Progesterone (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Progesterone (injection) is a steroid hormone that is FDA approved for the treatment of amenorrhea and abnormal uterine bleeding. Common adverse reactions include breakthrough bleeding; spotting; change in menstrual flow; amenorrhea; edema; change in weight (increase or decrease); changes in cervical erosion and cervical secretions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- This drug is indicated in amenorrhea and abnormal uterine bleeding due to hormonal imbalance in the absence of organic pathology, such as submucous fibroids or uterine cancer.
Dosage
- Progesterone is administered by intramuscular injection. It differs from other commonly used steroids in that it is irritating at the place of injection.
Amenorrhea
- Five to 10 mg are given for six to eight consecutive days. If there has been sufficient ovarian activity to produce a proliferative endometrium, one can expect withdrawal bleeding 48 to 72 hours after the last injection. This may be followed by spontaneous normal cycles.
Functional Uterine Bleeding
- Five to 10 mg are given daily for six doses. Bleeding may be expected to cease within six days. When estrogen is given as well, the administration of progesterone is begun after two weeks of estrogen therapy. If menstrual flow begins during the course of injections of progesterone, they are discontinued.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever the solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Progesterone (injection) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Progesterone (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Progesterone (injection) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Progesterone (injection) in pediatric patients.
Contraindications
Current or past history of thrombophlebitis, thromboembolic disorders, or cerebral apoplexy.
- Liver dysfunction or disease.
- Known or suspected malignancy of breast or genital organs.
- Undiagnosed vaginal bleeding.
- Known sensitivity to progesterone injection.
Warnings
- The physician should be alert to the earliest manifestations of thrombotic disorders (thrombophlebitis, cerebrovascular disorders, pulmonary embolism, and retinal thrombosis). Should any of these occur or be suspected, the drug should be discontinued immediately.
- Medication should be discontinued pending examination if there is a sudden partial or complete loss of vision, or if there is a sudden onset of proptosis, diplopia or migraine. If examination reveals papilledema or retinal vascular lesions, medication should be withdrawn.
Precautions
General
- The pretreatment physical examination should include special reference to breast and pelvic organs, as well as a Papanicolaou smear.
- Because progestational drugs may cause some degree of fluid retention, conditions which might be influenced by this condition, such as epilepsy, migraine, asthma, cardiac, or renal dysfunction, require careful observation.
- In cases of breakthrough bleeding, as in all cases of irregular bleeding per vaginum, nonfunctional causes should be borne in mind, and adequate diagnostic measures undertaken.
- Patients who have a history of psychic depression should be carefully observed and the drug discontinued if the depression recurs to a serious degree.
- The age of the patient constitutes no absolute limiting factor although treatment with progestin may mask the onset of the climacteric.
- The pathologist should be advised of progestin therapy when relevant specimens are submitted.
- There are possible risks which may be associated with the use of progestin treatment, including adverse effects on carbohydrate and lipid metabolism. The dosage used may be important in minimizing these adverse effects.
- A decrease in glucose tolerance has been observed in a small percentage of patients on estrogen-progestin combination treatment. The mechanism of this decrease is obscure. For this reason, diabetic patients should be carefully observed while receiving such therapy.
Adverse Reactions
Clinical Trials Experience
- Breakthrough bleeding; spotting; change in menstrual flow; amenorrhea; edema; change in weight (increase or decrease); changes in cervical erosion and cervical secretions; cholestatic jaundice; breast tenderness and galactorrhea; pain, irritation, and/or redness at the injection area; skin sensitivity reactions consisting of urticaria, pruritus, edema and generalized rash; acne, alopecia and hirsutism; rash (allergic) with and without pruritus; anaphylactoid reactions; mental depression; pyrexia; insomnia; nausea; and somnolence.
- A statistically significant association has been demonstrated between use of estrogen-progestin combination drugs and pulmonary embolism and cerebral thrombosis and embolism. For this reason patients on progestin therapy should be carefully observed. There is also evidence suggestive of an association with neuro-ocular lesions, e.g., retinal thrombosis and optic neuritis.
- The following adverse reactions have been observed in patients receiving estrogen-progestin combination drugs: Rise in blood pressure in susceptible individual, premenstrual syndrome, changes in libido, changes in appetite, cystitis-like syndrome, headache, nervousness, fatigue, backache, hirsutism, loss of scalp hair, erythema multiforme, erythema nodosum, hemorrhagic eruption, itching, and dizziness.
