Encorafenib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Encorafenib is a kinase inhibitor that is FDA approved for the treatment of patients, in combination with binimetinib, with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test. Common adverse reactions include fatigue, nausea, vomiting, abdominal pain, and arthralgia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Encorafenib is indicated, in combination with binimetinib, for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test.
Limitations of Use
- Encorafenib is not indicated for treatment of patients with wild-type BRAF melanoma.
Dosage
- The recommended dose is 450 mg orally once daily in combination with binimetinib. Take encorafenib with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding encorafenib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding encorafenib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of encorafenib have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding encorafenib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding encorafenib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
New Primary Malignancies
- New primary malignancies, cutaneous and non-cutaneous, have been observed in patients treated with BRAF inhibitors and can occur with encorafenib.
Cutaneous Malignancies
- In COLUMBUS, cutaneous squamous cell carcinoma (cuSCC), including keratoacanthoma (KA), occurred in 2.6%, and basal cell carcinoma occurred in 1.6% of patients who received encorafenib in combination with binimetinib. Median time to first occurrence of cuSCC/KA was 5.8 months (range 1 to 9 months).
- For patients who received encorafenib as a single agent, cuSCC/KA was reported in 8%, basal cell carcinoma in 1%, and a new primary melanoma in 5% of patients.
- Perform dermatologic evaluations prior to initiating treatment, every 2 months during treatment, and for up to 6 months following discontinuation of treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Dose modification is not recommended for new primary cutaneous malignancies.
Non-Cutaneous Malignancies
- Based on its mechanism of action, encorafenib may promote malignancies associated with activation of RAS through mutation or other mechanisms. Monitor patients receiving encorafenib for signs and symptoms of non-cutaneous malignancies. Discontinue encorafenib for RAS mutation-positive non-cutaneous malignancies.
Tumor Promotion in BRAF Wild-Type Tumors
- In vitro experiments have demonstrated paradoxical activation of MAP-kinase signaling and increased cell proliferation in BRAF wild-type cells, which are exposed to BRAF inhibitors. Confirm evidence of BRAF V600E or V600K mutation prior to initiating encorafenib.
Hemorrhage
- Hemorrhage can occur when encorafenib is administered in combination with binimetinib. In COLUMBUS, hemorrhage occurred in 19% of patients receiving encorafenib in combination with binimetinib; Grade 3 or greater hemorrhage occurred in 3.2% of patients. The most frequent hemorrhagic events were gastrointestinal, including rectal hemorrhage (4.2%), hematochezia (3.1%), and hemorrhoidal hemorrhage (1%). Fatal intracranial hemorrhage in the setting of new or progressive brain metastases occurred in 1.6% of patients.
- Withhold, reduce dose, or permanently discontinue based on severity of adverse reaction.
Uveitis
- Uveitis, including iritis and iridocyclitis, has been reported in patients treated with encorafenib in combination with binimetinib. In COLUMBUS, the incidence of uveitis among patients treated with encorafenib in combination with binimetinib was 4%.
- Assess for visual symptoms at each visit. Perform an ophthalmologic evaluation at regular intervals and for new or worsening visual disturbances, and to follow new or persistent ophthalmologic findings. Withhold, reduce dose, or permanently discontinue based on severity of adverse reaction.
QT Prolongation
- Encorafenib is associated with dose-dependent QTc interval prolongation in some patients. In COLUMBUS, an increase in QTcF to > 500 ms was measured in 0.5% (1/192) of patients who received encorafenib in combination with binimetinib.
- Monitor patients who already have or who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, severe or uncontrolled heart failure and those taking other medicinal products associated with QT prolongation. Correct hypokalemia and hypomagnesemia prior to and during encorafenib administration. Withhold, reduce dose, or permanently discontinue for QTc > 500 ms.
Embryo-Fetal Toxicity
- Based on its mechanism of action, encorafenib can cause fetal harm when administered to a pregnant woman. Encorafenib produced embryo-fetal developmental changes in rats and rabbits and was an abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 26 (in the rat) and 178 (in the rabbit) times the human exposure at the recommended dose of 450 mg, with no clear findings at lower doses.
