Nuclear pore
Nuclear pores are large protein complexes that cross the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are about on average 2000 nuclear pore complexes in the nuclear envelope of a vertebrate cell, but it varies depending on the number of transcriptions of the cell. The proteins that make up the nuclear pore complex are known as nucleoporins. About half of the nucleoporins typically contain either an alpha solenoid or a beta-propeller fold, or in some cases both as separate structural domains. The other half exhibit structural characteristics typical of "natively unfolded" proteins, i.e. they are highly flexible proteins that lack ordered secondary structure.[1] These disordered proteins are the FG nucleoporins, so called because their amino-acid sequence contains many repeats of the peptide phenylalanine—glycine.[2]
Nuclear pores allow the transport of water-soluble molecules across the nuclear envelope. This transport includes RNA and ribosomes moving from nucleus to the cytoplasm and proteins (such as DNA polymerase and lamins), carbohydrates, signal molecules and lipids moving into the nucleus. It is notable that the nuclear pore complex (NPC) can actively conduct 1000 translocations per complex per second. Although smaller molecules simply diffuse through the pores, larger molecules may be recognized by specific signal sequences and then be diffused with the help of nucleoporins into or out of the nucleus. This is known as the RAN cycle. Each of the eight protein subunits surrounding the actual pore (the outer ring) projects a spoke-shaped protein into the pore channel. The center of the pore often appears to contains a plug-like structure. It is yet unknown whether this corresponds to an actual plug or is merely cargo caught in transit.
Size and complexity
The entire pore complex has a diameter of about 120 nm, the diameter of the opening is about 50 nm wide and its "depth" is about 200 nm.[citation needed] The molecular mass of the NPC is about 125 million dalton and contains approximately 50 different proteins components.
Transport through the nuclear pore complex
Small particles (< 50 kDa) are able to pass through the nuclear pore complex by passive diffusion. Larger particles are also able to pass through the large diameter of the pore but at almost negligible rates.[3] Efficient passage through the complex requires several protein factors.[4] Karyopherins, which may act as importins or exportins are part of the Importin-β super-family which all share a similar three-dimensional structure.
Three models have been suggested to explain the translocation mechanism:
- Affinity gradients along the central plug
- Brownian affinity gating
- Selective phase
Import of proteins
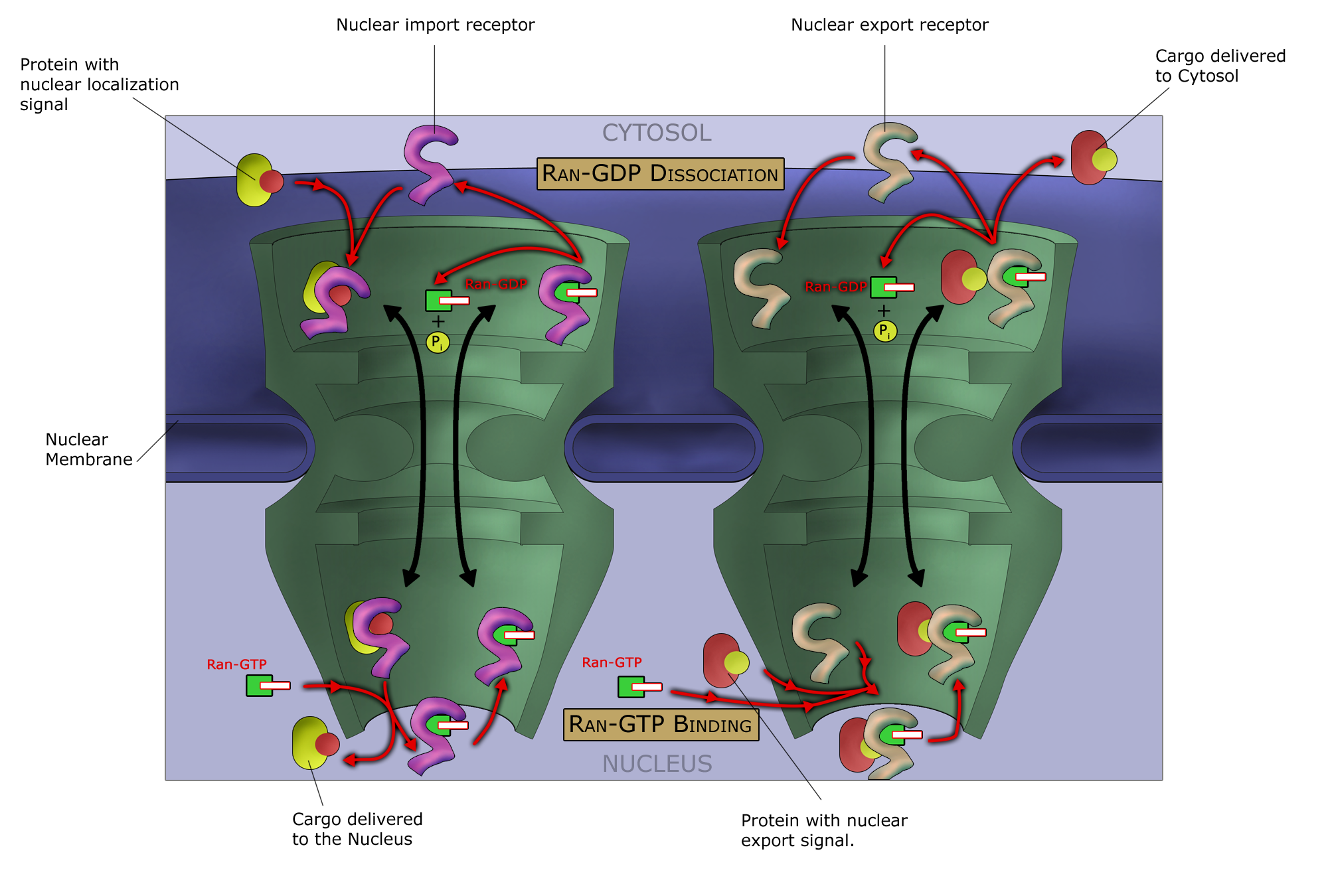
Any cargo with a nuclear localization signal (NLS) exposed will be destined for quick and efficient transport through the pore. Several NLS sequences are known, generally containing a conserved polypeptide sequence with basic residues such as PKKKRKV. Any material with an NLS will be taken up by importins to the nucleus.
