Carnitine
 | |
| Clinical data | |
|---|---|
| Routes of administration | oral and iv |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | < 10% |
| Protein binding | None |
| Metabolism | slightly |
| Excretion | Urine (> 95%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
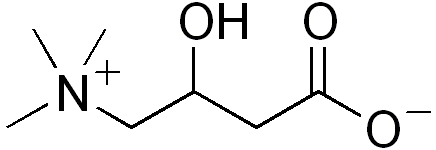
| Formula | C7H15NO3 |
| Molar mass | 161.199 g/mol |
|
WikiDoc Resources for Carnitine |
|
Articles |
|---|
|
Most recent articles on Carnitine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Carnitine at Clinical Trials.gov Clinical Trials on Carnitine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Carnitine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Carnitine Discussion groups on Carnitine Directions to Hospitals Treating Carnitine Risk calculators and risk factors for Carnitine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Carnitine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Carnitine, also known as L-carnitine or levocarnitine, is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine.[1] It helps in the consumption and disposal of fat in the body because it is responsible for the transport of fatty acids from the cytosol into the mitochondria. It is often sold as a nutritional supplement. Carnitine was originally found as a growth factor for mealworms and labeled vitamin Bt. Natural carnitine is the L-stereoisomer.
Production
Carnitine is biosynthesized within the body from the amino acids lysine or methionine primarily in the liver and kidneys.[2] Vitamin C (ascorbic acid) is essential to the synthesis of carnitine. It has been speculated that during growth or pregnancy the requirement of carnitine could exceed its natural production.
Role in fatty acid metabolism
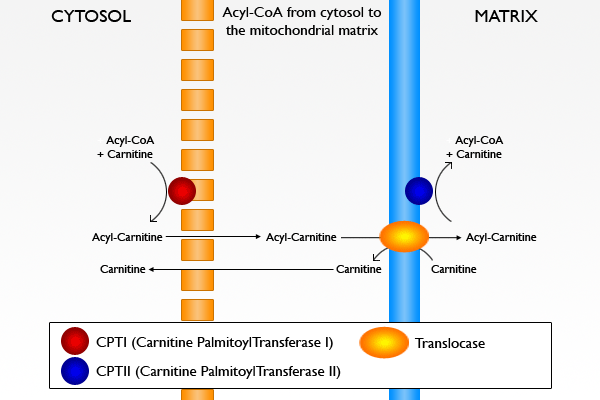
Carnitine transports long-chain acyl groups from fatty acids into the mitochondrial matrix, so that they can be broken down through beta-oxidation to acetate to obtain usable energy via the citric acid cycle. In some organisms such as fungi, the acetate is used in the glyoxylate cycle for gluconeogenesis and formation of carbohydrates. Fatty acids must be activated before binding to the carnitine molecule to form acyl-carnitine. The free fatty acid in the cytosol is attached with a thioester bond to coenzyme A (CoA). This reaction is catalyzed by the enzyme fatty acyl-CoA synthetase and driven to completion by inorganic pyrophosphatase.
The acyl group on CoA can now be transferred to carnitine and the resulting acyl-carnitine transported into the mitochondrial matrix. This occurs via a series of similar steps:
- Acyl-CoA is conjugated to carnitine by carnitine acyltransferase I (palmitoyltransferase) located on the outer mitochondrial membrane
- Acyl-carnitine is shuttled inside by a carnitine-acylcarnitine translocase
- Acyl-carnitine is converted to acyl-CoA by carnitine acyltransferase II (palmitoyltransferase) located on the inner mitochondrial membrane. The liberated carnitine returns to the cytosol.
Dysfunction of this process leads to the genetic disorders primary carnitine deficiency, carnitine palmitoyltransferase I deficiency, carnitine palmitoyltransferase II deficiency and carnitine-acylcarnitine translocase deficiency.[3]
Carnitine acyltransferase I undergoes allosteric inhibition as a result of malonyl-CoA, an intermediate in fatty acid biosynthesis, in order to prevent futile cycling between beta-oxidation and fatty acid synthesis.
