Hemin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* Hemin for injection should only be used by physicians experienced in the management of porphyrias in hospitals where the recommended clinical and laboratory diagnostic and monitoring techniques are available.
|
Overview
Hemin is an blood modifier agent that is FDA approved for the treatment of acute intermittent porphyria. There is a Black Box Warning for this drug as shown here. Common adverse reactions include phlebitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Hemin for injection is indicated for the amelioration of recurrent attacks of acute intermittent porphyria temporally related to the menstrual cycle in susceptible women.
- Manifestations such as pain, hypertension, tachycardia, abnormal mental status and mild to progressive neurologic signs may be controlled in selected patients with this disorder.
- Similar findings have been reported in other patients with acute intermittent porphyria, porphyria variegata and hereditary coproporphyria. Hemin is not indicated in porphyria cutanea tarda.
Dosing Information
- Before administering Hemin, an appropriate period of alternate therapy (i.e., 400 g glucose/day for 1 to 2 days) must be considered. If improvement is unsatisfactory for the treatment of acute attacks of porphyria, an intravenous infusion of Hemin containing a dose of 1 to 4 mg/kg/day of hematin should be given over a period of 10 to 15 minutes for 3 to 14 days based on the clinical signs. In more severe cases this dose may be repeated no earlier than every 12 hours. No more than 6 mg/kg of hematin should be given in any 24 hour period.
- After reconstitution each mL of Hemin contains the equivalent of approximately 7 mg of hematin. The drug may be administered directly from the vial.

- Since reconstituted Hemin is not transparent, any undissolved particulate matter is difficult to see when inspected visually. Therefore, terminal filtration through a sterile 0.45 micron or smaller filter is recommended.
Preparation of Solution:
- Reconstitute Hemin by aseptically adding 43 mL of Sterile Water for Injection, USP, to the dispensing vial. Immediately after adding diluent, the product should be shaken well for a period of 2 to 3 minutes to aid dissolution.
- NOTE: Because Hemin contains no preservative and because Hemin undergoes rapid chemical decomposition in solution, it should not be reconstituted until immediately before use. After the first withdrawal from the vial, any solution remaining must be discarded.
- No drug or chemical agent should be added to a Hemin fluid admixture unless its effect on the chemical and physical stability has first been determined.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hemin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hemin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and efficacy in pediatric patients under 16 years of age have not been established
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Guideline-Supported off-Label Use of Hemin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hemin in pediatric patients.
Contraindications
- Hemin is contraindicated in patients with known hypersensitivity to this drug.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* Hemin for injection should only be used by physicians experienced in the management of porphyrias in hospitals where the recommended clinical and laboratory diagnostic and monitoring techniques are available.
|
- Hemin is made from human blood. Products made from human blood may contain infectious agents, such as viruses, that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening blood donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating certain viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Recordati Rare Diseases, (1-888-575-8344). The physician should discuss the risks and benefits of this product with the patient.
- Because this product is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
- Hemin therapy is intended to limit the rate of porphyria/heme biosynthesis possibly by inhibiting the enzyme δ-aminolevulinic acid synthetase. For this reason, drugs such as estrogens, barbituric acid derivatives and steroid metabolites which increase the activity of enzyme δ-aminolevulinic acid synthetase should be avoided.
- Also, because hemin for injection has exhibited transient, mild anticoagulant effects during clinical studies, concurrent anticoagulant therapy should be avoided. The extent and duration of the hypocoagulable state induced by Hemin has not been established.
PRECAUTIONS
General
- Clinical benefit from Hemin depends on prompt administration. Attacks of porphyria may progress to a point where irreversible neuronal damage has occurred. Hemin therapy is intended to prevent an attack from reaching the critical stage of neuronal degeneration. Hemin is not effective in repairing neuronal damage.
- Recommended dosage guidelines should be strictly followed. Reversible renal shutdown has been observed in a case where an excessive hematin dose (12.2 mg/kg) was administered in a single infusion. Oliguria and increased nitrogen retention occurred although the patient remained asymptomatic. No worsening of renal function has been seen with administration of recommended dosages of hematin.
- A large arm vein or a central venous catheter should be utilized for the administration of Hemin to avoid the possibility of phlebitis.
- Since reconstituted Hemin is not transparent, any undissolved particulate matter is difficult to see when inspected visually. Therefore, terminal filtration through a sterile 0.45 micron or smaller filter is recommended.
- Because increased levels of iron and serum ferritin have been reported in post-marketing experience, physicians should monitor iron and serum ferritin in patients receiving multiple administrations of Hemin.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Phlebitis with or without leucocytosis and with or without mild pyrexia has occurred after administration of hematin through small arm veins.
Postmarketing Experience
- Reversible renal shutdown has occurred with administration of excessive doses.
- There have been post-marketing literature reports of thrombocytopenia and coagulopathy (including prolonged prothrombin time and prolonged partial thromboplastin time) in patients receiving Hemin.Iron overload and serum ferritin increased have also been reported.
Drug Interactions
There is limited information regarding Drug Interaction of Hemin in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with hematin. It is also not known whether hematin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. For this reason Hemin should not be given to a pregnant woman unless the expected benefits are sufficiently important to the health and welfare of the patient to outweigh the unknown hazard to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hemin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hemin during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Hemin is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients under 16 years of age have not been established.
Geriatic Use
- Clinical studies in Hemin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Hemin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hemin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Hemin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Hemin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hemin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hemin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
Tests for Diagnosis and Monitoring of Therapy
- Before Hemin therapy is begun, the presence of acute porphyria must be diagnosed using the following criteria:
- Presence of clinical symptoms.
- Positive Watson-Schwartz or Hoesch test. (A negative Watson-Schwartz or Hoesch test indicates a porphyric attack is highly unlikely. When in doubt quantitative measures of δ-aminolevulinic acid and porphobilinogen in serum or urine may aid in diagnosis.)
- Urinary concentrations of the following compounds may be monitored during Hemin therapy. Drug effect will be demonstrated by a decrease in one or more of the following compounds.
IV Compatibility
There is limited information regarding the compatibility of Hemin and IV administrations.
Overdosage
- Reversible renal shutdown has been observed in a case where an excessive hematin dose (12.2 mg/kg) was administered in a single infusion. Treatment of this case consisted of ethacrynic acid and mannitol
Pharmacology
| |

