Pirfenidone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pirfenidone is a respiratory agent that is FDA approved for the treatment of idiopathic pulmonary fibrosis (IPF). Common adverse reactions include nausea, rash, abdominal pain, upper respiratory tract infection, diarrhea, fatigue, headache, dyspepsia, dizziness, vomiting, anorexia, gastroesophageal reflux disease, sinusitis, insomnia, weight decreased, and arthralgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Pirfenidone is indicated for the treatment of idiopathic pulmonary fibrosis (IPF).
- Dosage:
- 267 mg (1 capsule) ORALLY 3 times daily for 7 days
- Then 534 mg (2 capsules) ORALLY 3 times daily on days 8 through 14
- Then 801 mg (3 capsules) ORALLY 3 times daily thereafter
- All doses should be taken with food
- Doses greater than 2403 mg/day not recommended
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pirfenidone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pirfenidone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pirfenidone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pirfenidone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pirfenidone in pediatric patients.
Contraindications
None
Warnings
Elevated Liver Enzymes
Increases in ALT and AST >3 × ULN have been reported in patients treated with pirfenidone. Rarely these have been associated with concomitant elevations in bilirubin. Patients treated with pirfenidone 2403 mg/day in the three Phase 3 trials had a higher incidence of elevations in ALT or AST ≥3 × ULN than placebo patients (3.7% vs. 0.8%, respectively). Elevations ≥10 × ULN in ALT or AST occurred in 0.3% of patients in the pirfenidone 2403 mg/day group and in 0.2% of patients in the placebo group. Increases in ALT and AST ≥3 × ULN were reversible with dose modification or treatment discontinuation. No cases of liver transplant or death due to liver failure that were related to pirfenidone have been reported. However, the combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury, that could lead to death or the need for liver transplants in some patients. Conduct liver function tests (ALT, AST, and bilirubin) prior to the initiation of therapy with pirfenidone in all patients, then monthly for the first 6 months and every 3 months thereafter. Dosage modifications or interruption may be necessary for liver enzyme elevations.
Photosensitivity Reaction or Rash
Patients treated with pirfenidone 2403 mg/day in the three Phase 3 studies had a higher incidence of photosensitivity reactions (9%) compared with patients treated with placebo (1%). The majority of the photosensitivity reactions occurred during the initial 6 months. Instruct patients to avoid or minimize exposure to sunlight (including sunlamps), to use a sunblock (SPF 50 or higher), and to wear clothing that protects against sun exposure. Additionally, instruct patients to avoid concomitant medications known to cause photosensitivity. Dosage reduction or discontinuation may be necessary in some cases of photosensitivity reaction or rash.
Gastrointestinal Disorders
In the clinical studies, gastrointestinal events of nausea, diarrhea, dyspepsia, vomiting, gastroesophageal reflux disease, and abdominal pain were more frequently reported by patients in the pirfenidone treatment groups than in those taking placebo. Dosage reduction or interruption for gastrointestinal events was required in 18.5% of patients in the 2403 mg/day group, as compared to 5.8% of patients in the placebo group; 2.2% of patients in the pirfenidone 2403 mg/day group discontinued treatment due to a gastrointestinal event, as compared to 1.0% in the placebo group. The most common (>2%) gastrointestinal events that led to dosage reduction or interruption were nausea, diarrhea, vomiting, and dyspepsia. The incidence of gastrointestinal events was highest early in the course of treatment (with highest incidence occurring during the initial 3 months) and decreased over time. Dosage modifications may be necessary in some cases of gastrointestinal adverse reactions.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of pirfenidone has been evaluated in more than 1400 subjects with over 170 subjects exposed to pirfenidone for more than 5 years in clinical trials.
pirfenidone was studied in 3 randomized, double-blind, placebo-controlled trials (Studies 1, 2, and 3) in which a total of 623 patients received 2403 mg/day of pirfenidone and 624 patients received placebo. Subjects ages ranged from 40 to 80 years (mean age of 67 years). Most patients were male (74%) and Caucasian (95%). The mean duration of exposure to pirfenidone was 62 weeks (range: 2 to 118 weeks) in these 3 trials.
At the recommended dosage of 2403 mg/day, 14.6% of patients on pirfenidone compared to 9.6% on placebo permanently discontinued treatment because of an adverse event. The most common (>1%) adverse reactions leading to discontinuation were rash and nausea. The most common (>3%) adverse reactions leading to dosage reduction or interruption were rash, nausea, diarrhea, and photosensitivity reaction.
