Piperacillin-Tazobactam
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Piperacillin-Tazobactam is an antibiotic that is FDA approved for the treatment of Intra-abdominal infections, skin and skin structure infections, female pelvic infections, community-acquired pneumonia, nosocomial pneumonia. Common adverse reactions include hepatitis, jaundice, hemolytic anemia, agranulocytosis, pancytopenia, hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock), interstitial nephritis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Piperacillin and tazobactam for injection USP is a combination product consisting of a penicillin-class antibacterial, piperacillin, and a β-lactamase inhibitor, tazobactam, indicated for the treatment of patients with moderate to severe infections caused by susceptible isolates of the designated bacteria in the conditions listed below.
Intra-abdominal Infections
- Appendicitis (complicated by rupture or abscess) and peritonitis caused by β-lactamase producing isolates of Escherichia coli or the following members of the Bacteroidesfragilisgroup: B. fragilis, B. ovatus, B. thetaiotaomicron, or B. vulgatus. The individual members of this group were studied in fewer than 10 cases.
Skin and Skin Structure Infections
- Uncomplicated and complicated skin and skin structure infections, including cellulitis, cutaneous abscesses and ischemic/diabetic foot infections caused by β-lactamase producing isolates of Staphylococcus aureus.
Female Pelvic Infections
- Postpartum endometritis or pelvic inflammatory disease caused by β-lactamase producing isolates of Escherichia coli.
Community-acquired Pneumonia
- Community-acquired pneumonia (moderate severity only) caused by β-lactamase producing isolates of Haemophilusinfluenzae.
Nosocomial Pneumonia
- Nosocomial pneumonia (moderate to severe) caused by β-lactamase producing isolates of Staphylococcus aureus and by piperacillin/tazobactam-susceptible Acinetobacterbaumannii, Haemophilus influenzae, Klebsiella pneumoniae, and Pseudomonas aeruginosa(Nosocomial pneumonia caused by P. aeruginosa should be treated in combination with an aminoglycoside).
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of piperacillin and tazobactam for injection USP and other antibacterial drugs, piperacillin and tazobactam for injection USP should be used only to treat infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- Piperacillin and tazobactam for injection should be administered by intravenous infusion over 30 minutes.
Adult Patients
- The usual total daily dose of piperacillin and tazobactam for injection for adults is 3.375 g every six hours totaling 13.5 g (12 g piperacillin/1.5 g tazobactam). The usual duration of piperacillin and tazobactam for injection treatment is from 7 to 10 days.
- Piperacillin and tazobactam for injection should be administered by intravenous infusion over 30 minutes.
Nosocomial Pneumonia
- Initial presumptive treatment of patients with nosocomial pneumonia should start with piperacillin and tazobactam for injection at a dosage of 4.5 g every six hours plus an aminoglycoside, totaling 18 g (16 g piperacillin/2 g tazobactam). The recommended duration of piperacillin and tazobactam for injection treatment for nosocomial pneumonia is 7 to 14 days. Treatment with the aminoglycoside should be continued in patients from whom P. aeruginosais isolated.
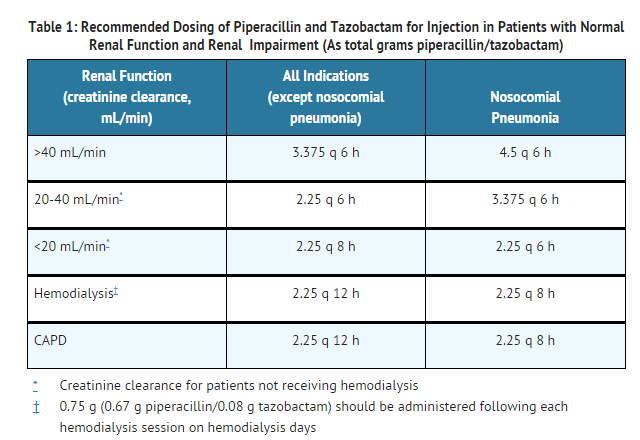
Renal Impairment
- In patients with renal impairment (creatinine clearance ≤ 40 mL/min) and dialysis patients (hemodialysis and CAPD), the intravenous dose of piperacillin and tazobactam for injection should be reduced to the degree of actual renal function impairment. The recommended daily doses of piperacillin and tazobactam for injection for patients with renal impairment are as follows:
- For patients on hemodialysis, the maximum dose is 2.25 g every twelve hours for all indications other than nosocomial pneumonia and 2.25 g every eight hours for nosocomial pneumonia. Since hemodialysis removes 30% to 40% of the administered dose, an additional dose of 0.75 g piperacillin and tazobactam for injection (0.67 g piperacillin/0.08 g tazobactam) should be administered following each dialysis period on hemodialysis days. No additional dosage of piperacillin and tazobactam for injection is necessary for CAPD patients.