- Advise women of the potential risk to a fetus. Advise females of reproductive potential to use an effective, non-hormonal method of contraception since encorafenib can render hormonal contraceptives ineffective, during treatment and for 2 weeks after the final dose of encorafenib.
Risks Associated with Encorafenib as a Single Agent
- Encorafenib when used as a single agent is associated with an increased risk of certain adverse reactions compared to when encorafenib is used in combination with binimetinib. Grades 3 or 4 dermatologic reactions occurred in 21% of patients treated with encorafenib single agent compared to 2% of patients treated with encorafenib in combination with binimetinib.
- If binimetinib is temporarily interrupted or permanently discontinued, reduce the dose of encorafenib as recommended.
Risks Associated with Combination Treatment
- Encorafenib is indicated for use in combination with binimetinib. Refer to the binimetinib prescribing information for additional risk information that applies to combination use treatment.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of encorafenib in combination with binimetinib is described in 192 patients with BRAF V600 mutation-positive unresectable or metastatic melanoma who received encorafenib (450 mg once daily) in combination with binimetinib (45 mg twice daily) in a randomized open-label, active-controlled trial (COLUMBUS).
- The COLUMBUS trial excluded patients with a history of Gilbert's syndrome, abnormal left ventricular ejection fraction, prolonged QTc (>480 msec), uncontrolled hypertension, and history or current evidence of retinal vein occlusion. The median duration of exposure was 11.8 months for patients treated with encorafenib in combination with binimetinib and 6.2 months for patients treated with vemurafenib.
- The most common (≥ 25%) adverse reactions in patients receiving encorafenib in combination with binimetinib were fatigue, nausea, vomiting, abdominal pain, and arthralgia.
- Adverse reactions leading to dose interruptions of encorafenib occurred in 30% of patients receiving encorafenib in combination with binimetinib; the most common were nausea (7%), vomiting (7%) and pyrexia (4%). Adverse reactions leading to dose reductions of encorafenib occurred in 14% of patients receiving encorafenib in combination with binimetinib; the most common were arthralgia (2%), fatigue (2%) and nausea (2%). Five percent (5%) of patients receiving encorafenib in combination with binimetinib experienced an adverse reaction that resulted in permanent discontinuation of encorafenib; the most common were hemorrhage in 2% and headache in 1% of patients.
- Table 4 and Table 5 present adverse drug reactions and laboratory abnormalities, respectively, identified in COLUMBUS. The COLUMBUS trial was not designed to demonstrate a statistically significant difference in adverse reaction rates for encorafenib in combination with binimetinib, as compared to vemurafenib, for any specific adverse reaction listed in Table 4.

- Encorafenib when used as a single agent increases the risk of certain adverse reactions compared to encorafenib in combination with binimetinib. In patients receiving encorafenib 300 mg orally once daily as a single agent, the following adverse reactions were observed at a higher rate (≥ 5%) compared to patients receiving encorafenib in combination with binimetinib: palmar-plantar erythrodysesthesia syndrome (51% vs. 7%), hyperkeratosis (57% vs. 23%), dry skin (38% vs. 16%), erythema (16% vs. 7%), rash (41% vs. 22%), alopecia (56% vs. 14%), pruritus (31% vs. 13%), arthralgia (44% vs. 26%), myopathy (33% vs. 23%), back pain (15% vs. 9%), dysgeusia (13% vs. 6%), and acneiform dermatitis (8% vs. 3%).
- Other clinically important adverse reactions occurring in < 10% of patients who received encorafenib in combination with binimetinib were:
- Nervous system disorders: Facial paresis
- Gastrointestinal disorders: Pancreatitis
- Skin and subcutaneous tissue disorders: Panniculitis
- Immune system disorders: Drug hypersensitivity

Postmarketing Experience
There is limited information regarding Encorafenib Postmarketing Experience in the drug label.