The most common adverse reactions with an incidence of ≥10% and more frequent in the pirfenidone than placebo treatment group are listed in TABLE 2.
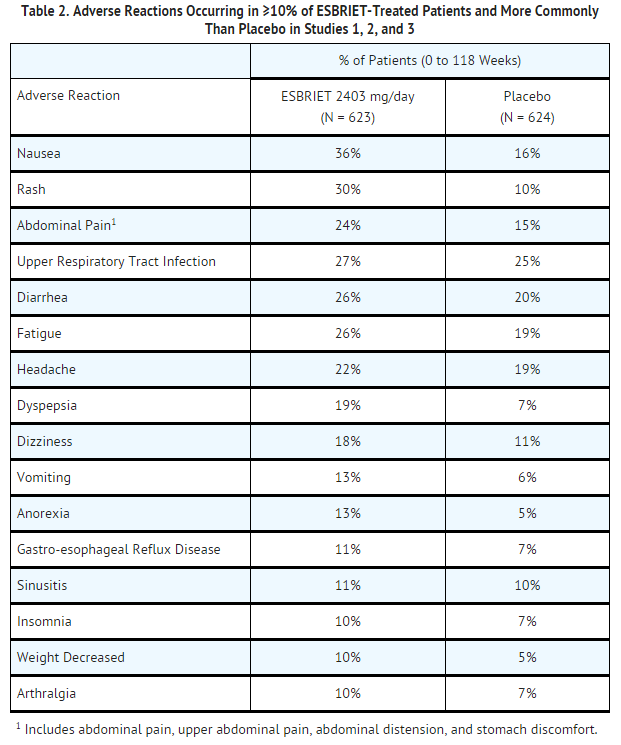
Adverse reactions occurring in ≥5 to <10% of pirfenidone-treated patients and more commonly than placebo are photosensitivity reaction (9% vs. 1%), decreased appetite (8% vs. 3%), pruritus (8% vs. 5%), asthenia (6% vs. 4%), dysgeusia (6% vs. 2%), and non-cardiac chest pain (5% vs. 4%).
Postmarketing Experience
In addition to adverse reactions identified from clinical trials the following adverse reactions have been identified during postapproval use of pirfenidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
- Blood and Lymphatic System Disorders
- Immune System Disorders
- Hepatobiliary Disorders
Drug Interactions
CYP1A2 Inhibitors
Pirfenidone is metabolized primarily (70 to 80%) via CYP1A2 with minor contributions from other CYP isoenzymes including CYP2C9, 2C19, 2D6 and 2E1.
Strong CYP1A Inhibitors
The concomitant administration of pirfenidone and fluvoxamine or other strong CYP1A2 inhibitors (e.g., enoxacin) is not recommended because it significantly increases exposure to pirfenidone. Use of fluvoxamine or other strong CYP1A2 inhibitors should be discontinued prior to administration of pirfenidone and avoided during pirfenidone treatment. In the event that fluvoxamine or other strong CYP1A2 inhibitors are the only drug of choice, dosage reductions are recommended. Monitor for adverse reactions and consider discontinuation of pirfenidone as needed.
Moderate CYP1A Inhibitors
Concomitant administration of pirfenidone and ciprofloxacin (a moderate inhibitor of CYP1A2) moderately increases exposure to pirfenidone. If ciprofloxacin at the dosage of 750 mg twice daily cannot be avoided, dosage reductions are recommended. Monitor patients closely when ciprofloxacin is used at a dosage of 250 mg or 500 mg once daily.
Concomitant CYP1A2 and other CYP Inhibitors
Agents or combinations of agents that are moderate or strong inhibitors of both CYP1A2 and one or more other CYP isoenzymes involved in the metabolism of pirfenidone (i.e., CYP2C9, 2C19, 2D6, and 2E1) should be discontinued prior to and avoided during pirfenidone treatment.
CYP1A2 Inducers
The concomitant use of pirfenidone and a CYP1A2 inducer may decrease the exposure of pirfenidone and this may lead to loss of efficacy. Therefore, discontinue use of strong CYP1A2 inducers prior to pirfenidone treatment and avoid the concomitant use of pirfenidone and a strong CYP1A2 inducer.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies of pirfenidone in pregnant women. Pirfenidone was not teratogenic in rats and rabbits. Because animal reproduction studies are not always predictive of human response, pirfenidone should be used during pregnancy only if the benefit outweighs the risk to the patient.
