rituxan infusion.
Previously Untreated, Low-Grade or Follicular, NHL
In Study 4, patients in the R-CVP arm experienced a higher incidence of infusional toxicity and neutropenia compared to patients in the CVP arm. The following adverse reactions occurred more frequently ( ≥ 5%) in patients receiving R-CVP compared to CVP alone: rash (17% vs. 5%), cough (15% vs. 6%), flushing (14% vs. 3%), rigors (10% vs. 2%), pruritus (10% vs. 1%), neutropenia (8% vs. 3%), and chest tightness (7% vs. 1%). [See Clinical Studies (14.2)]. In Study 5, detailed safety data collection was limited to serious adverse reactions, Grade ≥ 2 infections, and Grade ≥ 3 adverse reactions. In patients receiving Rituxan as single-agent maintenance therapy following Rituxan plus chemotherapy, infections were reported more frequently compared to the observation arm (37% vs. 22%). Grade 3-4 adverse reactions occurring at a higher incidence (≥ 2%) in the Rituxan group were infections (4% vs. 1%) and neutropenia (4% vs. <1%). In Study 6, the following adverse reactions were reported more frequently ( ≥ 5%) in patients receiving Rituxan following CVP compared to patients who received no further therapy: fatigue (39% vs. 14%), anemia (35% vs. 20%), peripheral sensory neuropathy (30% vs. 18%), infections (19% vs. 9%), pulmonary toxicity (18% vs. 10%), hepato-biliary toxicity (17% vs. 7%), rash and/or pruritus (17% vs. 5%), arthralgia (12% vs. 3%), and weight gain (11% vs. 4%). Neutropenia was the only Grade 3 or 4 adverse reaction that occurred more frequently ( ≥ 2%) in the Rituxan arm compared with those who received no further therapy (4% vs. 1%). [See Clinical Studies (14.3)].
DLBCL
In Studies 7 and 8, the following adverse reactions, regardless of severity, were reported more frequently ( ≥ 5%) in patients age ≥ 60 years receiving R-CHOP as compared to CHOP alone: pyrexia (56% vs. 46%), lung disorder (31% vs. 24%), cardiac disorder (29% vs. 21%), and chills (13% vs. 4%). Detailed safety data collection in these studies was primarily limited to Grade 3 and 4 adverse reactions and serious adverse reactions. In Study 8, a review of cardiac toxicity determined that supraventricular arrhythmias or tachycardia accounted for most of the difference in cardiac disorders (4.5% for R-CHOP vs. 1.0% for CHOP). The following Grade 3 or 4 adverse reactions occurred more frequently among patients in the R-CHOP arm compared with those in the CHOP arm: thrombocytopenia (9% vs. 7%) and lung disorder (6% vs. 3%). Other Grade 3 or 4 adverse reactions occurring more frequently among patients receiving R-CHOP were viral infection (Study 8), neutropenia (Studies 8 and 9), and anemia (Study 9).
CLL
The data below reflect exposure to Rituxan in combination with fludarabine and cyclophosphamide in 676 patients with CLL in Study 11 or Study 12 [See Clinical Studies (14.5)]. The age range was 30–83 years and 71% were men. Detailed safety data collection in Study 11 was limited to Grade 3 and 4 adverse reactions and serious adverse reactions. Infusion-related adverse reactions were defined by any of the following adverse events occurring during or within 24 hours of the start of infusion: nausea, pyrexia, chills, hypotension, vomiting, and dyspnea. In Study 11, the following Grade 3 and 4 adverse reactions occurred more frequently in R-FC-treated patients compared to FC-treated patients: infusion reactions (9% in R-FC arm), neutropenia (30% vs. 19%), febrile neutropenia (9% vs. 6%), leukopenia (23% vs. 12%), and pancytopenia (3% vs. 1%). In Study 12, the following Grade 3 or 4 adverse reactions occurred more frequently in R-FC-treated patients compared to FC-treated patients: infusion reactions (7% in R-FC arm), neutropenia (49% vs. 44%), febrile neutropenia (15% vs. 12%), thrombocytopenia (11% vs. 9%), hypotension (2% vs. 0%), and hepatitis B (2% vs. < 1%). Fifty-nine percent of R-FC-treated patients experienced an infusion reaction of any severity.
Clinical Trials Experience in Rheumatoid Arthritis
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The data presented below reflect the experience in 2578 RA patients treated with Rituxan in controlled and long-term studies with a total exposure of 5014 patient-years. Among all exposed patients, adverse reactions reported in greater than 10% of patients include infusion-related reactions, upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis. In placebo-controlled studies, patients received 2 × 500 mg or 2 × 1000 mg intravenous infusions of Rituxan or placebo, in combination with methotrexate, during a 24-week period. From these studies, 938 patients treated with Rituxan (2 × 1000 mg) or placebo have been pooled (see Table 2). Adverse reactions reported in ≥ 5% of patients were hypertension, nausea, upper respiratory tract infection, arthralgia, pyrexia and pruritus (see Table 2). The rates and types of adverse reactions in patients who received Rituxan 2 × 500 mg were similar to those observed in patients who received Rituxan 2 × 1000 mg.

Infusion Reactions
In the Rituxan RA pooled placebo-controlled studies, 32% of Rituxan-treated patients experienced an adverse reaction during or within 24 hours following their first infusion, compared to 23% of placebo-treated patients receiving their first infusion. The incidence of adverse reactions during the 24-hour period following the second infusion, Rituxan or placebo, decreased to 11% and 13%, respectively. Acute infusion reactions (manifested by fever, chills, rigors, pruritus, urticaria/rash, angioedema, sneezing, throat irritation, cough, and/or bronchospasm, with or without associated hypotension or hypertension) were experienced by 27% of Rituxan-treated patients following their first infusion, compared to 19% of placebo-treated patients receiving their first placebo infusion. The incidence of these acute infusion reactions following the second infusion of Rituxan or placebo decreased to 9% and 11%, respectively. Serious acute infusion reactions were experienced by < 1% of patients in either treatment group. Acute infusion reactions required dose modification (stopping, slowing, or interruption of the infusion) in 10% and 2% of patients receiving rituximab or placebo, respectively, after the first course. The proportion of patients experiencing acute infusion reactions decreased with subsequent courses of Rituxan. The administration of intravenous glucocorticoids prior to Rituxan infusions reduced the incidence and severity of such reactions, however, there was no clear benefit from the administration of oral glucocorticoids for the prevention of acute infusion reactions. Patients in clinical studies also received antihistamines and acetaminophen prior to Rituxan infusions.