Natural sources
The highest concentrations of carnitine are found in red meat and dairy products. Other natural sources of carnitine include nuts and seeds (e.g. pumpkin, sunflower, sesame), legumes or pulses (beans, peas, lentils, peanuts), vegetables (artichokes, asparagus, beet greens, broccoli, brussels sprouts, collard greens, garlic, mustard greens, okra, parsley), fruits (apricots, bananas), cereals (buckwheat, corn, millet, oatmeal, rice bran, rye, whole wheat, wheat bran, wheat germ) and other 'health' foods (bee pollen, brewer's yeast, carob, and kale).
| Product | Quantity | Carnitine |
| Beef Steak | 3.5 oz | 95 mg |
| Ground Beef | 3.5 oz | 94 mg |
| Pork | 3.5 oz | 27.7 mg |
| Bacon | 3.5 oz | 23.3 mg |
| Cod Fish | 3.5 oz | 5.6 mg |
| Chicken Breast | 3.5 oz | 3.9 mg |
| American Cheese | 3.5 oz | 3.7 mg |
| Ice Cream | 3.5 fl oz | 3.7 mg |
| Whole Milk | 3.5 fl oz | 3.3 mg |
| Cottage Cheese | 3.5 fl oz | 1.1 mg |
| Whole Wheat Bread | 3.5 oz | 0.36 mg |
| Asparagus | 3.5 oz | 0.195 mg |
| White Bread | 3.5 oz | 0.147 mg |
| Macaroni | 3.5 oz | 0.126 mg |
| Peanut Butter | 3.5 oz | 0.083 mg |
| Rice (cooked) | 3.5 oz | 0.0449 mg |
| Eggs | 3.5 oz | 0.0121 mg |
| Orange Juice | 3.5 fl oz | 0.0019 mg |
Other sources
Other sources may be found in over the counter vitamins, energy drinks and various other products. Products containing L-carnitine cannot be marketed as "natural health products" in Canada. L-Carnitine products and supplements are not allowed to be imported into Canada(Health Canada).[4]
Effects on diabetes
L-Carnitine improved glucose disposal among 15 patients with type II diabetes and 20 healthy volunteers.[5] Glucose storage increased between both groups, but glucose oxidation increased only in the diabetic group. Finally, glucose uptake increased about 8% for both.
See also
References
- ↑ Steiber A, Kerner J, Hoppel C (2004). "Carnitine: a nutritional, biosynthetic, and functional perspective". Mol. Aspects Med. 25 (5–6): 455–73. PMID 15363636.
- ↑ "L-Carnitine". Retrieved 2007-06-01.
- ↑ Olpin S (2005). "Fatty acid oxidation defects as a cause of neuromyopathic disease in infants and adults". Clin. Lab. 51 (5–6): 289–306. PMID 15991803.
- ↑ "NHPD Monthly Communique, Vol. 1, Issue 1, September 2005". Retrieved 2007-06-01.
- ↑ Geltrude Mingrone, Aldo V. Greco, Esmeralda Capristo, Giuseppe Benedetti, Annalisa Giancaterini, Andrea De Gaetano, and Giovanni Gasbarrini (1999). "L-Carnitine Improves Glucose Disposal in Type 2 Diabetic Patients". Journal of the American College of Nutrition. 18 (1): 77–82.
External links
- article on Carnitine at University of Maryland Medical Center
- Molecule of the Month at University of Bristol
Template:Other alimentary tract and metabolism products
cs:Karnitin de:Carnitin eo:Karnitino fa:کارنیتین it:Carnitina nl:Carnitine sv:Carnitin
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Hydroxy acids
- Quaternary ammonium compounds
- Dietary supplements
- Amino acids
- Inborn errors of metabolism