A fertility and embryo-fetal development study with rats and an embryo-fetal development study with rabbits that received oral doses up to 3 and 2 times, respectively, the maximum recommended daily dose (MRDD) in adults (on mg/m2 basis at maternal doses up to 1000 and 300 mg/kg/day, respectively) revealed no evidence of impaired fertility or harm to the fetus due to pirfenidone. In the presence of maternal toxicity, acyclic/irregular cycles (e.g., prolonged estrous cycle) were seen in rats at doses approximately equal to and higher than the MRDD in adults (on a mg/m2 basis at maternal doses of 450 mg/kg/day and higher). In a pre- and post-natal development study, prolongation of the gestation period, decreased numbers of live newborn, and reduced pup viability and body weights were seen in rats at an oral dosage approximately 3 times the MRDD in adults (on a mg/m2 basis at a maternal dose of 1000 mg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pirfenidone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pirfenidone during labor and delivery.
Nursing Mothers
A study with radio-labeled pirfenidone in rats has shown that pirfenidone or its metabolites are excreted in milk. It is not known whether pirfenidone is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue pirfenidone, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of pirfenidone in pediatric patients have not been established.
Geriatic Use
Of the total number of subjects in the clinical studies receiving pirfenidone, 714 (67%) were 65 years old and over, while 231 (22%) were 75 years old and over. No overall differences in safety or effectiveness were observed between older and younger patients. No dosage adjustment is required based upon age.
Gender
Results of population pharmacokinetic analysis of pirfenidone showed no significant differences in pharmacokinetics between males and females.
Race
Population pharmacokinetic analysis showed that race has no significant effect on the pharmacokinetics of pirfenidone.
Renal Impairment
Pirfenidone should be used with caution in patients with mild (CLcr 50–80 mL/min), moderate (CLcr 30–50 mL/min), or severe (CLcr less than 30 mL/min) renal impairment. Monitor for adverse reactions and consider dosage modification or discontinuation of pirfenidone as needed. The safety, efficacy, and pharmacokinetics of pirfenidone have not been studied in patients with end-stage renal disease requiring dialysis. Use of pirfenidone in patients with end-stage renal diseases requiring dialysis is not recommended.
Hepatic Impairment
Pirfenidone should be used with caution in patients with mild (Child Pugh Class A) to moderate (Child Pugh Class B) hepatic impairment. Monitor for adverse reactions and consider dosage modification or discontinuation of pirfenidone as needed.
The safety, efficacy, and pharmacokinetics of pirfenidone have not been studied in patients with severe hepatic impairment. Pirfenidone is not recommended for use in patients with severe (Child Pugh Class C) hepatic impairment.
Females of Reproductive Potential and Males
Pirfenidone had no effects on fertility and reproductive performance in rats at dosages up to 1000 mg/kg/day (approximately 3 times the MRDD in adults on a mg/m2 basis).
Immunocompromised Patients
There is no FDA guidance one the use of Pirfenidone in patients who are immunocompromised.
Smokers
Smoking causes decreased exposure to pirfenidone, which may alter the efficacy profile of pirfenidone. Instruct patients to stop smoking prior to treatment with pirfenidone and to avoid smoking when using pirfenidone.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Pirfenidone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pirfenidone and IV administrations.
Overdosage
There is limited clinical experience with overdosage. Multiple dosages of pirfenidone up to a maximum tolerated dose of 4005 mg per day were administered as five 267 mg capsules three times daily to healthy adult volunteers over a 12-day dose escalation.
In the event of a suspected overdosage, appropriate supportive medical care should be provided, including monitoring of vital signs and observation of the clinical status of the patient.
Pharmacology
| |
Pirfenidone
| |
| Systematic (IUPAC) name | |
| 5-Methyl-1-phenylpyridin-2-one | |
| Identifiers | |
| CAS number | ? |
| ATC code | L04 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 185.22 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 50–58%[1] |
| Metabolism | Hepatic (70–80% CYP1A2-mediated; minor contributions from CYP2C9, CYP2C19, CYP2D6 and CYP2E1)[1] |
| Half life | 2.4 hours[1] |
| Excretion | Urine (80%)[1] |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
The mechanism of action of pirfenidone in the treatment of IPF has not been established.
