Radiation fibrosis
| Radiation fibrosis | |
 | |
|---|---|
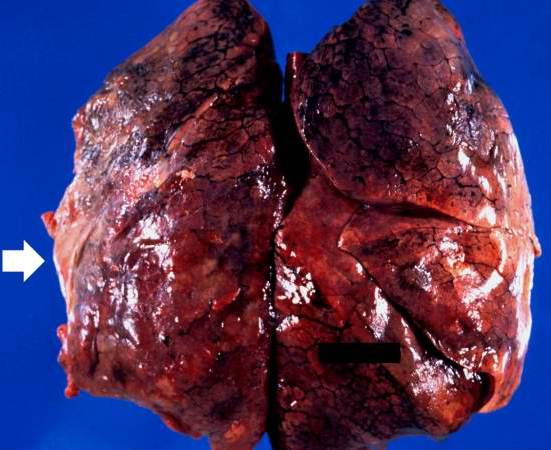
| A gross photograph of lung demonstrating areas of fibrosis on the pleural surface (arrow). Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Radiation fibrosis defined as the formation of scar tissue as a result of radiation therapy.
The lungs are extremely vascular; thus, the radiation changes seen in the vasculature can have a profound effect on the lungs. During the immediate post-irradiation period, endothelial cell swelling and vacuolization can be seen in the alveolar capillaries. The increased vascular permeability may lead to marked pulmonary congestion and edema and the other changes similar to those encountered in adult respiratory distress syndrome.
Long-term pulmonary consequences of radiation injury include fibrosis of the alveolar walls as well as the vascular changes (vessel narrowing). The respiratory dysfunction due to the combined alveolar wall fibrosis and thickening as well as the poor perfusion due to the vascular lesions can severely inhibit pulmonary function. This radiation pneumonitis creates a profound alveolocapillary block.
Vascular changes, which are dose/rate dependent, are prominent in all irradiated tissues.
Endothelial cells are not specifically radiosensitive but with high exposure vascular changes can occur. There is endothelial swelling and vacuolation or even death of the endothelial cells which can then lead to secondary thrombosis or hemorrhage.
At later time points, intimal hyperplasia and fibrosis occurs which results in thickening of the vessel wall and narrowing of the vessel lumen. These vascular changes can lead to poor blood flow to the tissues.
Radiation can lead to alterations in the mitotic process resulting in cells that have abnormal mitotic figures. These changes can lead to death of the cell. Subtle genetic injuries, such as DNA strand breaks, are responsible for translocations and deletions. These changes lead to the mutagenic, teratogenic, and carcinogenic potentials of ionizing radiation that become evident many years after the radiation exposure. During this long time interval, sequential mitotic divisions are occurring that will ultimately lead to these untoward consequences. This phenomenon is known as radiation "latency."
Radiation Fibrosis in Lungs: A Case Example
Clinical Summary
A 60-year-old white female had developed retraction of her left nipple six years earlier, at which time breast carcinoma was found. A radical mastectomy was performed.
Examination of the surgical specimens showed metastases in regional lymph nodes and local irradiation was thus administered. Two years later, carcinoma of the right breast was found. Following a modified mastectomy, more irradiation was given. A year later the patient developed recurrences for which chemotherapy (cytoxan and adriamycin) was given.
After a two year period without problems, the patient developed decreased exercise tolerance, dyspnea on exertion, shortness of breath, paroxysmal nocturnal dyspnea, and orthopnea increasing in severity over 10 days.
Chest examination revealed decreased breath sounds with dullness over the left base.
Chest x-ray showed a globose cardiac silhouette and left pleural effusion. A pericardiectomy was done because of suspected cardiac tamponade; however, the patient died soon after the operation.
Postmortem Findings
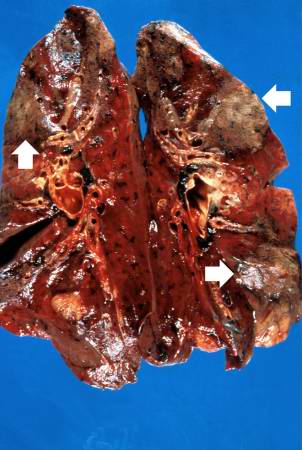
There was metastatic carcinoma in the pericardium, chest wall, diaphragm, both lungs, and mediastinal lymph nodes. Severe non obstructive cardiomyopathy, probably secondary to adriamycin, was found. Areas of pleural thickening with adhesions and interstitial fibrosis were found involving the anterior aspect of both lungs.