Infections
In the pooled, placebo-controlled studies, 39% of patients in the Rituxan group experienced an infection of any type compared to 34% of patients in the placebo group. The most common infections were nasopharyngitis, upper respiratory tract infections, urinary tract infections, bronchitis, and sinusitis. The incidence of serious infections was 2% in the Rituxan-treated patients and 1% in the placebo group. In the experience with Rituxan in 2578 RA patients, the rate of serious infections was 4.31 per 100 patient years. The most common serious infections ( ≥ 0.5%) were pneumonia or lower respiratory tract infections, cellulitis and urinary tract infections. Fatal serious infections included pneumonia, sepsis and colitis. Rates of serious infection remained stable in patients receiving subsequent courses. In 185 Rituxan-treated RA patients with active disease, subsequent treatment with a biologic DMARD, the majority of which were TNF antagonists, did not appear to increase the rate of serious infection. Thirteen serious infections were observed in 186.1 patient years (6.99 per 100 patient years) prior to exposure and 10 were observed in 182.3 patient years (5.49 per 100 patient years) after exposure.
Cardiac Adverse Reactions
In the pooled, placebo-controlled studies, the proportion of patients with serious cardiovascular reactions was 1.7% and 1.3% in the Rituxan and placebo treatment groups, respectively. Three cardiovascular deaths occurred during the double-blind period of the RA studies including all rituximab regimens (3/769 = 0.4%) as compared to none in the placebo treatment group (0/389). In the experience with Rituxan in 2578 RA patients, the rate of serious cardiac reactions was 1.93 per 100 patient years. The rate of myocardial infarction (MI) was 0.56 per 100 patient years (28 events in 26 patients), which is consistent with MI rates in the general RA population. These rates did not increase over three courses of Rituxan. Since patients with RA are at increased risk for cardiovascular events compared with the general population, patients with RA should be monitored throughout the infusion and Rituxan should be discontinued in the event of a serious or life-threatening cardiac event.
Hypophosphatemia and hyperuricemia
In the pooled, placebo-controlled studies, newly-occurring hypophosphatemia ( < 2.0 mg/dl) was observed in 12% (67/540) of patients on Rituxan versus 10% (39/398) of patients on placebo. Hypophosphatemia was more common in patients who received corticosteroids. Newly-occurring hyperuricemia (>10 mg/dl) was observed in 1.5% (8/540) of patients on Rituxan versus 0.3% (1/398) of patients on placebo. In the experience with Rituxan in RA patients, newly-occurring hypophosphatemia was observed in 21% (528/2570) of patients and newly-occurring hyperuricemia was observed in 2% (56/2570) of patients. The majority of the observed hypophosphatemia occurred at the time of the infusions and was transient.
Retreatment in Patients with RA
In the experience with Rituxan in RA patients, 2578 patients have been exposed to Rituxan and have received up to 10 courses of Rituxan in RA clinical trials, with 1890, 1043, and 425 patients having received at least two, three, and four courses, respectively. Most of the patients who received additional courses did so 24 weeks or more after the previous course and none were retreated sooner than 16 weeks. The rates and types of adverse reactions reported for subsequent courses of Rituxan were similar to rates and types seen for a single course of Rituxan. In RA Study 2, where all patients initially received Rituxan, the safety profile of patients who were retreated with Rituxan was similar to those who were retreated with placebo
Clinical Trials Experience in Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The data presented below reflect the experience in 197 patients with GPA and MPA treated with Rituxan or cyclophosphamide in a single controlled study, which was conducted in two phases: a 6 month randomized, double-blind, double-dummy, active-controlled remission induction phase and an additional 12 month remission maintenance phase. In the 6-month remission induction phase, 197 patients with GPA and MPA were randomized to either Rituxan 375 mg/ m2 once weekly for 4 weeks plus glucocorticoids, or oral cyclophosphamide 2 mg/kg daily (adjusted for renal function, white blood cell count, and other factors) plus glucocorticoids to induce remission. Once remission was achieved or at the end of the 6 month remission induction period, the cyclophosphamide group received azathioprine to maintain remission. The Rituxan group did not receive additional therapy to maintain remission. The primary analysis was at the end of the 6 month remission induction period and the safety results for this period are described below. Adverse reactions presented below in Table 3 were adverse events which occurred at a rate of greater than or equal to 10% in the Rituxan group. This table reflects experience in 99 GPA and MPA patients treated with Rituxan, with a total of 47.6 patient-years of observation and 98 GPA and MPA patients treated with cyclophosphamide, with a total of 47.0 patient-years of observation. Infection was the most common category of adverse events reported (47-62%) and is discussed below.

Infusion Reactions
Infusion-related reactions in the active-controlled, double-blind study were defined as any adverse event occurring within 24 hours of an infusion and considered to be infusion-related by investigators. Among the 99 patients treated with Rituxan, 12% experienced at least one infusion related reaction, compared with 11% of the 98 patients in the cyclophosphamide group. Infusion-related reactions included cytokine release syndrome, flushing, throat irritation, and tremor. In the Rituxan group, the proportion of patients experiencing an infusion related reaction was 12%, 5%, 4%, and 1% following the first, second, third, and fourth infusions, respectively. Patients were pre-medicated with antihistamine and acetaminophen before each Rituxan infusion and were on background oral corticosteroids which may have mitigated or masked an infusion reaction; however, there is insufficient evidence to determine whether premedication diminishes the frequency or severity of infusion reactions.
Infections
In the active-controlled, double-blind study, 62% (61/99) of patients in the Rituxan group experienced an infection of any type compared to 47% (46/98) patients in the cyclophosphamide group by Month 6. The most common infections in the Rituxan group were upper respiratory tract infections, urinary tract infections, and herpes zoster. The incidence of serious infections was 11% in the Rituxan-treated patients and 10% in the cyclophosphamide treated patients, with rates of approximately 25 and 28 per 100 patient-years, respectively. The most common serious infection was pneumonia.
Hypogammaglobulinemia (IgA, IgG or IgM below the lower limit of normal) has been observed in patients with GPA and MPA treated with Rituxan. At 6 months, in the Rituxan group, 27%, 58% and 51% of patients with normal immunoglobulin levels at baseline, had low IgA, IgG and IgM levels, respectively compared to 25%, 50% and 46% in the cyclophosphamide group. Retreatment in Patients with GPA and MPA In the active-controlled, double-blind study, subsequent courses of Rituxan were allowed for patients experiencing a relapse of disease. The limited data preclude any conclusions regarding the safety of subsequent courses of Rituxan with GPA and MPA [See Dosage and Administration (2.6), and Warnings and Precautions (5.14)].