Pediatric Patients
- For children with appendicitis and/or peritonitis 9 months of age or older, weighing up to 40 kg, and with normal renal function, the recommended piperacillin and tazobactam for injection dosage is 100 mg piperacillin/12.5 mg tazobactam per kilogram of body weight, every 8 hours. For pediatric patients between 2 months and 9 months of age, the recommended piperacillin and tazobactam for injection dosage based on pharmacokinetic modeling, is 80 mg piperacillin/10 mg tazobactam per kilogram of body weight, every 8 hours. * Pediatric patients weighing over 40 kg and with normal renal function should receive the adult dose.
- It has not been determined how to adjust piperacillin and tazobactam for injection dosage in pediatric patients with renal impairment.
Reconstitution and Dilution of Powder Formulations
Single dose vials
- Reconstitute piperacillin and tazobactam for injection vials with a compatible reconstitution diluent from the list provided below.
- 2.25 g, 3.375 g, and 4.5 g piperacillin and tazobactam for injection should be reconstituted with 10 mL, 15 mL, and 20 mL, respectively. Swirl until dissolved.
- Compatible Reconstitution Diluents for Single Dose Vials
- 0.9% sodium chloride for injection
- Sterile water for injection
- Dextrose 5%
- Bacteriostatic saline/parabens
- Bacteriostatic water/parabens
- Bacteriostatic saline/benzyl alcohol
- Bacteriostatic water/benzyl alcohol
- Reconstituted piperacillin and tazobactam for injection solutions for single dose vials should be further diluted (recommended volume per dose of 50 mL to 150 mL) in a compatible intravenous solution listed below.
- Administer by infusion over a period of at least 30 minutes. During the infusion it is desirable to discontinue the primary infusion solution.
- Compatible Intravenous Solutions for Single Dose Vials
- 0.9% sodium chloride for injection
- sterile water for injection‡
- Dextran 6% in saline
- Dextrose 5%
- LACTATED RINGER’S SOLUTION IS NOT COMPATIBLE WITH PIPERACILLIN AND TAZOBACTAM FOR INJECTION.
- ‡ Maximum recommended volume per dose of sterile water for injection is 50 mL.
- Piperacillin and tazobactam for injection should not be mixed with other drugs in a syringe or infusion bottle since compatibility has not been established.
- Piperacillin and tazobactam for injection is not chemically stable in solutions that contain only sodium bicarbonate and solutions that significantly alter the pH.
- Piperacillin and tazobactam for injection should not be added to blood products or albumin hydrolysates. Parenteral drug products should be inspected visually for particulate matter or discoloration prior to administration, whenever solution and container permit.
- Stability of Piperacillin and Tazobactam for Injection Powder Formulations Following Reconstitution
- Piperacillin and tazobactam for injection reconstituted from single dose vials is stable in glass and plastic containers (plastic syringes, I.V. bags and tubing) when used with compatible diluents.
- Single dose vials should be used immediately after reconstitution. Discard any unused portion after 24 hours if stored at room temperature (20°C to 25°C [68°F to 77°F]), or after 48 hours if stored at refrigerated temperature (2°C to 8°C [36°F to 46°F]). Vials should not be frozen after reconstitution.
- Stability studies in the I.V. bags have demonstrated chemical stability (potency, pH of reconstituted solution and clarity of solution) for up to 24 hours at room temperature and up to one week at refrigerated temperature. Piperacillin and tazobactam for injection contains no preservatives. Appropriate consideration of aseptic technique should be used.
