Hypertrophic cardiomyopathy cardiac catheterization
|
Hypertrophic Cardiomyopathy Microchapters |
|
Differentiating Hypertrophic Cardiomyopathy from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hypertrophic cardiomyopathy cardiac catheterization On the Web |
|
Hypertrophic cardiomyopathy cardiac catheterization in the news |
|
Blogs on Hypertrophic cardiomyopathy cardiac catheterization |
|
Directions to Hospitals Treating Hypertrophic cardiomyopathy |
|
Risk calculators and risk factors for Hypertrophic cardiomyopathy cardiac catheterization |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Left heart catheterization can be a useful diagnostic study to ascertain the severity of the dynamic outflow obstruction and its location.
Left Heart Catheterization
Upon cardiac catheterization, catheters can be placed in the left ventricle and the ascending aorta, to measure the pressure difference between these structures. In normal individuals, during ventricular systole, the pressure in the ascending aorta and the left ventricle will equalize, and the aortic valve is open. In individuals with aortic stenosis or with HCM with an outflow tract gradient, there will be a pressure gradient (difference) between the left ventricle and the aorta, with the left ventricular pressure higher than the aortic pressure. This gradient represents the degree of obstruction that has to be overcome in order to eject blood from the left ventricle.
The Brockenbrough–Braunwald–Morrow sign is observed in individuals with HCM with outflow tract gradient. This sign can be used to differentiate HCM from aortic stenosis. In individuals with aortic stenosis, after a premature ventricular contraction (PVC), the following ventricular contraction will be more forceful, and the pressure generated in the left ventricle will be higher. Because of the fixed obstruction that the stenotic aortic valve represents, the post-PVC ascending aortic pressure will increase as well. In individuals with HCM, however, the degree of obstruction will increase more than the force of contraction will increase in the post-PVC beat. The result of this is that the left ventricular pressure increases and the ascending aortic pressure decreases, with an increase in the LVOT gradient.
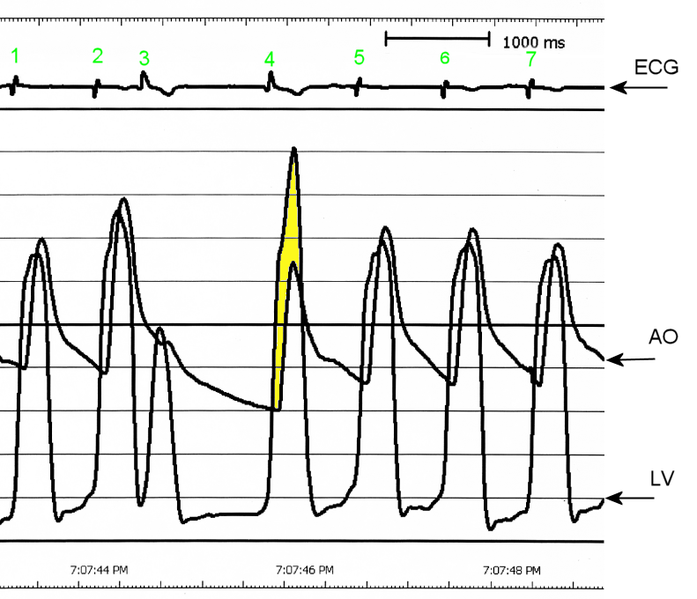
AO = Descending aorta; LV = Left ventricle; ECG = Electrocardiogram.
After the third QRS complex, the ventricle has more time to fill. Since there is more time to fill, the left ventricle will have more volume at the end of diastole (increased preload). Due to the Frank–Starling law of the heart, the contraction of the left ventricle (and pressure generated by the left ventricle) will be greater on the subsequent beat (beat #4 in this picture). Because of the dynamic nature of the outflow obstruction in HCM, the obstruction increases more that the left ventricular pressure increase. This causes a fall in the aortic pressure as the left ventricular pressure rises (seen as the yellow shaded area in the picture).
While the Brockenbrough–Braunwald–Morrow sign is most dramatically demonstrated using simultaneous intra-cardiac and intra-aortic catheters, it can be seen on routine physical examination as a decrease in the pulse pressure in the post-PVC beat in individuals with HCM.
Coronary Angiography
Among patients who have chest discomfort or an anginal equivalent, coronary angiography carries a class I recommendation to evaluate for the presence of obstructive coronary artery disease.
Detection of Concomitant Coronary Disease (DO NOT EDIT)[1]
| Class I |
| "1. Coronary arteriography (invasive or computed tomographic imaging) is indicated in patients with HCM with chest discomfort who have an intermediate to high likelihood of CAD when the identification of concomitant CAD will change management strategies. (Level of Evidence: C) " |
References
- ↑ Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011). "2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 58 (25): e212–60. doi:10.1016/j.jacc.2011.06.011. PMID 22075469. Retrieved 2011-12-19. Unknown parameter
|month=ignored (help)