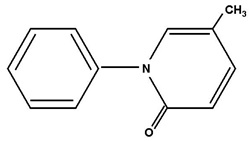
Structure
The chemical name of pirfenidone is 5-methyl-1-phenyl-2-1(H)-pyridone. It has a molecular formula of C12H11NO and a molecular weight of 185.23. The structural formula of pirfenidone is:
Pharmacodynamics
Cardiac Electrophysiology
The effect of pirfenidone on QT interval was evaluated in a randomized, placebo, and positive controlled parallel study in 160 healthy adult volunteers. Volunteers received pirfenidone 2403 mg/day (recommended dose) and 4005 mg/day (1.6 times recommended dose) or placebo for 10 days or a single dose of 400 mg moxifloxacin (active control).
Relative to placebo, the maximum mean change from baseline in study-specific QT interval was 3.2 milliseconds (ms) and 2.2 ms for pirfenidone 2403 mg/day and 4005 mg/day, respectively. No volunteer had a QTc interval greater than 480 ms or change from baseline greater than 60 ms. Although there was no evidence that pirfenidone prolonged the QTc interval in this study, a definitive conclusion may not be drawn as the positive control (moxifloxacin) did not perform as expected in this study, and pirfenidone at 4005 mg/day (1.7 times the maximum recommended dose) did not cover the maximum pirfenidone exposure increase with co-administration of fluvoxamine, a strong CYP1A2 inhibitor.
Pharmacokinetics
Absorption
After single oral-dose administration of 801 mg pirfenidone, the maximum observed plasma concentration (Cmax) was achieved between 30 minutes and 4 hours (median time of 0.5 hours). Food decreased the rate and extent of absorption. Median Tmax increased from 0.5 hours to 3 hours with food. Maximum plasma concentrations and AUC0-inf decreased by approximately 49% and 16% with food, respectively. A reduced incidence of adverse reactions was observed in the fed group when compared to the fasted group. In controlled studies with IPF patients, pirfenidone was taken with food.
The absolute bioavailability of pirfenidone has not been determined in humans.
Distribution
pirfenidone binds to human plasma proteins, primarily to serum albumin, in a concentration-independent manner over the range of concentrations observed in clinical trials. The overall mean binding was 58% at concentrations observed in clinical studies (1 to 10 µg/mL). Mean apparent oral volume of distribution is approximately 59 to 71 liters.
Metabolism
In vitro profiling studies in hepatocytes and liver microsomes have shown that pirfenidone is primarily metabolized in the liver by CYP1A2 and multiple other CYPs (CYP2C9, 2C19, 2D6, and 2E1). Oral administration of pirfenidone results in the formation of four metabolites. In humans, only pirfenidone and 5-carboxy-pirfenidone are present in plasma in significant quantities. The mean metabolite-to-parent ratio ranged from approximately 0.6 to 0.7.
No formal radiolabeled studies have assessed the metabolism of pirfenidone in humans. In vitro data suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
Elimination
The mean terminal half-life is approximately 3 hours in healthy subjects. Pirfenidone is excreted predominantly as metabolite 5-carboxy-pirfenidone, mainly in the urine (approximately 80% of the dose). The majority of pirfenidone was excreted as the 5-carboxy metabolite (approximately 99.6% of that recovered).
Nonclinical Toxicology
Carcinogenesis
Long-term studies were conducted in mice and rats with admixture of pirfenidone to the diet to evaluate its carcinogenic potential.
In a 24-month carcinogenicity study in B6C3F1 mice, pirfenidone caused statistically significant dose-related increases of the combination of hepatocellular adenoma and carcinoma and hepatoblastoma in male mice at doses of 800 mg/kg and above (AUC exposure approximately 0.4 times adult exposure at the MRDD). There were statistically significant dose-related increases of the combination of hepatocellular adenoma and carcinoma in female mice at doses of 2000 mg/kg and above (AUC exposure approximately 0.7 times adult exposure at the MRDD).
In a 24-month carcinogenicity study in Fischer rats, pirfenidone caused statistically significant dose-related increases of the combination of hepatocellular adenoma and carcinoma in male rats at doses of 750 mg/kg and above (AUC exposure approximately 1.9 times adult exposure at the MRDD). There were statistically significant increases of the combination of hepatocellular adenoma and carcinoma and the combination of uterine adenocarcinoma and adenoma at a dose of 1500 mg/kg/day (AUC exposure approximately 3.0 times adult exposure at the MRDD).
The relevance of these tumor findings in rodents to humans is unknown.