Drug Interactions
Effect of Other Drugs on Encorafenib
Strong or Moderate CYP3A4 Inhibitors
- Concomitant administration of encorafenib with a strong or moderate CYP3A4 inhibitor increased encorafenib plasma concentrations and may increase encorafenib adverse reactions. Avoid coadministration of encorafenib with strong or moderate CYP3A4 inhibitors, including grapefruit juice. If coadministration of strong or moderate CYP3A4 inhibitors cannot be avoided, modify dose as recommended.
Strong or Moderate CYP3A4 Inducers
- Concomitant administration of encorafenib with a strong or moderate CYP3A4 inducer may decrease encorafenib plasma concentrations and may decrease encorafenib efficacy. Avoid concomitant administration of strong or moderate CYP3A4 inducers with encorafenib.
Effect of Encorafenib on Other Drugs
Sensitive CYP3A4 Substrates
- Concomitant administration of encorafenib with sensitive CYP3A4 substrates may result in increased toxicity or decreased efficacy of these agents.
- Coadministration of encorafenib with hormonal contraceptives (CYP3A4 substrates) can result in decreased concentrations and loss of hormonal contraceptive efficacy. Avoid hormonal contraceptives.
Drugs That Prolong the QT Interval
- Encorafenib is associated with dose-dependent QTc interval prolongation. Avoid coadministration of encorafenib with medicinal products with a known potential to prolong QT/QTc interval.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- Based on its mechanism of action, encorafenib can cause fetal harm when administered to a pregnant woman. There are no available clinical data on the use of encorafenib during pregnancy. In animal reproduction studies, encorafenib produced embryo-fetal developmental changes in rats and rabbits and was an abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 26 (in the rat) and 178 (in the rabbit) times the human exposure at the clinical dose of 450 mg, with no clear findings at lower doses. Advise pregnant women of the potential risk to a fetus.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
- In reproductive toxicity studies, administration of encorafenib to rats during the period of organogenesis resulted in maternal toxicity, decreased fetal weights, and increased incidence of total skeletal variations at a dose of 20 mg/kg/day (approximately 26 times the human exposure based on area under the concentration-time curve [AUC] at the recommended clinical dose of 450 mg once daily). In pregnant rabbits, administration of encorafenib during the period of organogenesis resulted in maternal toxicity, decreased fetal body weights, increased incidence of total skeletal variations and increased post-implantation loss, including total loss of pregnancy at a dose of 75 mg/kg/day (approximately 178 times the human exposure based on AUC at the recommended clinical dose of 450 mg once daily). While formal placental transfer studies have not been performed, encorafenib exposure in the fetal plasma of both rats and rabbits was up to 1.7% and 0.8%, respectively, of maternal exposure.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Encorafenib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Encorafenib during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of encorafenib or its metabolites in human milk or the effects of encorafenib on the breastfed infant, or on milk production. Because of the potential for serious adverse reactions from encorafenib in breastfed infants, advise women not to breastfeed during treatment with encorafenib and for 2 weeks after the final dose.
Pediatric Use
- The safety and effectiveness of encorafenib have not been established in pediatric patients.
Geriatic Use
- Of the 690 patients with BRAF mutation-positive melanoma who received encorafenib at doses between 300 mg and 600 mg once daily in combination with binimetinib (45 mg twice daily) across multiple clinical trials, 20% were aged 65 to 74 years and 8% were aged 75 years and older. No overall differences in the safety or effectiveness of encorafenib plus binimetinib were observed in elderly patients as compared to younger patients.
Gender
There is no FDA guidance on the use of Encorafenib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Encorafenib with respect to specific racial populations.
Renal Impairment
- No dose adjustment is recommended for patients with mild to moderate renal impairment (CLcr 30 to < 90 mL/min). A recommended dose has not been established for patients with severe renal impairment (CLcr < 30 mL/min).
Hepatic Impairment
- Dose adjustment for encorafenib is not recommended in patients with mild hepatic impairment (Child-Pugh Class A). A recommended dose has not been established for patients with moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy Testing
- Verify the pregnancy status of females of reproductive potential prior to initiating encorafenib.
