Beta oxidation
|
WikiDoc Resources for Beta oxidation |
|
Articles |
|---|
|
Most recent articles on Beta oxidation Most cited articles on Beta oxidation |
|
Media |
|
Powerpoint slides on Beta oxidation |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Beta oxidation at Clinical Trials.gov Trial results on Beta oxidation Clinical Trials on Beta oxidation at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Beta oxidation NICE Guidance on Beta oxidation
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Beta oxidation Discussion groups on Beta oxidation Patient Handouts on Beta oxidation Directions to Hospitals Treating Beta oxidation Risk calculators and risk factors for Beta oxidation
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Beta oxidation |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in the mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Krebs Cycle.
Occurs in mitochondrial matrix.
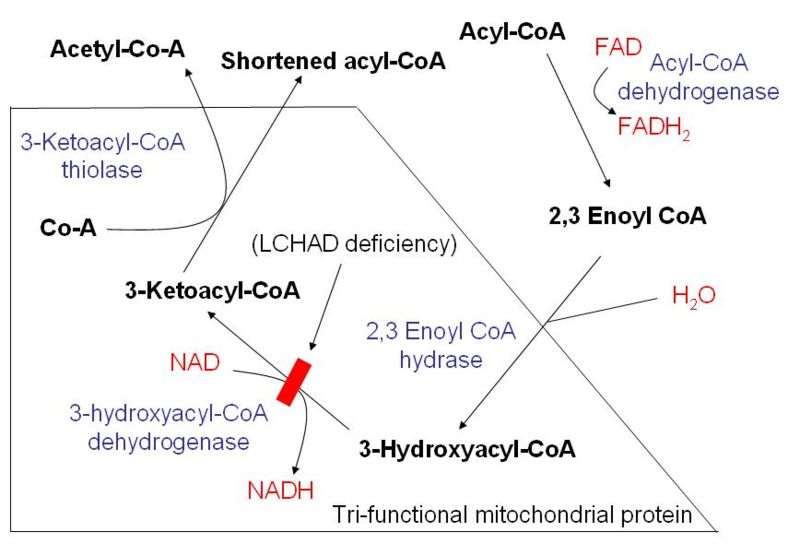
Four recurring steps
Once inside the mitochondria, the β-oxidation of fatty acids occurs via four recurring steps:
| Description | Diagram | Enzyme | End product |
| Oxidation by FAD: The first step is the oxidation of the fatty acid by Acyl-CoA-Dehydrogenase. The enzyme catalyzes the formation of a double bond between the C-2 and C-3. |  |
acyl CoA dehydrogenase | trans-Δ2-enoyl-CoA |
| Hydration: The next step is the hydration of the bond between C-2 and C-3. The reaction is stereospecific, forming only the L isomer. |  |
enoyl CoA hydratase | L-3-hydroxyacyl CoA |
| Oxidation by NAD+: The third step is the oxidation of L-3-hydroxyacyl CoA by NAD+. This converts the hydroxyl group into a keto group. | 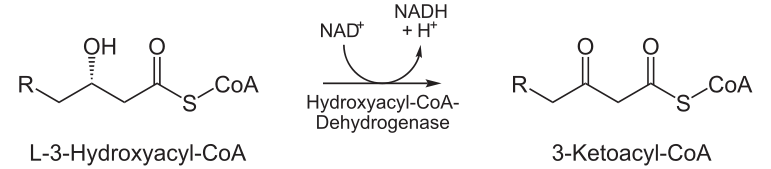 |
L-3-hydroxyacyl CoA dehydrogenase | 3-ketoacyl CoA |
| Thiolysis: The final step is the cleavage of 3-ketoacyl CoA by the thiol group of another molecule of CoA. The thiol is inserted between C-2 and C-3. | 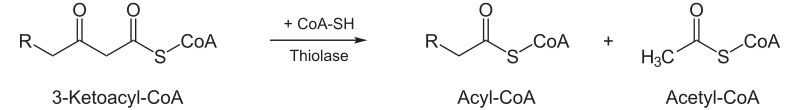 |
Β-ketothiolase | An acetyl CoA molecule, and an acyl CoA molecule, which is two carbons shorter |
This process continues until the entire chain is cleaved into acetyl CoA units. For every cycle, the Acyl CoA unit is shortened by two carbon atoms. Concomitantly, one molecule of FADH2, NADH and acetyl CoA are formed.