Mutagenesis
Pirfenidone was not mutagenic or clastogenic in the following tests: mutagenicity tests in bacteria, a chromosomal aberration test in Chinese hamster lung cells, and a micronucleus test in mice.
Clinical Studies
The efficacy of pirfenidone was evaluated in patients with IPF in three phase 3, randomized, double-blind, placebo-controlled, multicenter trials (Studies 1, 2, and 3).
Study 1 was a 52-week trial comparing pirfenidone 2403 mg/day (n=278) versus placebo (n=277) in patients with IPF. Study 2 and Study 3 were nearly identical to each other in design, with few exceptions, including an intermediate dose treatment arm in Study 2. Study 2 compared treatment with either pirfenidone 2403 mg/day (n=174) or pirfenidone 1197 mg/day (n=87) to placebo (n=174), while Study 3 compared pirfenidone 2403 mg/day (n=171) to placebo (n=173). Study drug was administered three times daily with food for a minimum of 72 weeks. Patients continued on treatment until the last patient completed 72 weeks of treatment, which included observations to approximately 120 weeks of study treatment. The primary endpoint was the change in percent predicted forced vital capacity (%FVC) from baseline to study end, measured at 52 weeks in Study 1, and at 72 weeks in Studies 2 and 3.
Studies 1, 2 and 3 enrolled adult patients who had a clinical and radiographic diagnosis of IPF (with or without accompanying surgical lung biopsy), without evidence or suspicion of an alternative diagnosis for interstitial lung disease. Eligible patients were to have %FVC greater than or equal to 50% at baseline and a percent predicted diffusing capacity of the lungs for carbon monoxide (%DLCO) greater than or equal to 30% (Study 1) or 35% (Studies 2 and 3) at baseline. In all three trials, over 80% of patients completed study treatment.
A total of 1247 patients with IPF were randomized to receive pirfenidone 2403 mg/day (n=623) or placebo (n=624) in these three trials. Baseline characteristics were generally balanced across treatment groups. The study population ranged from 40 to 80 years of age (mean age 67 years). Most patients were male (74%), white (95%), and current or former smokers (65%). Approximately 93% of patients met criteria for definite IPF on high resolution computed tomography (HRCT). Baseline mean %FVC and %DLCO were 72% and 46%, respectively. Approximately 15% subjects discontinued from each treatment group.
Change from Baseline in Percent Predicted Forced Vital Capacity
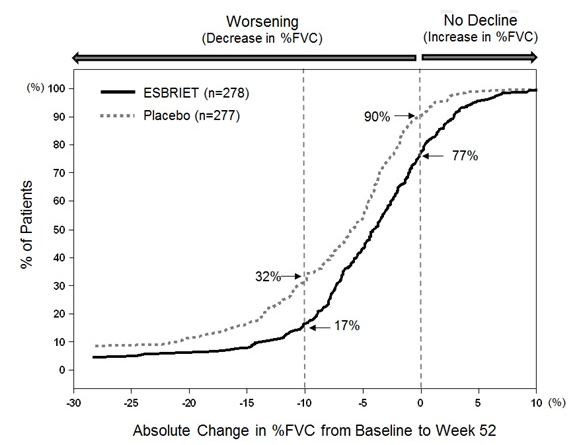
In Study 1, the primary efficacy analysis for the change in %FVC from baseline to Week 52 demonstrated a statistically significant treatment effect of pirfenidone 2403 mg/day (n=278) compared with placebo (n=277) using a rank ANCOVA with the lowest rank imputation for missing data due to death. In Study 2, there was a statistically significant difference at Week 72 for the change in %FVC from baseline. In Study 3, there was no statistically significant difference at Week 72 for the change in %FVC from baseline.
FIGURE 1 presents the cumulative distribution for all cut-offs for the change from baseline in %FVC at Week 52 for Study 1. For all categorical declines in lung function, the proportion of patients declining was lower on pirfenidone than on placebo. Study 2 showed similar results.