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Rituxan with the incidence of antibodies to other products may be misleading. Using an ELISA assay, anti-human anti-chimeric antibody (HACA) was detected in 4 of 356 (1.1%) patients with low-grade or follicular NHL receiving single-agent Rituxan. Three of the four patients had an objective clinical response. A total of 273/2578 (11%) patients with RA tested positive for HACA at any time after receiving Rituxan. HACA positivity was not associated with increased infusion reactions or other adverse reactions. Upon further treatment, the proportions of patients with infusion reactions were similar between HACA positive and negative patients, and most reactions were mild to moderate. Four HACA positive patients had serious infusion reactions, and the temporal relationship between HACA positivity and infusion reaction was variable. A total of 23/99 (23%) Rituxan-treated patients with GPA and MPA tested positive for HACA by 18 months. The clinical relevance of HACA formation in Rituxan-treated patients is unclear.
Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to Rituxan.
- Hematologic: prolonged pancytopenia, marrow hypoplasia, Grade 3-4 prolonged or late-onset neutropenia, hyperviscosity syndrome in Waldenstrom's macroglobulinemia, prolonged hypogammaglobulinemia
- Cardiac: fatal cardiac failure.
- Immune/Autoimmune Events: uveitis, “[optic neuritis]], systemic vasculitis, pleuritis, lupus-like syndrome, serum sickness, polyarticular arthritis, and vasculitis with rash.
- Infection: viral infections, including progressive multifocal leukoencephalopathy (PML), increase in fatal infections in HIV-associated lymphoma, and a reported increased incidence of Grade 3 and 4 infections
- Neoplasia: disease progression of Kaposi's sarcoma.
- Skin: severe mucocutaneous reactions.
- Gastrointestinal: bowel obstruction and perforation.
- Pulmonary: fatal bronchiolitis obliterans and fatal interstitial lung disease.
- Nervous system: Posterior Reversible Encephalopathy Syndrome (PRES) /Reversible Posterior Leukoencephalopathy Syndrome (RPLS).
Drug Interactions
Formal drug interaction studies have not been performed with Rituxan. In patients with CLL, Rituxan did not alter systemic exposure to fludarabine or cyclophosphamide. In clinical trials of patients with RA, concomitant administration of methotrexate or cyclophosphamide did not alter the pharmacokinetics of rituximab.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Category C: There are no adequate and well-controlled studies of rituximab in pregnant women. Postmarketing data indicate that B-cell lymphocytopenia generally lasting less than six months can occur in infants exposed to rituximab in-utero. Rituximab was detected postnatally in the serum of infants exposed in-utero.
Non-Hodgkin's lymphoma, moderate-severe rheumatoid arthritis, Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis are serious conditions that require treatment. Rituximab should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Reproduction studies in cynomolgus monkeys at maternal exposures similar to human therapeutic exposures showed no evidence of teratogenic effects. However, B-cell lymphoid tissue was reduced in the offspring of treated dams. The B-cell counts returned to normal levels, and immunologic function was restored within 6 months of birth
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rituximab sandbox in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rituximab sandbox during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Rituximab sandbox in women who are nursing.
Pediatric Use
FDA has not required pediatric studies in polyarticular juvenile idiopathic arthritis (PJIA) patients ages 0 to 16 due to concerns regarding the potential for prolonged immunosuppression as a result of B-cell depletion in the developing juvenile immune system. Hypogammaglobulinemia has been observed in pediatric patients treated with Rituxan. The safety and effectiveness of Rituxan in pediatric patients have not been established.
Geriatic Use
Diffuse Large B-Cell NHL Among patients with DLBCL evaluated in three randomized, active-controlled trials, 927 patients received Rituxan in combination with chemotherapy. Of these, 396 (43%) were age 65 or greater and 123 (13%) were age 75 or greater. No overall differences in effectiveness were observed between these patients and younger patients. Cardiac adverse reactions, mostly supraventricular arrhythmias, occurred more frequently among elderly patients. Serious pulmonary adverse reactions were also more common among the elderly, including pneumonia and pneumonitis. Low-Grade or Follicular Non-Hodgkin's Lymphoma Patients with previously untreated follicular NHL evaluated in Study 5 were randomized to Rituxan as single-agent maintenance therapy (n=505) or observation (n=513) after achieving a response to Rituxan in combination with chemotherapy. Of these, 123 (24%) patients in the Rituxan arm were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients. Other clinical studies of Rituxan in low-grade or follicular, CD20-positive, B-cell NHL did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects. Chronic Lymphocytic Leukemia Among patients with CLL evaluated in two randomized active-controlled trials, 243 of 676 Rituxan-treated patients (36%) were 65 years of age or older; of these, 100 Rituxan-treated patients (15%) were 70 years of age or older. In exploratory analyses defined by age, there was no observed benefit from the addition of Rituxan to fludarabine and cyclophosphamide among patients 70 years of age or older in Study 11 or in Study 12; there was also no observed benefit from the addition of Rituxan to fludarabine and cyclophosphamide among patients 65 years of age or older in Study 12 [See Clinical Studies (14.5)]. Patients 70 years or older received lower dose intensity of fludarabine and cyclophosphamide compared to younger patients, regardless of the addition of Rituxan. In Study 11, the dose intensity of Rituxan was similar in older and younger patients, however in Study 12 older patients received a lower dose intensity of Rituxan. The incidence of Grade 3 and 4 adverse reactions was higher among patients receiving R-FC who were 70 years or older compared to younger patients for neutropenia [44% vs. 31% (Study 11); 56% vs. 39% (Study 12)], febrile neutropenia [16% vs. 6% (Study 10)], anemia [5% vs. 2% (Study 11); 21% vs. 10% (Study 12)], thrombocytopenia [19% vs. 8% (Study 12)], pancytopenia [7% vs. 2% (Study 11); 7% vs. 2% (Study 12)] and infections [30% vs. 14% (Study 12)]. Rheumatoid Arthritis Among the 2578 patients in global RA studies completed to date, 12% were 65–75 years old and 2% were 75 years old and older. The incidences of adverse reactions were similar between older and younger patients. The rates of serious adverse reactions, including serious infections, malignancies, and cardiovascular events were higher in older patients. Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis Of the 99 Rituxan-treated GPA and MPA patients, 36 (36%) were 65 years old and over, while 8 (8%) were 75 years and over. No overall differences in efficacy were observed between patients that were 65 years old and over and younger patients. The overall incidence and rate of all serious adverse events was higher in patients 65 years old and over. The clinical study did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Rituximab sandbox with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rituximab sandbox with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rituximab sandbox in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rituximab sandbox in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rituximab sandbox in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rituximab sandbox in patients who are immunocompromised.