- Piperacillin and tazobactam for injection reconstituted from single dose vials can be used in ambulatory intravenous infusion pumps. Stability of piperacillin and tazobactam for injection in an ambulatory intravenous infusion pump has been demonstrated for a period of 12 hours at room temperature. Each dose was reconstituted and diluted to a volume of 37.5 mL or 25 mL. One-day supplies of dosing solution were aseptically transferred into the medication reservoir (I.V. bags or cartridge). The reservoir was fitted to a preprogrammed ambulatory intravenous infusion pump per the manufacturer's instructions. Stability of piperacillin and tazobactam for injection is not affected when administered using an ambulatory intravenous infusion pump.
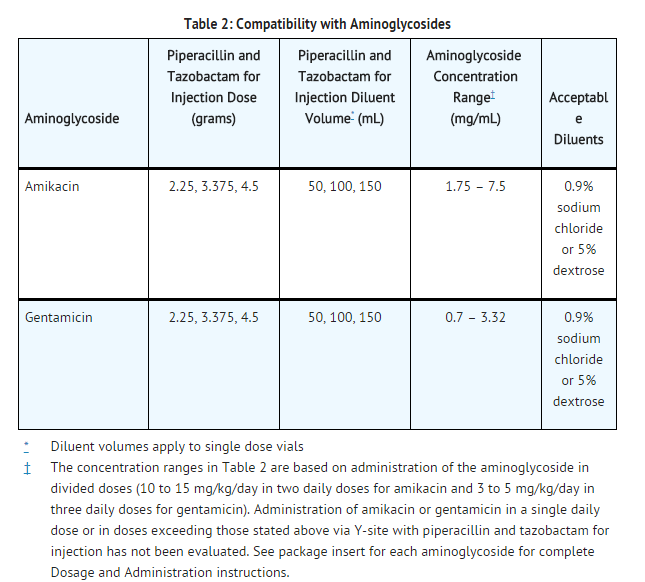
Compatibility with Aminoglycosides
- Due to the in vitro inactivation of aminoglycosides by piperacillin, piperacillin and tazobactam for injection and aminoglycosides are recommended for separate administration. Piperacillin and tazobactam for injection and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated.
- In circumstances where co-administration via Y-site is necessary, Y-site infusion only with the following aminoglycosides under the following conditions:
- Only the concentration and diluents for amikacin or gentamicin with the dosages of piperacillin and tazobactam for injection listed above have been established as compatible for coadministration via Y-site infusion. Simultaneous coadministration via Y-site infusion in any manner other than listed above may result in inactivation of the aminoglycoside by piperacillin and tazobactam for injection.
- Piperacillin and tazobactam for injection is not compatible with tobramycin for simultaneous coadministration via Y-site infusion. Compatibility of piperacillin and tazobactam for injection with other aminoglycosides has not been established.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Piperacillin-Tazobactam in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Piperacillin-Tazobactam in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Piperacillin-Tazobactam in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Piperacillin-Tazobactam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Piperacillin-Tazobactam in pediatric patients.
Contraindications
- Piperacillin and tazobactam for injection is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or β-lactamase inhibitors.
Warnings
Hypersensitivity Reactions
- Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with piperacillin and tazobactam for injection. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. Before initiating therapy with piperacillin and tazobactam for injection, careful inquiry should be made concerning previous hypersensitivity reactions. If an allergic reaction occurs, piperacillin and tazobactam for injection should be discontinued and appropriate therapy instituted.
Serious Skin Reactions
- Serious skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in patients receiving piperacillin and tazobactam for injection. If patients develop a skin rash they should be monitored closely and piperacillin and tazobactam for injection discontinued if lesions progress.
Clostridium difficile Associated Diarrhea
- Clostridium difficileassociated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including piperacillin and tazobactam for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficilemay need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Hematologic Effects
- Bleeding manifestations have occurred in some patients receiving β-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, piperacillin and tazobactam for injection should be discontinued and appropriate therapy instituted.
- The leukopenia/neutropenia associated with piperacillin and tazobactam for injection administration appears to be reversible and most frequently associated with prolonged administration.
- Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, ie, ≥ 21 days.
Central Nervous System Effects
- As with other penicillins, patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure).
Electrolyte Effects
- Piperacillin and tazobactam for injection contains a total of 2.35 mEq (54 mg) of Na+ per gram of piperacillin in the combination product. This should be considered when treating patients requiring restricted salt intake.
- Periodic electrolyte determinations should be performed in patients with low potassium reserves, and the possibility of hypokalemia should be kept in mind with patients who have potentially low potassium reserves and who are receiving cytotoxic therapy or diuretics.