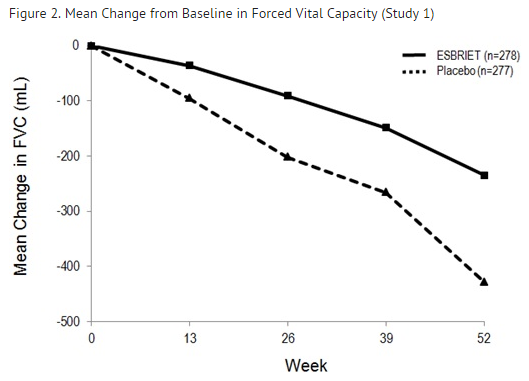
Mean Change from Baseline in FVC (mL)
In Study 1, a reduction in the mean decline in FVC (in mL) was observed in patients receiving pirfenidone 2403 mg/day (-235 mL) compared to placebo (-428 mL) (mean treatment difference 193 mL) at Week 52 (see FIGURE 2). In Study 2, a reduction in the decline in FVC volume was also observed in patients receiving pirfenidone 2403 mg/day compared with placebo (mean treatment difference 157 mL) at Week 72. There was no statistically significant difference in decline in FVC volume seen in Study 3.
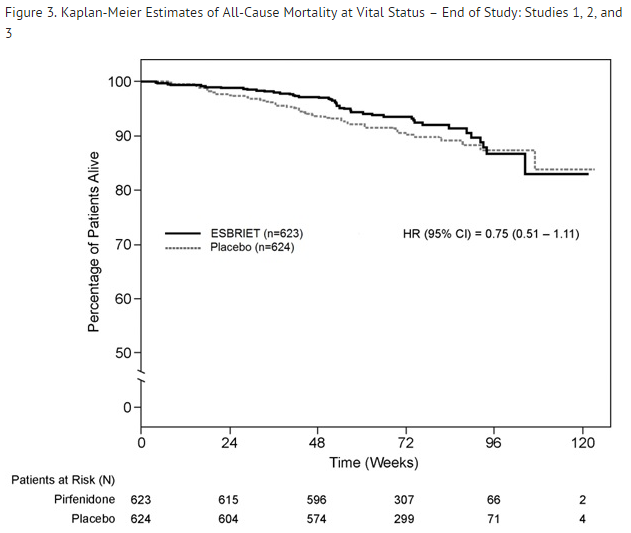
Survival
Survival was evaluated for pirfenidone compared to placebo in Studies 1, 2, and 3 as an exploratory analysis to support the primary endpoint (FVC). All-cause mortality was assessed over the study duration and available follow-up period, irrespective of cause of death and whether patients continued treatment. All-cause mortality did not show a statistically significant difference (see FIGURE 3).
How Supplied
Bottle for a 30-day supply
- Bottle containing 270 capsules and closed with a child-resistant closure
- NDC 64116-121-01
14-day Titration Blister Pack
- Carton containing a total of 63 capsules in two blister cards
- NDC 64116-121-02
4-Week Maintenance Blister Pack
- Carton containing a total of 252 capsules in four blister cards
- NDC 64116-121-03
Storage
Store at 25°C (77°F)
Images
Drug Images
{{#ask: Page Name::Pirfenidone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pirfenidone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Liver Enzyme Elevations
Advise patients that they may be required to undergo liver function testing periodically. Instruct patients to immediately report any symptoms of a liver problem (e.g., skin or the white of eyes turn yellow, urine turns dark or brown [tea colored], pain on the right side of stomach, bleed or bruise more easily than normal, lethargy).
Photosensitivity Reaction or Rash
Advise patients to avoid or minimize exposure to sunlight (including sunlamps) during use of pirfenidone because of concern for photosensitivity reactions or rash. Instruct patients to use a sunblock and to wear clothing that protects against sun exposure. Instruct patients to report symptoms of photosensitivity reaction or rash to their physician. Temporary dosage reductions or discontinuations may be required.
Gastrointestinal Events
Instruct patients to report symptoms of persistent gastrointestinal effects including nausea, diarrhea, dyspepsia, vomiting, gastroesophageal reflux disease, and abdominal pain. Temporary dosage reductions or discontinuations may be required.
Smokers
Encourage patients to stop smoking prior to treatment with pirfenidone and to avoid smoking when using pirfenidone.
Take with Food
Instruct patients to take pirfenidone with food to help decrease nausea and dizziness.
Precautions with Alcohol
Alcohol-Pirfenidone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Esbriet [2]
Look-Alike Drug Names
There is limited information regarding Pirfenidone Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 "pirfenidone 267 mg hard capsules". electronic Medicines Compendium. Intermune UK & I Ltd. 3 January 2014. Retrieved 6 March 2014.
- ↑ "FDA LABEL: ESBRIET- pirfenidone capsule".
{{#subobject:
|Label Page=Pirfenidone |Label Name=Pirfenidone capsules 267 mg.png
}}