-
This is a gross photograph of cut sections of lung. There are several areas of fibrosis (arrows) within the lung parenchyma.
-
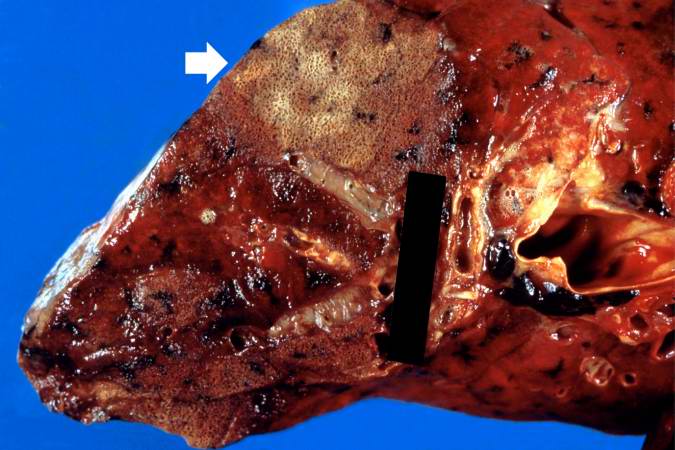
This is a gross photograph showing a closer view of a cut section of lung. An area of fibrosis (arrow) is evident in this photograph.
-
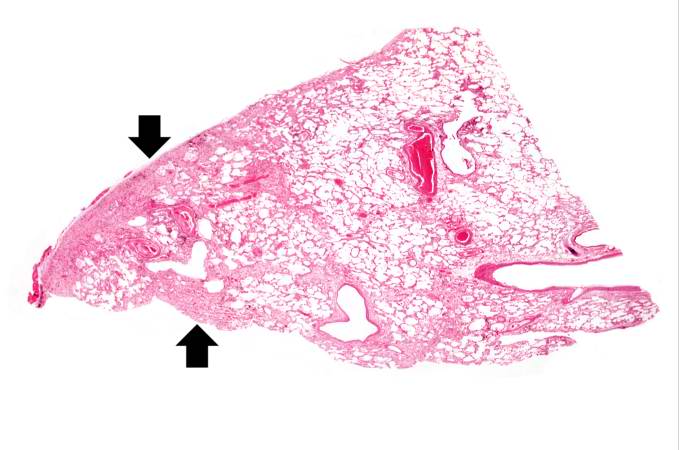
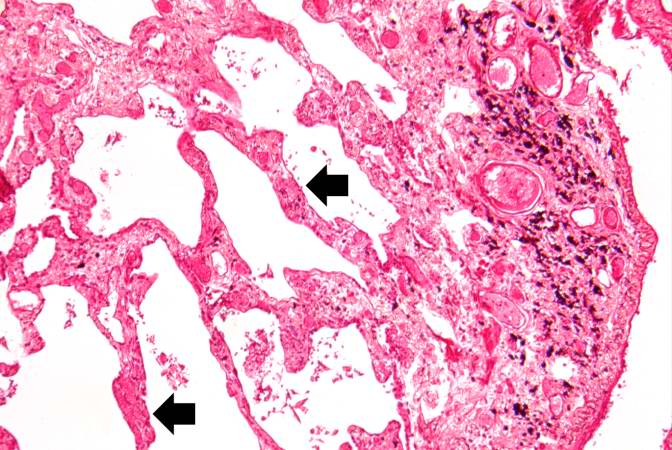
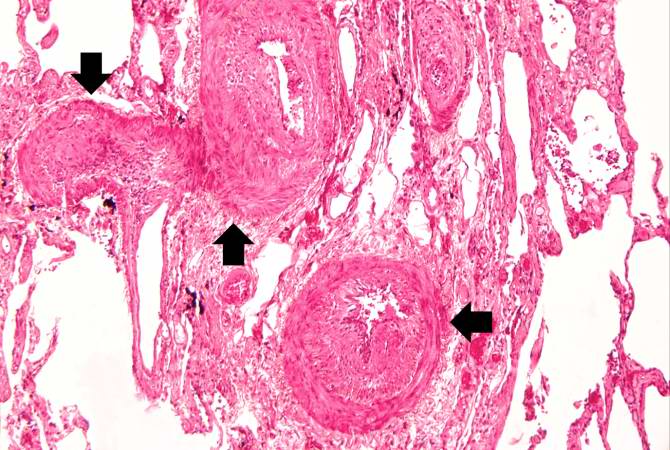
This is a low-power photomicrograph of lung section. Note the thickening of the alveolar septa (arrows).