Development of Drug-Resistant Bacteria
- Prescribing piperacillin and tazobactam for injection in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of development of drug-resistant bacteria.
Adverse Reactions
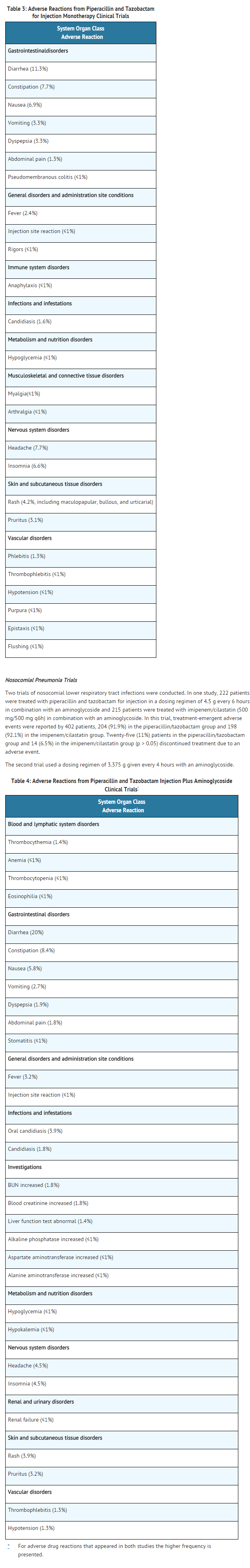
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- During the initial clinical investigations, 2621 patients worldwide were treated with piperacillin and tazobactam for injection in phase 3 trials. In the key North American monotherapy clinical trials (n=830 patients), 90% of the adverse events reported were mild to moderate in severity and transient in nature. However, in 3.2% of the patients treated worldwide, piperacillin and tazobactam for injection was discontinued because of adverse events primarily involving the skin (1.3%), including rash and pruritus; the gastrointestinal system (0.9%), including diarrhea, nausea, and vomiting; and allergic reactions (0.5%).
Pediatrics
- Studies of piperacillin and tazobactam for injection in pediatric patients suggest a similar safety profile to that seen in adults. In a prospective, randomized, comparative, open-label clinical trial of pediatric patients with severe intra-abdominal infections (including appendicitis and/or peritonitis), 273 patients were treated with piperacillin and tazobactam for injection (112.5 mg/kg every 8 hours) and 269 patients were treated with cefotaxime (50 mg/kg) plus metronidazole (7.5 mg/kg) every 8 hours. In this trial, treatment-emergent adverse events were reported by 146 patients, 73 (26.7%) in the piperacillin and tazobactam for injection group and 73 (27.1%) in the cefotaxime/metronidazole group. Six patients (2.2%) in the piperacillin and tazobactam for injection group and 5 patients (1.9%) in the cefotaxime/metronidazole group discontinued due to an adverse event.
Adverse Laboratory Events (Seen During Clinical Trials)
- Of the trials reported, including that of nosocomial lower respiratory tract infections in which a higher dose of piperacillin and tazobactam for injection was used in combination with an aminoglycoside, changes in laboratory parameters include:
- Hematologic–decreases in hemoglobin and hematocrit, thrombocytopenia, increases in platelet count, eosinophilia, leukopenia, neutropenia. These patients were withdrawn from therapy; some had accompanying systemic symptoms (e.g., fever, rigors, chills).
- Coagulation–positive direct Coombs’ test, prolonged prothrombin time, prolonged partial thromboplastin time.
- Hepatic–transient elevations of AST (SGOT), ALT (SGPT), alkaline phosphatase, bilirubin.
- Renal–increases in serum creatinine, blood urea nitrogen
- Additional laboratory events include abnormalities in electrolytes (i.e., increases and decreases in sodium, potassium, and calcium), hyperglycemia, decreases in total protein or albumin, blood glucose decreased, gamma-glutamyltransferase increased, hypokalemia, and bleeding time prolonged.
Postmarketing Experience
- In addition to the adverse drug reactions identified in clinical trials in Table 3 and Table 4, the following adverse reactions have been identified during postapproval use of piperacillin and tazobactam for injection.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish causal relationship to drug exposure.