Administration and Monitoring
Administration
Administer only as an Intravenous Infusion Do not administer as an intravenous push or bolus. Premedicate before each infusion Rituxan should only be administered by a healthcare professional with appropriate medical support to manage severe infusion reactions that can be fatal if they occur [see Warnings and Precautions (5.1)].
- First Infusion: Initiate infusion at a rate of 50 mg/hr. In the absence of infusion toxicity, increase infusion rate by 50 mg/hr increments every 30 minutes, to a maximum of 400 mg/hr.
- Subsequent Infusions:
- Standard Infusion: Initiate infusion at a rate of 100 mg/hr. In the absence of infusion toxicity, increase rate by 100 mg/hr increments at 30-minute intervals, to a maximum of 400 mg/hr.
- For previously untreated follicular NHL and DLBCL patients:
- If patients did not experience a Grade 3 or 4 infusion related adverse event during Cycle 1, a 90-minute infusion can be administered in Cycle 2 with a glucocorticoid-containing chemotherapy regimen.
- Initiate at a rate of 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes. If the 90-minute infusion is tolerated in Cycle 2, the same rate can be used
- when administering the remainder of the treatment regimen (through Cycle 6 or 8).
- Patients who have clinically significant cardiovascular disease or who have a circulating lymphocyte count ≥5000/mm3 before Cycle 2 should not be administered the 90-minute infusion [see Clinical Studies (14.4)].
- Interrupt the infusion or slow the infusion rate for infusion reactions [see Boxed Warning, Warnings and Precautions (5.1)]. Continue the infusion at one-half the previous rate upon improvement of symptoms.
Monitoring
FDA Package Insert for Rituximab contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
There has been no experience with overdosage in human clinical trials. Single doses of up to 500 mg/m2 have been administered in clinical trials.
Pharmacology
Mechanism of Action
Rituximab binds specifically to the antigen CD20 (human B-lymphocyte-restricted differentiation antigen, Bp35), a hydrophobic transmembrane protein with a molecular weight of approximately 35 kD located on pre-B and mature B lymphocytes. The antigen is expressed on > 90% of B-cell non-Hodgkin's lymphomas (NHL), but the antigen is not found on hematopoietic stem cells, pro-B-cells, normal plasma cells or other normal tissues. CD20 regulates an early step(s) in the activation process for cell cycle initiation and differentiation, and possibly functions as a calcium ion channel. CD20 is not shed from the cell surface and does not internalize upon antibody binding. Free CD20 antigen is not found in the circulation. B cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis. In this setting, B cells may be acting at multiple sites in the autoimmune/inflammatory process, including through production of rheumatoid factor (RF) and other autoantibodies, antigen presentation, T-cell activation, and/or proinflammatory cytokine production. Mechanism of Action: The Fab domain of rituximab binds to the CD20 antigen on B lymphocytes, and the Fc domain recruits immune effector functions to mediate B-cell lysis in vitro. Possible mechanisms of cell lysis include complement-dependent cytotoxicity (CDC) and antibody-dependent cell mediated cytotoxicity (ADCC). The antibody has been shown to induce apoptosis in the DHL-4 human B-cell lymphoma line. Normal Tissue Cross-reactivity: Rituximab binding was observed on lymphoid cells in the thymus, the white pulp of the spleen, and a majority of B lymphocytes in peripheral blood and lymph nodes. Little or no binding was observed in the non-lymphoid tissues examined.
Structure
Rituxan® (rituximab) is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against the CD20 antigen. Rituximab has an approximate molecular weight of 145 kD. Rituximab has a binding affinity for the CD20 antigen of approximately 8.0 nM. Rituximab is produced by mammalian cell (Chinese Hamster Ovary) suspension culture in a nutrient medium containing the antibiotic gentamicin. Gentamicin is not detectable in the final product. Rituxan is a sterile, clear, colorless, preservative-free liquid concentrate for intravenous administration. Rituxan is supplied at a concentration of 10 mg/mL in either 100 mg/10 mL or 500 mg/50 mL single-use vials. The product is formulated in polysorbate 80 (0.7 mg/mL), sodium citrate dihydrate (7.35 mg/mL), sodium chloride (9 mg/mL) and Water for Injection. The pH is 6.5.
Pharmacodynamics
In NHL patients, administration of Rituxan resulted in depletion of circulating and tissue-based B cells. Among 166 patients in Study 1, circulating CD19-positive B cells were depleted within the first three weeks with sustained depletion for up to 6 to 9 months post treatment in 83% of patients. B-cell recovery began at approximately 6 months and median B-cell levels returned to normal by 12 months following completion of treatment. There were sustained and statistically significant reductions in both IgM and IgG serum levels observed from 5 through 11 months following rituximab administration; 14% of patients had IgM and/or IgG serum levels below the normal range.
In RA patients, treatment with Rituxan induced depletion of peripheral B lymphocytes, with the majority of patients demonstrating near complete depletion (CD19 counts below the lower limit of quantification, 20 cells/µl) within 2 weeks after receiving the first dose of Rituxan. The majority of patients showed peripheral B-cell depletion for at least 6 months. A small proportion of patients (~4%) had prolonged peripheral B-cell depletion lasting more than 3 years after a single course of treatment. Total serum immunoglobulin levels, IgM, IgG, and IgA were reduced at 6 months with the greatest change observed in IgM. At Week 24 of the first course of Rituxan treatment, small proportions of patients experienced decreases in IgM (10%), IgG (2.8%), and IgA (0.8%) levels below the lower limit of normal (LLN). In the experience with Rituxan in RA patients during repeated Rituxan treatment, 23.3%, 5.5%, and 0.5% of patients experienced decreases in IgM, IgG, and IgA concentrations below LLN at any time after receiving Rituxan, respectively. The clinical consequences of decreases in immunoglobulin levels in RA patients treated with Rituxan are unclear. Treatment with rituximab in patients with RA was associated with reduction of certain biologic markers of inflammation such as interleukin-6 (IL-6), C-reactive protein (CRP), serum amyloid protein (SAA), S100 A8/S100 A9 heterodimer complex (S100 A8/9), anti-citrullinated peptide (anti-CCP), and RF.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis
In GPA and MPA patients, peripheral blood CD19 B-cells depleted to less than 10 cells/µl following the first two infusions of Rituxan, and remained at that level in most (84%) patients through Month 6. By Month 12, the majority of patients (81%) showed signs of B-cell return with counts >10 cells/μL. By Month 18, most patients (87%) had counts >10 cells/μL.