Contraception
- Encorafenib can cause fetal harm when administered to a pregnant woman
- Advise females of reproductive potential to use effective contraception during treatment with encorafenib and for 2 weeks after the final dose. Counsel patients to use a non-hormonal method of contraception since encorafenib has the potential to render hormonal contraceptives ineffective.
Infertility
- Based on findings in male rats at doses approximately 13 times the human exposure at the 450 mg clinical dose, use of encorafenib may impact fertility in males.
Immunocompromised Patients
There is no FDA guidance one the use of Encorafenib in patients who are immunocompromised.
Administration and Monitoring
Administration
Patient Selection
- Confirm the presence of a BRAF V600E or V600K mutation in tumor specimens prior to initiating encorafenib. Information on FDA-approved tests for the detection of BRAF V600E and V600K mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
Recommended Dosage
- The recommended dosage of encorafenib is 450 mg (six 75 mg capsules) orally taken once daily in combination with binimetinib until disease progression or unacceptable toxicity. Refer to the binimetinib prescribing information for recommended binimetinib dosing information.
- Encorafenib may be taken with or without food. Do not take a missed dose of encorafenib within 12 hours of the next dose of encorafenib.
- Do not take an additional dose if vomiting occurs after encorafenib administration but continue with the next scheduled dose.
Dosage Modifications for Adverse Reactions
- If binimetinib is withheld, reduce encorafenib to a maximum dose of 300 mg once daily until binimetinib is resumed.
- Dose reductions for adverse reactions associated with encorafenib are presented in Table 1.

- Dosage modifications for adverse reactions associated with encorafenib are presented in Table 2.

- Refer to the binimetinib prescribing information for dose modifications for adverse reactions associated with binimetinib.
Dose Modifications for Coadministration with Strong or Moderate CYP3A4 Inhibitors
- Avoid coadministration with strong or moderate CYP3A4 inhibitors during treatment with encorafenib. If coadministration with a strong or moderate CYP3A4 inhibitor is unavoidable, reduce the encorafenib dose according to the recommendations in Table 3. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the encorafenib dose that was taken prior to initiating the CYP3A4 inhibitor.

Monitoring
There is limited information regarding Encorafenib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Encorafenib and IV administrations.
Overdosage
- Since encorafenib is 86% bound to plasma proteins, hemodialysis is likely to be ineffective in the treatment of overdose with encorafenib.
Pharmacology
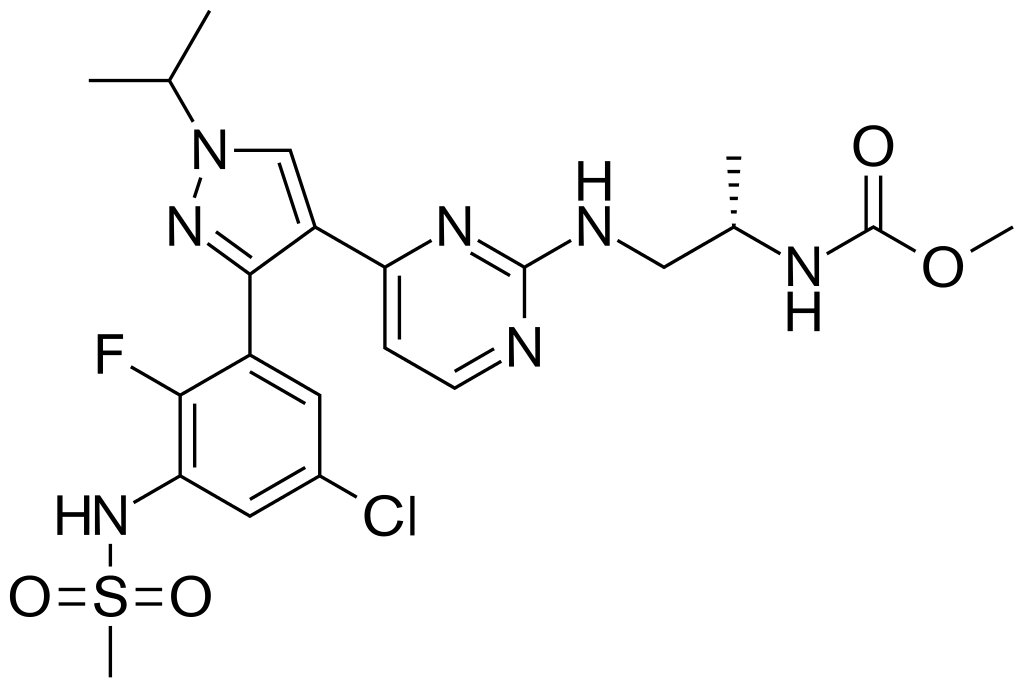
| |
Encorafenib
| |
| Systematic (IUPAC) name | |
| Methyl [(2S)-1-{[4-(3-{5-chloro-2-fluoro-3-[(methylsulfonyl)amino]phenyl}-1-isopropyl-1H-pyrazol-4-yl)-2-pyrimidinyl]amino}-2-propanyl]carbamate | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 540.011 g/mol |
