Laquinimod
| |
| Names | |
|---|---|
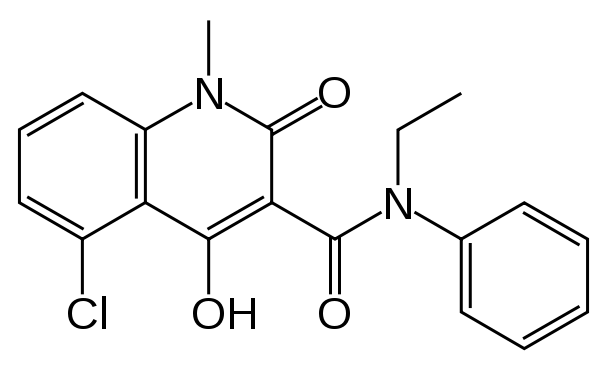
| IUPAC names
5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-
N-phenyl-1,2-dihydroquinoline-3-carboxamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H17ClN2O3 | |
| Molar mass | 356.803 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Laquinimod |
|
Articles |
|---|
|
Most recent articles on Laquinimod |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Laquinimod at Clinical Trials.gov Clinical Trials on Laquinimod at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Laquinimod
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Laquinimod Discussion groups on Laquinimod Patient Handouts on Laquinimod Directions to Hospitals Treating Laquinimod Risk calculators and risk factors for Laquinimod
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Laquinimod |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Laquinimod is an experimental immunomodulator developed by Active Biotech and Teva. It is being investigated as an oral treatment for multiple sclerosis (MS).
Laquinimod is the successor of Active Biotech's failed experimental immunomodulator linomide.[1]
The compound has been investigated in two Phase II trials using successive magnetic resonance scans (MRI). Laquinimod seems to be able to reduce the MS disease activity on MRI.[2][3] However, the response to a given dose was discrepant between both studies.[4]
Phase III studies for MS started in December 2007.[5] In 2011, Teva announced its clinical trials involving laquinimod had failed, being unable to significantly reduce relapses into MS among patients beyond a placebo.[6] However, the final results of above-mentioned phase III trial proved oral laquinimod administered once daily slowed the progression of disability and reduced the rate of relapse in patients with relapsing–remitting multiple sclerosis.[7]
See also
- Fingolimod, a marketed drug with the same mechanism of action
- Ponesimod, an experimental drug with the same mechanism
References
- ↑ Tan IL; Lycklama à Nijeholt GJ; Polman CH; et al. (April 2000). "Linomide in the treatment of multiple sclerosis: MRI results from prematurely terminated phase-III trials". Mult Scler. 6 (2): 99–104. doi:10.1191/135245800678827626. PMID 10773855. Unknown parameter
|author-separator=ignored (help) - ↑ Comi G; Pulizzi A; Rovaris M; et al. (June 2008). "Effect of laquinimod on MRI-monitored disease activity in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study". Lancet. 371 (9630): 2085–2092. doi:10.1016/S0140-6736(08)60918-6. PMID 18572078. Unknown parameter
|author-separator=ignored (help) - ↑ Polman C; Barkhof F; Sandberg-Wollheim M; et al. (March 2005). "Treatment with laquinimod reduces development of active MRI lesions in relapsing MS". Neurology. 64 (6): 987–91. doi:10.1212/01.WNL.0000154520.48391.69. PMID 15781813. Unknown parameter
|author-separator=ignored (help) - ↑ Keegan BM, Weinshenker BG (June 2008). "Laquinimod, a new oral drug for multiple sclerosis". Lancet. 371 (9630): 2059–2060. doi:10.1016/S0140-6736(08)60894-6. PMID 18572062.
- ↑ Clinical trial number NCT00509145 for "Safety and Efficacy of Orally Administered Laquinimod Versus Placebo for Treatment of Relapsing Remitting Multiple Sclerosis (RRMS) (ALLEGRO)" at ClinicalTrials.gov
- ↑ Kresege, Naomi (1 August 2011). "Teva's Copaxone Successor Fails in Latest Clinical Trial". Bloomberg. Retrieved 2 August 2011.
Teva Pharmaceutical Industries Ltd. (TEVA)’s experimental multiple sclerosis pill failed to reduce relapses more than placebo in a clinical trial, dealing a blow to the company’s effort to find a successor to an older drug.
- ↑ Template:Cite doi
External links
|
Multiple sclerosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Laquinimod On the Web |
|
American Roentgen Ray Society Images of Laquinimod |