Pharmacokinetics
Pharmacokinetics were characterized in 203 NHL patients receiving 375 mg/m2 Rituxan weekly by intravenous infusion for 4 doses. Rituximab was detectable in the serum of patients 3 to 6 months after completion of treatment. The pharmacokinetic profile of rituximab when administered as 6 infusions of 375 mg/m2 in combination with 6 cycles of CHOP chemotherapy was similar to that seen with rituximab alone. Based on a population pharmacokinetic analysis of data from 298 NHL patients who received rituximab once weekly or once every three weeks, the estimated median terminal elimination half-life was 22 days (range, 6.1 to 52 days). Patients with higher CD19-positive cell counts or larger measurable tumor lesions at pretreatment had a higher clearance. However, dose adjustment for pretreatment CD19 count or size of tumor lesion is not necessary. Age and gender had no effect on the pharmacokinetics of rituximab. Pharmacokinetics were characterized in 21 patients with CLL receiving rituximab according to the recommended dose and schedule. The estimated median terminal half-life of rituximab was 32 days (range, 14 to 62 days).
Following administration of 2 doses of Rituxan in patients with RA, the mean ( ± S.D.; % CV) concentrations after the first infusion (Cmax first) and second infusion (Cmax second) were 157 ( ± 46; 29%) and 183 ( ± 55; 30%) mcg/mL, and 318 ( ± 86; 27%) and 381 ( ± 98; 26%) mcg/mL for the 2 × 500 mg and 2 × 1000 mg doses, respectively. Based on a population pharmacokinetic analysis of data from 2005 RA patients who received Rituxan, the estimated clearance of rituximab was 0.335 L/day; volume of distribution was 3.1 L and mean terminal elimination half-life was 18.0 days (range, 5.17 to 77.5 days). Age, weight and gender had no effect on the pharmacokinetics of rituximab in RA patients.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis
Based on the population pharmacokinetic analysis of data in 97 GPA and MPA patients who received 375 mg/m2 rituximab once weekly by intravenous infusion for four weeks, the estimated median terminal elimination half-life was 23 days (range, 9 to 49 days). Rituximab mean clearance and volume of distribution were 0. 312 L/day (range, 0.115 to 0.728 L/day) and 4.50 L (range, 2.21 to 7.52 L) respectively. Male patients and patients with higher BSA or positive HACA levels have higher clearance. However, further dose adjustment based on gender or HACA status is not necessary. The pharmacokinetics of rituximab have not been studied in children and adolescents. No formal studies were conducted to examine the effects of either renal or hepatic impairment on the pharmacokinetics of rituximab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to establish the carcinogenic or mutagenic potential of Rituxan or to determine potential effects on fertility in males or females.
Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
An embryo-fetal developmental toxicity study was performed on pregnant cynomolgus monkeys. Pregnant animals received rituximab via the intravenous route during early gestation (organogenesis period; post-coitum days 20 through 50). Rituximab was administered as loading doses on post-coitum (PC) days 20, 21 and 22, at 15, 37.5 or 75 mg/kg/day, and then weekly on PC Days 29, 36, 43 and 50, at 20, 50 or 100 mg/kg/week. The 100 mg/kg/week dose resulted in 80% of the exposure (based on AUC) of those achieved following a dose of 2 grams in humans. Rituximab crosses the monkey placenta. Exposed offspring did not exhibit any teratogenic effects but did have decreased lymphoid tissue B cells. A subsequent pre- and postnatal reproductive toxicity study in cynomolgus monkeys was completed to assess developmental effects including the recovery of B cells and immune function in infants exposed to rituximab in utero. Animals were treated with a loading dose of 0, 15, or 75 mg/kg every day for 3 days, followed by weekly dosing with 0, 20, or 100 mg/kg dose. Subsets of pregnant females were treated from PC Day 20 through postpartum Day 78, PC Day 76 through PC Day 134, and from PC Day 132 through delivery and postpartum Day 28. Regardless of the timing of treatment, decreased B cells and immunosuppression were noted in the offspring of rituximab-treated pregnant animals. The B-cell counts returned to normal levels, and immunologic function was restored within 6 months postpartum.
Clinical Studies
Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of Rituxan in relapsed, refractory CD20+ NHL were demonstrated in 3 single-arm studies enrolling 296 patients.
Study 1
A multicenter, open-label, single-arm study was conducted in 166 patients with relapsed or refractory, low-grade or follicular, B-cell NHL who received 375 mg/m2 of Rituxan given as an intravenous infusion weekly for 4 doses. Patients with tumor masses > 10 cm or with > 5000 lymphocytes/µL in the peripheral blood were excluded from the study. Results are summarized in Table 4. The median time to onset of response was 50 days. Disease-related signs and symptoms (including B-symptoms) resolved in 64% (25/39) of those patients with such symptoms at study entry.
Study 2
In a multicenter, single-arm study, 37 patients with relapsed or refractory, low-grade NHL received 375 mg/m2 of Rituxan weekly for 8 doses. Results are summarized in Table 4.
Study 3
In a multicenter, single-arm study, 60 patients received 375 mg/m2 of Rituxan weekly for 4 doses. All patients had relapsed or refractory, low-grade or follicular, B-cell NHL and had achieved an objective clinical response to Rituxan administered 3.8–35.6 months (median 14.5 months) prior to retreatment with Rituxan. Of these 60 patients, 5 received more than one additional course of Rituxan. Results are summarized in Table 4.
In pooled data from studies 1 and 3, 39 patients with bulky (single lesion > 10 cm in diameter) and relapsed or refractory, low-grade NHL received Rituxan 375 mg/m2 weekly for 4 doses. Results are summarized in Table 4.

Previously Untreated, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of Rituxan in previously untreated, low-grade or follicular, CD20+ NHL were demonstrated in 3 randomized, controlled trials enrolling 1,662 patients.
Study 4
A total of 322 patients with previously untreated follicular NHL were randomized (1:1) to receive up to eight 3-week cycles of CVP chemotherapy alone (CVP) or in combination with Rituxan 375 mg/m2 on Day 1 of each cycle (R-CVP) in an open-label, multicenter study. The main outcome measure of the study was progression-free survival (PFS) defined as the time from randomization to the first of progression, relapse, or death. Twenty-six percent of the study population was >60 years of age, 99% had Stage III or IV disease, and 50% had an International Prognostic Index (IPI) score ≥2. The results for PFS as determined by a blinded, independent assessment of progression are presented in Table 5. The point estimates may be influenced by the presence of informative censoring. The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.