-
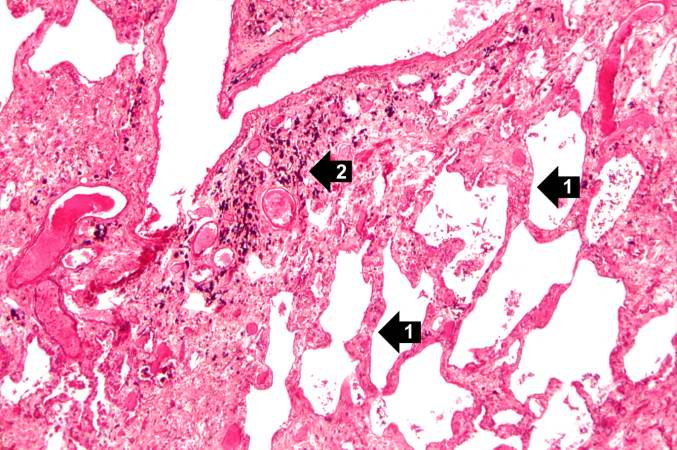
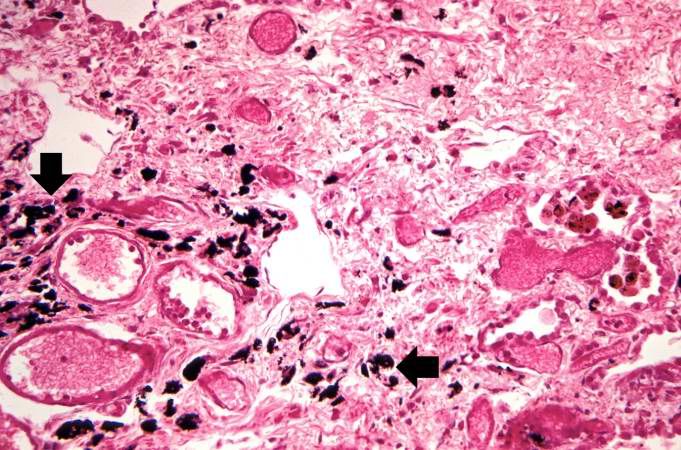
This is a higher-power photomicrograph of lung section. Note the thickening of the alveolar septa (1) and accumulations of anthracotic pigment (2).
-
This is another high-power photomicrograph of lung section showing the thickening of the alveolar septa (arrows) and accumulations of black anthracotic pigment.
-
This high-power photomicrograph of lung section shows the thickening of the alveolar septum (arrows) by fibrous connective tissue.
-
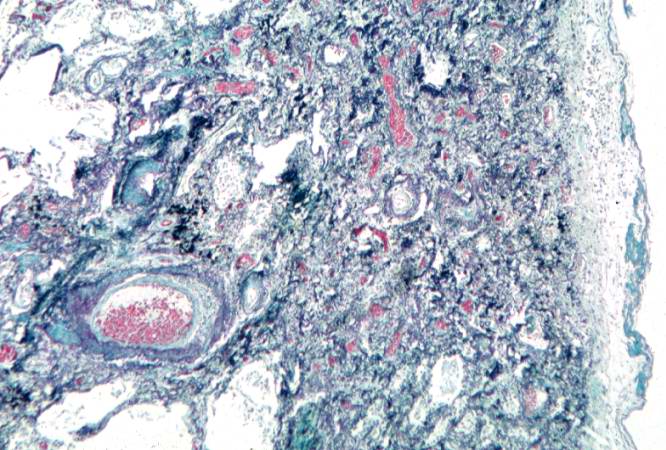
This is a photomicrograph of a trichrome-stained section of lung demonstrating the extensive fibrosis throughout this section (green-blue stained material is fibrous connective tissue).
-
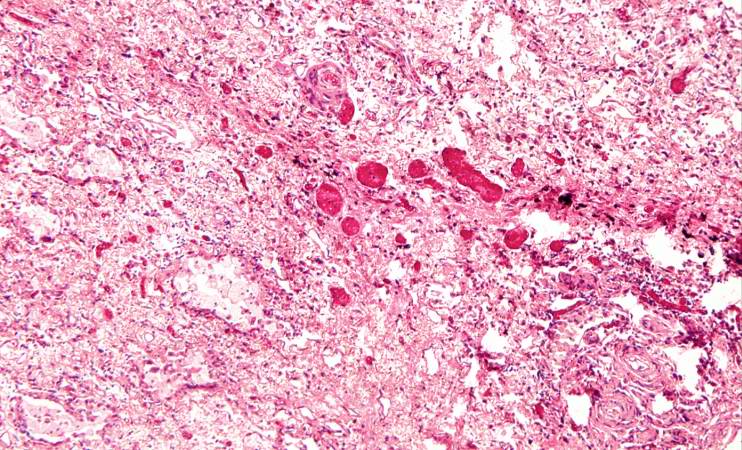
This is a photomicrograph of an area of tissue exhibiting diffuse fibrosis and thickening of the alveolar septa.