- Gastrointestinal
- Hematologic
- Immune
- Hypersensitivity reactions, anaphylactic/anaphylactoid reactions (including shock)
- Renal
- Skin and Appendages
Additional Experience with Piperacillin
- The following adverse reaction has also been reported for piperacillin for injection:
- Skeletal–prolonged muscle relaxation.
- Post-marketing experience with piperacillin and tazobactam for injection in pediatric patients suggests a similar safety profile to that seen in adults.
Drug Interactions
Aminoglycosides
- Piperacillin may inactivate aminoglycosides by converting them to microbiologically inert amides.
- In vivo inactivation:
- When aminoglycosides are administered in conjunction with piperacillin to patients with end-stage renal disease requiring hemodialysis, the concentrations of the aminoglycosides (especially tobramycin) may be significantly reduced and should be monitored.
- Sequential administration of piperacillin and tazobactam for injection and tobramycin to patients with either normal renal function or mild to moderate renal impairment has been shown to modestly decrease serum concentrations of tobramycin but no dosage adjustment is considered necessary.
- In vitro inactivation:
- Due to the in vitro inactivation of aminoglycosides by piperacillin, piperacillin and tazobactam for injection and aminoglycosides are recommended for separate administration. Piperacillin and tazobactam for injection and aminoglycosides should be reconstituted, diluted, and administered separately when concomitant therapy with aminoglycosides is indicated. Piperacillin and tazobactam for injection is compatible with amikacin and gentamicin for simultaneous Y-site infusion in certain diluents and at specific concentrations. Piperacillin and tazobactam for injection is not compatible with tobramycin for simultaneous Y-site infusion.
Probenecid
- Probenecid administered concomitantly with piperacillin and tazobactam for injection prolongs the half-life of piperacillin by 21% and that of tazobactam by 71% because probenecid inhibits tubular renal secretion of both piperacillin and tazobactam. Probenecid should not be co-administered with piperacillin and tazobactam for injection unless the benefit outweighs the risk.
Anticoagulants
- Coagulation parameters should be tested more frequently and monitored regularly during simultaneous administration of high doses of heparin, oral anticoagulants, or other drugs that may affect the blood coagulation system or the thrombocyte function.
Vecuronium
- Piperacillin when used concomitantly with vecuronium has been implicated in the prolongation of the neuromuscular blockade of vecuronium. Piperacillin and tazobactam for injection could produce the same phenomenon if given along with vecuronium. Due to their similar mechanism of action, it is expected that the neuromuscular blockade produced by any of the non-depolarizing muscle relaxants could be prolonged in the presence of piperacillin. Monitor for adverse reactions related to neuromuscular blockade.
Methotrexate
- Limited data suggests that co-administration of methotrexate and piperacillin may reduce the clearance of methotrexate due to competition for renal secretion. The impact of tazobactam on the elimination of methotrexate has not been evaluated. If concurrent therapy is necessary, serum concentrations of methotrexate as well as the signs and symptoms of methotrexate toxicity should be frequently monitored.
Effects on Laboratory Tests
- There have been reports of positive test results using the Bio-Rad Laboratories Platelia Aspergillus EIA test in patients receiving piperacillin/tazobactam injection who were subsequently found to be free of Aspergillus infection. Cross-reactions with non-Aspergillus polysaccharides and polyfuranoses with the Bio-Rad Laboratories Platelia Aspergillus EIA test have been reported. Therefore, positive test results in patients receiving piperacillin/tazobactam should be interpreted cautiously and confirmed by other diagnostic methods.
- As with other penicillins, the administration of piperacillin and tazobactam for injection may result in a false-positive reaction for glucose in the urine using a copper-reduction method (CLINITEST®). It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
Use in Specific Populations
Pregnancy
Piperacillin/tazobactam
- Teratology studies have been performed in mice and rats and have revealed no evidence of harm to the fetus when piperacillin/tazobactam is administered intravenously up to a dose of 3000/750 mg/kg piperacillin/tazobactam which is 1 to 2 times and 2 to 3 times the human dose of piperacillin and tazobactam, respectively, based on body-surface area (mg/m2).
- Piperacillin and tazobactam cross the placenta in humans.
- There are, however, no adequate and well-controlled studies with the piperacillin/tazobactam combination or with piperacillin or tazobactam alone in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Piperacillin-Tazobactam in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Piperacillin-Tazobactam during labor and delivery.