The classical scheme of NLS-protein importation begins with Importin-α first binding to the NLS sequence, and acts as a bridge for Importin-β to attach. The importinβ—importinα—cargo complex is then directed towards the nuclear pore and diffuses through it. Once the complex is in the nucleus, RanGTP binds to Importin-β and displaces it from the complex. Then the cellular apoptosis susceptibility protein (CAS), an exportin which in the nucleus is bound to RanGTP, displaces Importin-α from the cargo. The NLS-protein is thus free in the nucleoplasm. The Importinβ-RanGTP and Importinα-CAS-RanGTP complex diffuses back to the cytoplasm where GTPs are hydrolyzed to GDP leading to the release of Importinβ and Importinα which become available for a new NLS-protein import round.
Although cargo passes through the pore with the assistance of chaperone proteins, the translocation through the pore itself is not energy dependent. However, the whole import cycle needs the hydrolysis of 2 GTPs and is thus energy dependent and has to be considered as active transport. The import cycle is powered by the nucleo-cytoplasmic RanGTP gradient. This gradient arises from the exclusive nuclear localization of RanGEFs, proteins that exchange GDP to GTP on Ran molecules. Thus there is an elevated RanGTP concentration in the nucleus compared to the cytoplasm.
Export of proteins
Some nuclear proteins need to be exported from the nucleus to the cytoplasm, as do ribosome subunits and messenger RNAs. Thus there is an export mechanism similar to the import mechanism.
In the classical export scheme, proteins with an nuclear export sequence (NES) can bind in the nucleus to form a heterotrimeric complex with an exportin and RanGTP (for example the exportin CRM1). The complex can then diffuse to the cytoplasm where GTP is hydrolysed and the NES-protein is released. CRM1-RanGDP diffuses back to the nucleus where GDP is exchanged to GTP by RanGEFs. This process is also energy dependent as it consumes one GTP. Export with the exportin CRM1 can be inhibited by Leptomycin B.
Export of RNA
Different export pathways from NPC for each RNA class exist. RNA export is also signal mediated (NES), the NES is in RNA-binding proteins (except for tRNA which has no adapter). It is notable that all viral RNAs and cellular RNAs (tRNA, rRNA, U snRNA, microRNA) except mRNA are dependent on RanGTP. Conseved mRNA export factors are necessary for mRNA nuclear export. Export factors are Mex67/Tap (large subunit) and Mtr2/p15 (small subunit). An adapter binds to the large export factor subunit mediating the export process.
Additional images
-
The Ran-GTP cycle
References
- ↑ Denning D, Patel S, Uversky V, Fink A, Rexach M (2003). "Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded". Proc Natl Acad Sci U S A. 100 (5): 2450–5. PMID 12604785.
- ↑ Peters R (2006). "Introduction to nucleocytoplasmic transport: molecules and mechanisms". Methods Mol Biol. 322: 235–58. PMID 16739728.
- ↑ Rodriguez M, Dargemont C, Stutz F (2004). "Nuclear export of RNA". Biol Cell. 96 (8): 639–55. PMID 15519698.
- ↑ Reed R, Hurt E (2002). "A conserved mRNA export machinery coupled to pre-mRNA splicing". Cell. 108 (4): 523–31. PMID 11909523.
External links
- Histology image: 20104loa – Histology Learning System at Boston University
- Nuclear+pore at the US National Library of Medicine Medical Subject Headings (MeSH)
- Nuclear Pore Complex animations
- Nuclear Pore Complex illustrations
de:Kernpore eo:Nuklea poro sr:Комплекс нуклеусне поре fi:Tumahuokonen