β-oxidation of unsaturated fatty acids
β-oxidation of unsaturated fatty acids poses a problem since the location of a cis bond can prevent the formation of a trans-δ2 bond. These situations are handled by an additional two enzymes.
Whatever the conformation of the hydrocarbon chain, β-oxidation occurs normally until the acyl CoA (because of the presence of a double bond) is not an appropriate substrate for acyl CoA dehydrogenase, or enoyl CoA hydratase:
- If the acyl CoA contains a cis-Δ3 bond, then cis-δ3-Enoyl CoA isomerase will convert the bond to a trans-Δ2 bond, which is a regular substrate.
- If the acyl CoA contains a cis-Δ4 double bond, then its dehydrogenation yields a 2,4-dienoyl intermediate, which is not a substrate for enoyl CoA hydratase. However, the enzyme 2,4 Dienoyl CoA reductase reduces the intermediate, using NADPH, into trans-Δ3-enoyl CoA. As in the above case, this compound is converted into a suitable intermediate by cis-Δ3-Enoyl CoA isomerase.
To summarize:
- odd numbered double bonds are handled by the isomerase.
- even numbered bonds by the reductase (which creates an odd numbered double bond) and the isomerase.
β-oxidation of odd-numbered chains
Chains with an odd-number of carbons are oxidized in the same manner as even-numbered chains, but the final products are propionyl CoA and acetyl CoA.
Propionyl CoA is converted into succinyl CoA (which is an intermediate in the citric acid cycle) in a reaction that involves Vitamin B12. Succinyl CoA can then enter the citric acid cycle.
Because it cannot be completely metabolized in the citric acid cycle, the products of its partial reaction must be removed in a process called cataplerosis. This allows regeneration of the citric acid cycle intermediates, possibly an important process in certain metabolic diseases.
Oxidation in peroxisomes
Fatty acid oxidation also occurs in peroxisomes. However, the oxidation ceases at octanyl CoA.
One significant difference is that oxidation in peroxisomes is not coupled to ATP synthesis. Instead, the high-potential electrons are transferred to O2, which yields H2O2. The enzyme catalase, found exclusively in peroxisomes, converts the hydrogen peroxide into water and oxygen. It is believed that very long chain (C-22)fatty acids undergo initial oxidation in peroxisomes which is followed by mitochondrial oxidation. Peroxisomal oxidation is induced by high fat diet and administration of hypolipidemic drugs like clofibrate.
Energy yield
The ATP yield for every oxidation cycle is 14 ATP, broken down as follows:
| Source | ATP | Total |
| 1 FADH2 | x 1.5 ATP | = 1.5 ATP (some sources say 2 ATP) |
| 1 NADH | x 2.5 ATP | = 2.5 ATP (some sources say 3 ATP) |
| 1 acetyl CoA | x 10 ATP | = 10 ATP (some sources say 12 ATP) |
| TOTAL | = 14 ATP |
For an even-numbered saturated fat (C2n), n - 1 oxidations are necessary and the final process yields an additional acetyl CoA. In addition, two equivalents of ATP are lost during the activation of the fatty acid. Therefore, the total ATP yield can be stated as:
- (n - 1) * 14 + 10 - 2 = total ATP
For instance, the ATP yield of palmitate (C16, n = 8) is:
- (8 - 1) * 14 + 10 - 2 = 106 ATP
Represented in table form:
| Source | ATP | Total |
| 7 FADH2 | x 1.5 ATP | = 10.5 ATP |
| 7 NADH | x 2.5 ATP | = 17.5 ATP |
| 8 acetyl CoA | x 10 ATP | = 80 ATP |
| Activation | = -2 ATP | |
| TOTAL | = 106 ATP |
For sources that use the larger ATP production numbers described above, the total would be 129 ATP equivalents per palmitate.
See also
External links
- The chemical logic behind fatty acid metabolism at ufp.pt
- JEREMY M. BERG,JOHN L. TYMOCZKO and LUBERT STRYER Biochemistry, 2002
- Animations at brookscole.com
de:Β-Oxidation it:Beta ossidazione lb:Beta Oxydatioun sv:Beta-oxidation