Nursing Mothers
- Piperacillin is excreted in low concentrations in human milk; tazobactam concentrations in human milk have not been studied. Caution should be exercised when piperacillin and tazobactam for injection is administered to a nursing woman.
Pediatric Use
- Use of piperacillin and tazobactam for injection in pediatric patients 2 months of age or older with appendicitis and/or peritonitis is supported by evidence from well-controlled studies and pharmacokinetic studies in adults and in pediatric patients. This includes a prospective, randomized, comparative, open-label clinical trial with 542 pediatric patients 2 to 12 years of age with complicated intra-abdominal infections, in which 273 pediatric patients received piperacillin/tazobactam. Safety and efficacy in pediatric patients less than 2 months of age have not been established.
- It has not been determined how to adjust piperacillin and tazobactam for injection dosage in pediatric patients with renal impairment.
Geriatic Use
- Patients over 65 years are not at an increased risk of developing adverse effects solely because of age. However, dosage should be adjusted in the presence of renal impairment.
- In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- Piperacillin and tazobactam for injection contains 54 mg (2.35 mEq) of sodium per gram of piperacillin in the combination product. At the usual recommended doses, patients would receive between 648 and 864 mg/day (28.2 and 37.6 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Piperacillin-Tazobactam with respect to specific gender populations.
Race
There is no FDA guidance on the use of Piperacillin-Tazobactam with respect to specific racial populations.
Renal Impairment
- In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of piperacillin and tazobactam for injection should be reduced to the degree of renal function impairment
Hepatic Impairment
- Dosage adjustment of piperacillin and tazobactam for injection is not warranted in patients with hepatic cirrhosis
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Piperacillin-Tazobactam in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Piperacillin-Tazobactam in patients who are immunocompromised.
Cystic Fibrosis
As with other semisynthetic penicillins, piperacillin therapy has been associated with an increased incidence of fever and rash in cystic fibrosis patients
Administration and Monitoring
Administration
Monitoring
- Monitor hematologic tests during prolonged therapy.
- Monitor for adverse reactions related to neuromuscular blockade.
- Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
IV Compatibility
There is limited information regarding IV Compatibility of Piperacillin-Tazobactam in the drug label.
Overdosage
- There have been postmarketing reports of overdose with piperacillin/tazobactam. The majority of those events experienced, including nausea, vomiting, and diarrhea, have also been reported with the usual recommended dosages. Patients may experience neuromuscular excitability or convulsions if higher than recommended doses are given intravenously (particularly in the presence of renal failure).
- Treatment should be supportive and symptomatic according the patient’s clinical presentation. Excessive serum concentrations of either piperacillin or tazobactam may be reduced by hemodialysis. Following a single 3.375 g dose of piperacillin/tazobactam, the percentage of the piperacillin and tazobactam dose removed by hemodialysis was approximately 31% and 39%, respectively.
Pharmacology
There is limited information regarding Piperacillin-Tazobactam Pharmacology in the drug label.
Mechanism of Action
- Piperacillin and tazobactam for injection USP is an antibacterial drug
Structure
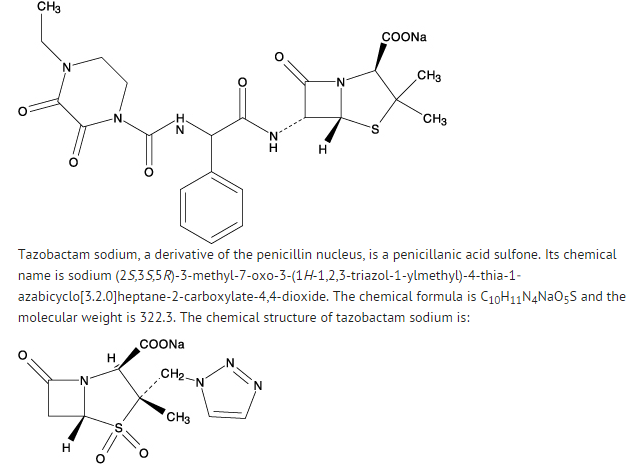
- Piperacillin and tazobactam for injection USP is an injectable antibacterial combination product consisting of the semisynthetic antibacterial piperacillin sodium and the β-lactamase inhibitor tazobactam sodium for intravenous administration.