Study 5
An open-label, multicenter, randomized (1:1) study was conducted in 1,018 patients with previously untreated follicular NHL who achieved a response (CR or PR) to Rituxan in combination with chemotherapy. Patients were randomized to Rituxan as single-agent maintenance therapy, 375 mg/m2 every 8 weeks for up to 12 doses or to observation. Rituxan was initiated at 8 weeks following completion of chemotherapy. The main outcome measure of the study was progression-free survival (PFS), defined as the time from randomization in the maintenance/observation phase to progression, relapse, or death, as determined by independent review. Of the randomized patients, 40% were ≥ 60 years of age, 70% had Stage IV disease, 96% had ECOG performance status (PS) 0–1, and 42% had FLIPI scores of 3–5. Prior to randomization to maintenance therapy, patients had received R-CHOP (75%), R-CVP (22%), or R-FCM (3%); 71% had a complete or unconfirmed complete response and 28% had a partial response. PFS was longer in patients randomized to Rituxan as single agent maintenance therapy (HR: 0.54, 95% CI: 0.42, 0.70). The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.

Study 6
A total of 322 patients with previously untreated low-grade, B-cell NHL who did not progress after 6 or 8 cycles of CVP chemotherapy were enrolled in an open-label, multicenter, randomized trial. Patients were randomized (1:1) to receive Rituxan, 375 mg/m2 intravenous infusion, once weekly for 4 doses every 6 months for up to 16 doses or no further therapeutic intervention. The main outcome measure of the study was progression-free survival defined as the time from randomization to progression, relapse, or death. Thirty-seven percent of the study population was >60 years of age, 99% had Stage III or IV disease, and 63% had an IPI score ≥2. There was a reduction in the risk of progression, relapse, or death (hazard ratio estimate in the range of 0.36 to 0.49) for patients randomized to Rituxan as compared to those who received no additional treatment.
Diffuse Large B-Cell NHL (DLBCL)
The safety and effectiveness of Rituxan were evaluated in three randomized, active-controlled, open-label, multicenter studies with a collective enrollment of 1854 patients. Patients with previously untreated diffuse large B-cell NHL received Rituxan in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
Study 7
A total of 632 patients age ≥ 60 years with DLBCL (including primary mediastinal B-cell lymphoma) were randomized in a 1:1 ratio to treatment with CHOP or R-CHOP. Patients received 6 or 8 cycles of CHOP, each cycle lasting 21 days. All patients in the R-CHOP arm received 4 doses of Rituxan 375 mg/m2 on Days –7 and –3 (prior to Cycle 1) and 48–72 hours prior to Cycles 3 and 5. Patients who received 8 cycles of CHOP also received Rituxan prior to Cycle 7. The main outcome measure of the study was progression-free survival, defined as the time from randomization to the first of progression, relapse, or death. Responding patients underwent a second randomization to receive Rituxan or no further therapy. Among all enrolled patients, 62% had centrally confirmed DLBCL histology, 73% had Stage III–IV disease, 56% had IPI scores ≥ 2, 86% had ECOG performance status of < 2, 57% had elevated LDH levels, and 30% had two or more extranodal disease sites involved. Efficacy results are presented in Table 6. These results reflect a statistical approach which allows for an evaluation of Rituxan administered in the induction setting that excludes any potential impact of Rituxan given after the second randomization. Analysis of results after the second randomization in Study 7 demonstrates that for patients randomized to R-CHOP, additional Rituxan exposure beyond induction was not associated with further improvements in progression-free survival or overall survival.
Study 8
A total of 399 patients with DLBCL, age ≥ 60 years, were randomized in a 1:1 ratio to receive CHOP or R-CHOP. All patients received up to eight 3-week cycles of CHOP induction; patients in the R-CHOP arm received Rituxan 375 mg/m2 on Day 1 of each cycle. The main outcome measure of the study was event-free survival, defined as the time from randomization to relapse, progression, change in therapy, or death from any cause. Among all enrolled patients, 80% had Stage III or IV disease, 60% of patients had an age-adjusted IPI ≥ 2, 80% had ECOG performance status scores < 2, 66% had elevated LDH levels, and 52% had extranodal involvement in at least two sites. Efficacy results are presented in Table 6.
Study 9
A total of 823 patients with DLBCL, aged 18–60 years, were randomized in a 1:1 ratio to receive an anthracycline-containing chemotherapy regimen alone or in combination with Rituxan. The main outcome measure of the study was time to treatment failure, defined as time from randomization to the earliest of progressive disease, failure to achieve a complete response, relapse, or death. Among all enrolled patients, 28% had Stage III–IV disease, 100% had IPI scores of ≤ 1, 99% had ECOG performance status of < 2, 29% had elevated LDH levels, 49% had bulky disease, and 34% had extranodal involvement. Efficacy results are presented in Table 6.
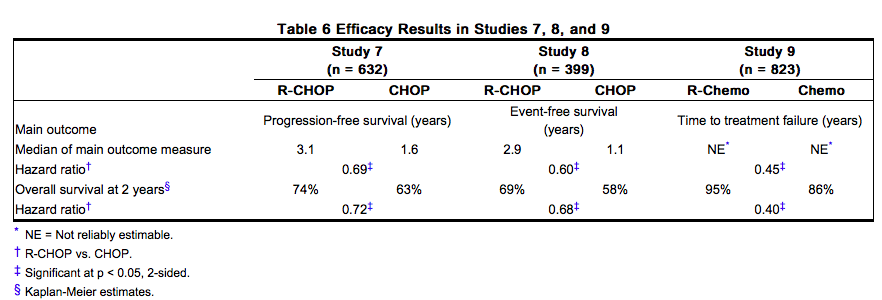
In Study 8, overall survival estimates at 5 years were 58% vs. 46% for R-CHOP and CHOP, respectively.