- The following laboratory results may be altered by the use of estrogen-progestin combination drugs: increased sulfobromophthalein retention and other hepatic function tests; coagulation tests: increase in prothrombin factors VII, VIII, IX, and X; metyrapone test; pregnanediol determinations; thyroid function: increase in PBI, and butanol extractable protein bound iodine and decrease in T3 uptake values.
Postmarketing Experience
There is limited information regarding Progesterone (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Progesterone (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Progesterone (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Progesterone (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Progesterone (injection) during labor and delivery.
Nursing Mothers
- Detectable amounts of drug have been identified in the milk of mothers receiving progestational drugs. The effect of this on the nursing infant has not been determined.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- The safety and effectiveness in geriatric patients (over age 65) have not been established.
Gender
There is no FDA guidance on the use of Progesterone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Progesterone (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Progesterone (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Progesterone (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Progesterone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Progesterone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular.
Monitoring
- Monitor in patient with renal insufficiency since progesterone metabolites are excreted mainly by the kidneys.
IV Compatibility
There is limited information regarding the compatibility of Progesterone (injection) and IV administrations.
Overdosage
There is limited information regarding Progesterone (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Progesterone (injection) Mechanism of Action in the drug label.
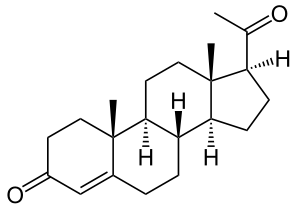
Structure
- Progesterone injection, a progestin, is a sterile solution of progesterone in a suitable vegetable oil available for intramuscular use.
- Progesterone occurs as a white or creamy white, crystalline powder. It is odorless and is stable in air. Practically insoluble in water, it is soluble in alcohol, acetone, and dioxane and sparingly soluble in vegetable oils.
- It has the following structural formula:

- Each mL contains: Progesterone 50 mg, benzyl alcohol 10% as preservative in sesame oil q.s.
Pharmacodynamics
There is limited information regarding Progesterone (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- After intramuscular administration of 10 mg of progesterone in oil maximum plasma concentrations (geometric mean of 7 ng/mL) were reached within approximately 8 hours after injection and plasma concentrations remained above baseline for about 24 hours after injection. Injection of 10, 25, and 50 mg resulted in geometric mean values for maximum plasma concentration (Cmax) of 7, 28, and 50 ng/mL, respectively.
Distribution
- Progesterone is extensively bound to plasma proteins, primarily albumin (50 to 54%) and cortisol-binding protein (43 to 48%).
Metabolism
- Progesterone is metabolized primarily in the liver by reduction to pregnanediol, pregnanetriol and pregnanolone. Subsequent conjugation results in the formation of glucuronide and sulfate metabolites. The mean plasma metabolic clearance rate in cycling women is 2510 ± 135 (SEM) L/day.
Excretion
- The glucuronide and sulfate conjugates of pregnanediol and pregnanolone are excreted in the urine and bile. Progesterone metabolites which are excreted in the bile may undergo enterohepatic recycling or may be excreted in the feces.
- The pharmacokinetic data was determined in a small number of patients, limiting the precision in which population values may be estimated.
Special Populations
- Renal Insufficiency
- The safety and effectiveness in patients with renal insufficiency have not been established. Since progesterone metabolites are excreted mainly by the kidneys, progesterone should be administered with caution and careful monitoring in this patient population.
- Hepatic Insufficiency
- The safety and effectiveness in patients with hepatic insufficiency have not been established. Since progesterone is metabolized by the liver, use in patients with liver dysfunction or disease is contraindicated.
Drug Interactions
- The metabolism of progesterone by human liver microsomes was inhibited by ketoconazole (IC50 < 01 μM). Ketoconazole is a known inhibitor of cytochrome P450 3A4 and these data suggest that ketoconazole or other known inhibitors of this enzyme may increase the bioavailability of progesterone. The clinical relevance of the in vitro findings is unknown.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term intramuscular administration of Medroxyprogesterone acetate (MPA) has been shown to produce mammary tumors in beagle dogs. There is no evidence of a carcinogenic effect associated with the oral administration of MPA to rats and mice.
- Medroxyprogesterone acetate was not mutagenic in a battery of in vitro or in vivo genetic toxicity assays.
- Progesterone at high doses is an antifertility drug and high doses would be expected to impair fertility until the cessation of treatment.
Clinical Studies
There is limited information regarding Progesterone (injection) Clinical Studies in the drug label.
How Supplied

Storage
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Progesterone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Progesterone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Progesterone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PROGESTERONE[1]
Look-Alike Drug Names
There is limited information regarding Progesterone (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.