- Piperacillin sodium is derived from D(-)-α-aminobenzyl-penicillin. The chemical name of piperacillin sodium is sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2carboxylate. The chemical formula is C23H26N5NaO7S and the molecular weight is 539.5. The chemical structure of piperacillin sodium is:
- Piperacillin and tazobactam for injection USP, is a white to yellowish sterile, cryodesiccated powder consisting of piperacillin and tazobactam as their sodium salts packaged in glass vials. The product does not contain excipients or preservatives. Dilute solutions are colorless to yellowish.
- Each piperacillin and tazobactam for injection USP 2.25 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 g of tazobactam. Each vial contains 4.69 mEq (108 mg) of sodium.
- Each piperacillin and tazobactam for injection USP 3.375 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 g of tazobactam. Each vial contains 7.04 mEq (162 mg) of sodium.
- Each piperacillin and tazobactam for injection USP 4.5 g single dose vial contains an amount of drug sufficient for withdrawal of piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 g of tazobactam. Each vial contains 9.39 mEq (216 mg) of sodium.
- Piperacillin and tazobactam for injection USP contains a total of 2.35 mEq (54 mg) of sodium per gram of piperacillin in the combination product.
Pharmacodynamics
- The pharmacodynamic parameter for piperacillin/tazobactam that is most predictive of clinical and microbiological efficacy is time above MIC.
Pharmacokinetics
There is limited information regarding Piperacillin-Tazobactam Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term carcinogenicity studies in animals have not been conducted with piperacillin/tazobactam, piperacillin, or tazobactam.
Piperacillin/Tazobactam
- Piperacillin/tazobactam was negative in microbial mutagenicity assays, the unscheduled DNA synthesis (UDS) test, a mammalian point mutation (Chinese hamster ovary cell HPRT) assay, and a mammalian cell (BALB/c-3T3) transformation assay. In vivo, piperacillin/tazobactam did not induce chromosomal aberrations in rats.
Piperacillin/Tazobactam
- Reproduction studies have been performed in rats and have revealed no evidence of impaired fertility when piperacillin/tazobactam is administered intravenously up to a dose of 1280/320 mg/kg piperacillin/tazobactam, which is similar to the maximum recommended human daily dose based on body-surface area (mg/m2).
Clinical Studies
There is limited information regarding Piperacillin-Tazobactam Clinical Studies in the drug label.
How Supplied
- Piperacillin and tazobactam for injection USP is supplied as single-dose vials in the following sizes:
- Each piperacillin and tazobactam for injection USP 2.25 g vial provides piperacillin sodium equivalent to 2 grams of piperacillin and tazobactam sodium equivalent to 0.25 g of tazobactam. Each vial contains 4.69 mEq (108 mg) of sodium.
- Supplied 10 per box – NDC 0781-3110-95
- Each piperacillin and tazobactam for injection USP 3.375 g single-dose vial provides piperacillin sodium equivalent to 3 grams of piperacillin and tazobactam sodium equivalent to 0.375 g of tazobactam. Each vial contains 7.04 mEq (162 mg) of sodium.
- Supplied 10 per box – NDC 0781-3113-95
- Each piperacillin and tazobactam for injection USP 4.5 g single-dose vial provides piperacillin sodium equivalent to 4 grams of piperacillin and tazobactam sodium equivalent to 0.5 g of tazobactam. Each vial contains 9.39 mEq (216 mg) of sodium.
- Supplied 10 per box – NDC 0781-3114-95
Storage
- Piperacillin and tazobactam for injection, USP vials should be stored at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] prior to reconstitution.
Images
Drug Images
{{#ask: Page Name::Piperacillin-Tazobactam |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Piperacillin-Tazobactam |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Piperacillin-Tazobactam Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Piperacillin/tazobactam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PIPERACILLIN AND TAZOBACTAM®[1]
Look-Alike Drug Names
There is limited information regarding Piperacillin-Tazobactam Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Piperacillin-Tazobactam |Label Name=Piper T 05.jpg
}}
{{#subobject:
|Label Page=Piperacillin-Tazobactam |Label Name=Piper T 06.jpg
}}
{{#subobject:
|Label Page=Piperacillin-Tazobactam |Label Name=Piper T 07.jpg
}}
{{#subobject:
|Label Page=Piperacillin-Tazobactam |Label Name=Piper T 08.png
}}