Ninety-Minute Infusions in Previously Untreated Follicular NHL and DLBCL
In Study 10, a total of 363 patients with previously untreated follicular NHL (n=113) or DLBCL (n=250) were evaluated in a prospective, open-label, multi-center, single-arm trial for the safety of 90-minute rituximab infusions. Patients with follicular NHL received rituximab 375 mg/m2 plus CVP chemotherapy. Patients with DLBCL received rituximab 375 mg/m2 plus CHOP chemotherapy. Patients with clinically significant cardiovascular disease were excluded from the study. Patients were eligible for a 90-minute infusion at Cycle 2 if they did not experience a Grade 3-4 infusion-related adverse event with Cycle 1 and had a circulating lymphocyte count ≤ 5000/mm3 before Cycle 2. All patients were pre-medicated with acetaminophen and an antihistamine and received the glucocorticoid component of their chemotherapy prior to Rituxan infusion. The main outcome measure was the development of Grade 3-4 infusion-related reactions on the day of, or day after, the 90-minute infusion at Cycle 2 [See Adverse Reactions (6.1)]. Eligible patients received their Cycle 2 rituximab infusion over 90 minutes as follows: 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes [See Dosage and Administration (2.1)]. Patients who tolerated the 90-minute rituximab infusion at Cycle 2 continued to receive subsequent rituximab infusions at the 90-minute infusion rate for the remainder of the treatment regimen (through Cycle 6 or Cycle 8). The incidence of Grade 3-4 infusion-related reactions at Cycle 2 was 1.1% (95% CI [0.3%, 2.8%]) among all patients, 3.5% (95% CI [1.0%, 8.8%]) for those patients treated with R-CVP, and 0.0% (95% CI [0.0%, 1.5%]) for those patients treated with R-CHOP. For Cycles 2-8, the incidence of Grade 3-4 infusion-related reactions was 2.8% (95% CI [1.3%, 5.0%]). No acute fatal infusion related reactions were observed.
Chronic Lymphocytic Leukemia (CLL)
The safety and effectiveness of Rituxan were evaluated in two randomized (1:1) multicenter open-label studies comparing FC alone or in combination with Rituxan for up to 6 cycles in patients with previously untreated CLL [Study 11 (n=817)] or previously treated CLL [Study 12 (n=552)]. Patients received fludarabine 25 mg/m2/day and cyclophosphamide 250 mg/m2/day on days 1, 2 and 3 of each cycle, with or without Rituxan. In both studies, seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of Rituxan-based therapy. In Study 11, 30% of patients were 65 years or older, 31% were Binet stage C, 45% had B symptoms, more than 99% had ECOG performance status (PS) 0–1, 74% were male, and 100% were White. In Study 12, 44% of patients were 65 years or older, 28% had B symptoms, 82% received a prior alkylating drug, 18% received prior fludarabine, 100% had ECOG PS 0–1, 67% were male and 98% were White. The main outcome measure in both studies was progression-free survival (PFS), defined as the time from randomization to progression, relapse, or death, as determined by investigators (Study 11) or an independent review committee (Study 12). The investigator assessed results in Study 12 were supportive of those obtained by the independent review committee. Efficacy results are presented in Table 7.
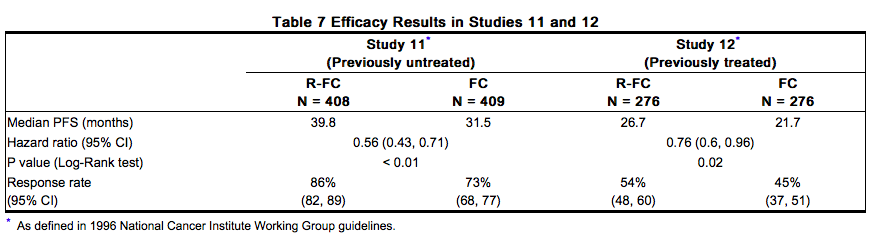
Across both studies, 243 of 676 Rituxan-treated patients (36%) were 65 years of age or older and 100 Rituxan-treated patients (15%) were 70 years of age or older. The results of exploratory subset analyses in elderly patients are presented in Table 8.

Rheumatoid Arthritis (RA)
Reducing the Signs and Symptoms: Initial and Re-Treatment Courses The efficacy and safety of Rituxan were evaluated in two randomized, double-blind, placebo-controlled studies of adult patients with moderately to severely active RA who had a prior inadequate response to at least one TNF inhibitor. Patients were 18 years of age or older, diagnosed with active RA according to American College of Rheumatology (ACR) criteria, and had at least 8 swollen and 8 tender joints. In RA Study 1, patients were randomized to receive either Rituxan 2 × 1000 mg + MTX or placebo + MTX for 24 weeks. Further courses of Rituxan 2 × 1000 mg + MTX were administered in an open label extension study at a frequency determined by clinical evaluation, but no sooner than 16 weeks after the preceding course of Rituxan. In addition to the intravenous premedication, glucocorticoids were administered orally on a tapering schedule from baseline through Day 14. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24 of the placebo-controlled period are shown in Table 9. In RA Study 2, all patients received the first course of Rituxan 2 × 1000 mg + MTX. Patients who experienced ongoing disease activity were randomized to receive a second course of either Rituxan 2 × 1000 mg + MTX or placebo + MTX, the majority between Weeks 24–28. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24, before the re-treatment course, and at Week 48, after retreatment, are shown in Table 9.

Improvement was also noted for all components of ACR response following treatment with Rituxan, as shown in Table 10.

The time course of ACR 20 response for Study 1 is shown in Figure 2. Although both treatment groups received a brief course of intravenous and oral glucocorticoids, resulting in similar benefits at Week 4, higher ACR 20 responses were observed for the Rituxan group by Week 8. A similar proportion of patients achieved these responses through Week 24 after a single course of treatment (2 infusions) with Rituxan. Similar patterns were demonstrated for ACR 50 and 70 responses.

Radiographic Response
In RA Study 1, structural joint damage was assessed radiographically and expressed as changes in Genant-modified Total Sharp Score (TSS) and its components, the erosion score (ES) and the joint space narrowing (JSN) score. Rituxan + MTX slowed the progression of structural damage compared to placebo + MTX after 1 year as shown in Table 11.

In RA Study 1 and its open-label extension, 70% of patients initially randomized to Rituxan + MTX and 72% of patients initially randomized to placebo + MTX were evaluated radiographically at Year 2. As shown in Table 11, progression of structural damage in Rituxan + MTX patients was further reduced in the second year of treatment. Following 2 years of treatment with Rituxan + MTX, 57% of patients had no progression of structural damage. During the first year, 60% of Rituxan + MTX treated patients had no progression, defined as a change in TSS of zero or less compared to baseline, compared to 46% of placebo + MTX treated patients. In their second year of treatment with Rituxan + MTX, more patients had no progression than in the first year (68% vs. 60%), and 87% of the Rituxan + MTX treated patients who had no progression in the first year also had no progression in the second year.