-
This is another high-power photomicrograph of an area of tissue with diffuse fibrosis and thickening of the alveolar septa. There are also accumulations of anthracotic pigment in this area (arrows).
-
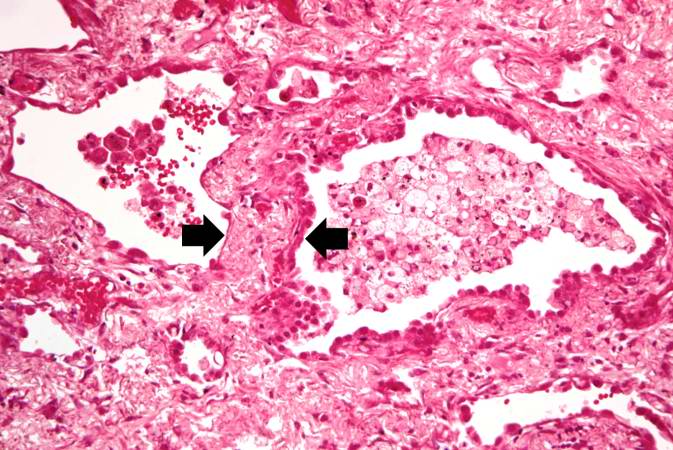
This medium-power photomicrograph shows fibrosis and severe intimal changes in blood vessels (arrows).
-
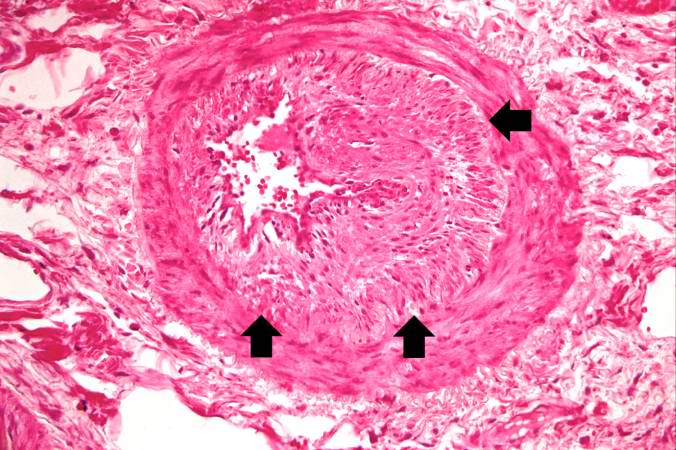
This high-power photomicrograph shows intimal changes (arrows) in this blood vessel in the lung.
-
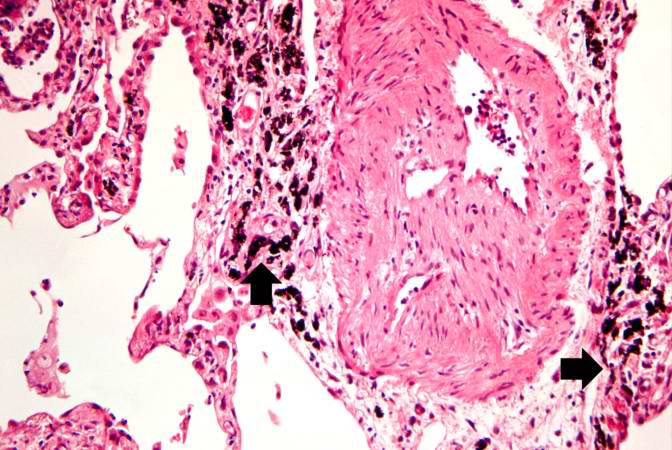
This is a high-power photomicrograph of a recanalized blood vessel in the lung. Notice the anthracotic pigment adjacent to the vessel (arrows).
Template:Skin and subcutaneous tissue symptoms and signs Template:Nervous and musculoskeletal system symptoms and signs Template:Urinary system symptoms and signs Template:Cognition, perception, emotional state and behaviour symptoms and signs Template:Speech and voice symptoms and signs Template:General symptoms and signs