Asparagine
|
WikiDoc Resources for Asparagine |
|
Articles |
|---|
|
Most recent articles on Asparagine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Asparagine at Clinical Trials.gov Clinical Trials on Asparagine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Asparagine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Asparagine Discussion groups on Asparagine Patient Handouts on Asparagine Directions to Hospitals Treating Asparagine Risk calculators and risk factors for Asparagine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Asparagine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Asparagine (abbreviated as Asn or N; Asx or B represent either asparagine or aspartic acid) is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side chain's functional group. It is considered a non-essential amino acid.
A reaction between asparagine and reducing sugars or reactive carbonyls produces acrylamide (acrylic amide) in food when heated to sufficient temperature, i.e. baking. These occur primarily in baked goods such as french fries, potato chips, and roasted coffee.
Its codons are AAU and AAC.[1]
History
Asparagine was first isolated in 1806 from asparagus juice, in which it is abundant -- hence its name -- becoming the first amino acid to be isolated. The characteristic smell observed in the urine of individuals after their consumption of asparagus is attributed to various metabolic byproducts of asparagine: in 1891, Marceli Nencki claimed that the substance responsible was methanethiol, and Robert White's 1975 research indicated that the substances were various thioesters. Other likely possibilities include asparagine aminosuccinic monoamide. Allison and McWhirter's 1956 research[2] indicated that some individuals do not produce this odor after asparagus consumption, and that this is autosomal; however, a re-examination in 1980 showed that these individuals are, rather, not able to detect the odor.
Structural function in proteins
Since the asparagine side chain can make efficient hydrogen bond interactions with the peptide backbone, asparagines are often found near the beginning and end of alpha-helices, and in turn motifs in beta sheets. Its role can be thought as "capping" the hydrogen bond interactions which would otherwise need to be satisfied by the polypeptide backbone. Glutamines have an extra methylene group, have more conformational entropy and thus are less useful in this regard.
Asparagine also provides key sites for N-linked glycosylation, modification of the protein chain with the addition of carbohydrate chains.
Sources
Dietary Sources
Asparagine is not an essential amino acid, which means that it can be synthesized from central metabolic pathway intermediates in humans and is not required in the diet. Asparagine is found in:
- Animal sources: dairy, beef, poultry, eggs, fish, lactalbumin, seafood
- Vegetarian sources: asparagus, potatoes, legumes, nuts, seeds, soy, whey, whole grains,
Biosynthesis
The precursor to asparagine is oxaloacetate. Oxaloacetate is converted to aspartate using a transaminase enzyme. The enzyme transfers the amino group from glutamate to oxaloacetate producing α-ketoglutarate and aspartate. The enzyme asparagine synthetase produces asparagine, AMP, glutamate, and pyrophosphate from aspartate, glutamine, and ATP. In the asparagine synthetase reaction, ATP is used to activate aspartate, forming β-aspartyl-AMP. Glutamine donates an ammonium group which reacts with β-aspartyl-AMP to form asparagine and free AMP.
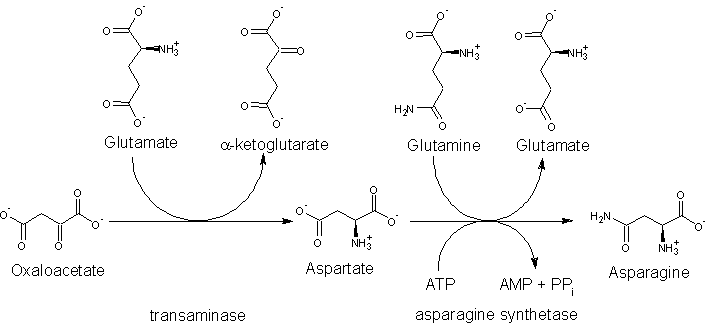
Degradation
Aspartate is a glucogenic amino acid. L-asparaginase hydrolyzes the amide group to form aspartate and ammonium. A transaminase converts the aspartate to oxaloacetate which can then be metabolized in the citric acid cycle or gluconeogenesis.
Function
The nervous system needs asparagine to maintain the equilibrium, as well as in amino acid transformation. It also plays an important role in the synthesis of ammonia.
References
- ↑ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. Retrieved 2007-05-17.
- ↑ ALLISON AC, MCWHIRTER KG (1956). "Two unifactorial characters for which man is polymorphic". Nature. 178 (4536): 748–9. PMID 13369530.
External links
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) |
ca:Asparagina de:Asparagin eo:Asparagino ko:아스파라긴 id:Asparagin it:Asparagina he:אספרגין lv:Asparagīns lb:Asparagin lt:Asparaginas nl:Asparagine sk:Asparagín sr:Аспарагин fi:Asparagiini sv:Asparagin uk:Аспарагін