Lesser Efficacy of 500 Vs. 1000 mg Treatment Courses for Radiographic Outcomes
RA Study 3 is a randomized, double-blind, placebo-controlled study which evaluated the effect of placebo + MTX compared to Rituxan 2 × 500 mg + MTX and Rituxan 2 × 1000 mg + MTX treatment courses in MTX-naïve RA patients with moderately to severely active disease. Patients received a first course of two infusions of rituximab or placebo on Days 1 and 15. MTX was initiated at 7.5 mg/week and escalated up to 20 mg/week by Week 8 in all three treatment arms. After a minimum of 24 weeks, patients with ongoing disease activity were eligible to receive re-treatment with additional courses of their assigned treatment. After one year of treatment, the proportion of patients achieving ACR 20/50/70 responses were similar in both Rituxan dose groups and were higher than in the placebo group. However, with respect to radiographic scores, only the Rituxan 1000 mg treatment group demonstrated a statistically significant reduction in TSS: a change of 0.36 units compared to 1.08 units for the placebo group, a 67% reduction.
Physical Function Response
RA Study 4 is a randomized, double-blind, placebo-controlled study in adult RA patients with moderately to severely active disease with inadequate response to MTX. Patients were randomized to receive an initial course of Rituxan 500 mg, Rituxan 1000 mg, or placebo in addition to background MTX. Physical function was assessed at Weeks 24 and 48 using the Health Assessment Questionnaire Disability Index (HAQ-DI). From baseline to Week 24, a greater proportion of Rituxan-treated patients had an improvement in HAQ-DI of at least 0.22 (a minimal clinically important difference) and a greater mean HAQ-DI improvement compared to placebo, as shown in Table 12. HAQ-DI results for the Rituxan 500 mg treatment group were similar to the Rituxan 1000 mg treatment group; however radiographic responses were not assessed (see Dosing Precaution in the Radiographic Responses section above). These improvements were maintained at 48 weeks.

Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
A total of 197 patients with active, severe GPA and MPA (two forms of ANCA Associated Vasculidities) were treated in a randomized, double-blind, active-controlled multicenter, non-inferiority study, conducted in two phases – a 6 month remission induction phase and a 12 month remission maintenance phase. Patients were 15 years of age or older, diagnosed with GPA (75% of patients) or MPA (24% of patients) according to the Chapel Hill Consensus conference criteria (1% of the patients had unknown vasculitis type). All patients had active disease, with a Birmingham Vasculitis Activity Score for Granulomatosis with Polyangiitis (BVAS/GPA) ≥ 3, and their disease was severe, with at least one major item on the BVAS/GPA. Ninety-six (49%) of patients had new disease and 101 (51%) of patients had relapsing disease. Patients in both arms received 1000 mg of pulse intravenous methylprednisolone per day for 1 to 3 days within 14 days prior to initial infusion. Patients were randomized in a 1:1 ratio to receive either Rituxan 375 mg/m2 once weekly for 4 weeks or oral cyclophosphamide 2 mg/kg daily for 3 to 6 months in the remission induction phase. Patients were pre-medicated with antihistamine and acetaminophen prior to Rituxan infusion. Following intravenous corticosteroid administration, all patients received oral prednisone (1 mg/kg/day, not exceeding 80 mg/day) with pre-specified tapering. Once remission was achieved or at the end of the 6 month remission induction period, the cyclophosphamide group received azathioprine to maintain remission. The Rituxan group did not receive additional therapy to maintain remission. The main outcome measure for both GPA and MPA patients was achievement of complete remission at 6 months defined as a BVAS/GPA of 0, and off glucocorticoid therapy. The pre-specified non-inferiority margin was a treatment difference of 20%. As shown in Table 13, the study demonstrated non-inferiority of Rituxan to cyclophosphamide for complete remission at 6 months.
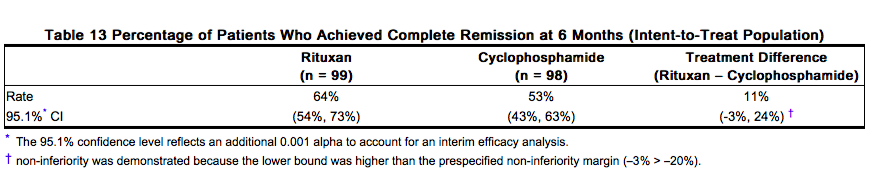
Complete Remission (CR) at 12 and 18 months
In the Rituxan group, 44% of patients achieved CR at 6 and 12 months, and 38% of patients achieved CR at 6, 12, and 18 months. In patients treated with cyclophosphamide (followed by azathioprine for maintenance of CR), 38% of patients achieved CR at 6 and 12 months, and 31% of patients achieved CR at 6, 12, and 18 months.
Retreatment with Rituxan
Based upon investigator judgment, 15 patients received a second course of Rituxan therapy for treatment of relapse of disease activity which occurred between 8 and 17 months after the first course of Rituxan. The limited data preclude any conclusions regarding the efficacy of subsequent courses of Rituxan in patients with GPA and MPA [see Warnings and Precautions (5.14)].
How Supplied
Rituxan vials [100 mg/10 mL single-use vials (NDC 50242-051-21) and 500 mg/50 mL single-use vials (NDC 50242-053-06)] are stable at 2°C–8°C (36°F–46°F). Do not use beyond expiration date stamped on carton. Rituxan vials should be protected from direct sunlight. Do not freeze or shake.
Storage
Rituxan solutions for infusion may be stored at 2°C–8°C (36°F–46°F) for 24 hours. Rituxan solutions for infusion have been shown to be stable for an additional 24 hours at room temperature. However, since Rituxan solutions do not contain a preservative, diluted solutions should be stored refrigerated (2°C–8°C). No incompatibilities between Rituxan and polyvinylchloride or polyethylene bags have been observed.
Images
Drug Images
{{#ask: Page Name::Rituximab sandbox |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rituximab sandbox |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be provided the Rituxan Medication Guide and provided an opportunity to read prior to each treatment session. It is important that the patient's overall health be assessed at each visit and the risks of Rituxan therapy and any questions resulting from the patient's reading of the Medication Guide be discussed. See FDA approved patient labeling (Medication Guide). Rituxan is detectable in serum for up to six months following completion of therapy. Individuals of childbearing potential should use effective contraception during treatment and for 12 months after Rituxan therapy.
Precautions with Alcohol
Alcohol-Rituximab sandbox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Rituximab sandbox Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Rituximab sandbox Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Rituximab sandbox |Label Name=Rituximab_label_01.jpg
}}
{{#subobject:
|Label Page=Rituximab sandbox |Label Name=Rituximab_label_02.jpg
}}
{{#subobject:
|Label Page=Rituximab sandbox |Label Name=Rituximab_panel_01.png
}}
{{#subobject:
|Label Page=Rituximab sandbox |Label Name=Rituximab_panel_02.png
}}