| |
Hemin
| |
| Systematic (IUPAC) name | |
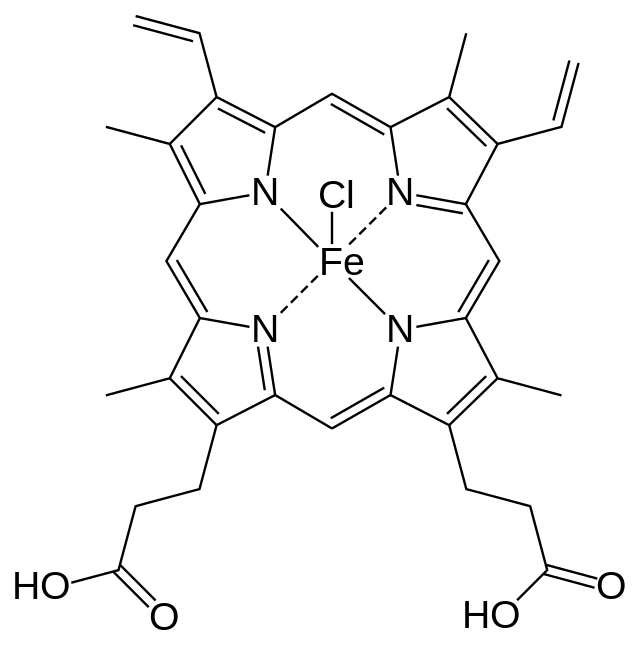
| Chloro[3,7,12,17-tetramethyl-8,13-divinylporphyrin-2,18-dipropanoato(2−)]iron(III) | |
| Identifiers | |
| CAS number | |
| ATC code | B06 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 651.94 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous infusion |
Mechanism of Action
- Heme acts to limit the hepatic and/or marrow synthesis of porphyrin. This action is likely due to the inhibition of δ-aminolevulinic acid synthetase, the enzyme which limits the rate of the porphyrin/heme biosynthetic pathway. The exact mechanism by which hematin produces symptomatic improvement in patients with acute episodes of the hepatic porphyrias has not been elucidated
Structure
- Hemin for injection) is an enzyme inhibitor derived from processed red blood cells. Hemin for injection was known previously as hematin. The term hematin has been used to describe the chemical reaction product of hemin and sodium carbonate solution. Hemin is an iron containing metalloporphyrin. Chemically hemin is represented as chloro [7,12-diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoato(2-)-N21,N22,N23,N24] iron. The structural formula for hemin is:

Pharmacodynamics
There is limited information regarding Hemin Pharmacodynamics in the drug label.
Pharmacokinetics
- Following intravenous administration of hematin in non-jaundiced human patients, an increase in fecal urobilinogen can be observed which is roughly proportional to the amount of hematin administered. This suggests an enterohepatic pathway as at least one route of elimination. Bilirubin metabolites are also excreted in the urine following hematin injections.
- Hemin for injection therapy for the acute porphyrias is not curative. After discontinuation of Hemin treatment, symptoms generally return although in some cases remission is prolonged. Some neurological symptoms have improved weeks to months after therapy although little or no response was noted at the time of treatment.
- Other aspects of human pharmacokinetics have not been defined.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Hemin was not mutagenic in bacteria systems in vitro and was not clastogenic in mammalian systems in vitro and in vivo. No data are available on potential for carcinogenicity or impairment of fertility in animals or humans.
Clinical Studies
There is limited information regarding Hemin Clinical Studies in the drug label.
How Supplied
- Hemin is supplied as a sterile, lyophilized black powder in single dose dispensing vials (NDC 55292-701-54) in a carton (NDC 55292-701-55). When mixed as directed with Sterile Water for Injection, USP, each 43 mL provides the equivalent of approximately 301 mg hematin (7 mg/mL).
Storage
- Store lyophilized powder at 20-25°C (68-77°F).
- Caution: The packaging (vial stopper) of this product contains natural rubber latex which may cause allergic reactions.
Images
Drug Images
{{#ask: Page Name::Hemin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
NDC 55292-701-54
Single Dose Vial
Hemin For Injection Hemin®
313 mg Hemin per Vial
For Intravenous Infusion Only Sterile Powder for Injection
RECORDATI RARE DISEASES GROUP
Rx only
Each vial contains:
Hemin ......313 mg
Sodium Carbonate ..... 215 mg
Sorbitol ..... 300 mg
pH may have been adjusted with hydrochloric acid.
Contains no preservatives.
When mixed as directed, each 43 mL provides the equivalent of approximately 301 mg hematin (7 mg/mL).
See package insert for full prescribing information and appropriate caution statements regarding administration.
Caution: Vial stopper contains latex.
The patient and physician should discuss the risks and benefits of this product.
Store powder at 20-25°C (68-77°F). See USP controlled room temperature.
DIRECTIONS FOR MIXING: Add 43 mL of Sterile Water for Injection, USP. Shake to aid dissolution. Infusion may be given from this vial.
USE IMMEDIATELY AFTER MIXING. Discard any unused portion.
Mfd. by: Fresenius Kabi USA, LLC Raleigh, NC 27616
For: Recordati Rare Diseases Inc. Lebanon, NJ 08833, U.S.A.
U.S. Lic. No. 1899
780-04245-4
Lot:
Exp.:

NDC 55292-701-55
Contains One Vial
Hemin For Injection Hemin®
313 mg Hemin per Vial
For Intravenous Infusion Only Sterile Powder for Injection
RECORDATI RARE DISEASES GROUP
Rx only
Each vial contains:
Hemin ..... 313 mg
Sodium Carbonate ...... 215 mg
Sorbitol ..... 300 mg
pH may have been adjusted with hydrochloric acid.
Contains no preservatives.
When mixed as directed, each 43 mL provides the equivalent of approximately 301 mg hematin (7 mg/mL).
Manufactured by: Fresenius Kabi USA, LLC Raleigh, NC 27616
For: Recordati Rare Diseases Inc. Lebanon, NJ 08833, U.S.A.
U.S. Lic. No. 1899
® Trademark of Recordati Rare Diseases Inc.
Lot No.
Exp. Date
Store powder at 20-25°C (68-77°F). See USP controlled room temperature.
Mixing directions: 1. Prep stopper. 2. Insert vent needle in “air” target. 3. Add 43 mL of Sterile Water for Injection, USP. 4. Immediately after adding diluent shake to aid dissolution. 5. May be administered directly from original single dose dispensing vial. 6. Administer by intermittent intravenous infusion over a period of from 10 to 15 minutes.
USE IMMEDIATELY AFTER MIXING. Discard any unused portion.
See package insert for full prescribing information and appropriate caution statements regarding administration.
Caution: Vial stopper contains latex.
The patient and physician should discuss the risks and benefits of this product.

{{#ask: Label Page::Hemin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information in this label
Precautions with Alcohol
Alcohol-Hemin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Panhematin
Look-Alike Drug Names
There is limited information regarding Hemin Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.