| Synonyms | LGX818 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Approved |
| Routes | ? |
Mechanism of Action
- Encorafenib is a kinase inhibitor that targets BRAF V600E, as well as wild-type BRAF and CRAF in in vitro cell-free assays with IC50 values of 0.35, 0.47, and 0.3 nM, respectively. Mutations in the BRAF gene, such as BRAF V600E, can result in constitutively activated BRAF kinases that may stimulate tumor cell growth. Encorafenib was also able to bind to other kinases in vitro including JNK1, JNK2, JNK3, LIMK1, LIMK2, MEK4, and STK36 and substantially reduce ligand binding to these kinases at clinically achievable concentrations (≤ 0.9 µM).
- Encorafenib inhibited in vitro growth of tumor cell lines expressing BRAF V600 E, D, and K mutations. In mice implanted with tumor cells expressing BRAF V600E, encorafenib induced tumor regressions associated with RAF/MEK/ERK pathway suppression.
- Encorafenib and binimetinib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared with either drug alone, co-administration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of encorafenib and binimetinib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone.
Structure
- The molecular formula is C22H27ClFN7O4S and the molecular weight is 540 daltons. The chemical structure of encorafenib is shown below:

Pharmacodynamics
Cardiac Electrophysiology
- A dedicated study to evaluate the QT prolongation potential of encorafenib has not been conducted. Encorafenib is associated with dose-dependent QTc interval prolongation. Following administration of the recommended dose of encorafenib in combination with binimetinib, based on a central tendency analysis of QTc in a study of adult patients with melanoma, the largest mean (90% CI) QTcF change from baseline (ΔQTcF) was 18 (14 to 22) ms.
Pharmacokinetics
- The pharmacokinetics of encorafenib were studied in healthy subjects and patients with solid tumors, including advanced and unresectable or metastatic cutaneous melanoma harboring a BRAF V600E or V600K mutation. After a single dose, systemic exposure of encorafenib was dose proportional over the dose range of 50 mg to 700 mg. After once-daily dosing, systemic exposure of encorafenib was less than dose proportional over the dose range of 50 mg to 800 mg. Steady-state was reached within 15 days, with exposure being 50% lower compared to Day 1; intersubject variability (CV%) of AUC ranged from 12% to 69%.
Absorption
- After oral administration, the median Tmax of encorafenib is 2 hours. At least 86% of the dose is absorbed.
Effect of Food
- Administration of a single dose of encorafenib 100 mg (0.2 times the recommended dose) with a high-fat, high-calorie meal (comprised of approximately 150 calories from protein, 350 calories from carbohydrates, and 500 calories from fat) decreased the mean maximum encorafenib concentration (Cmax) by 36% with no effect on AUC.
Distribution
- Encorafenib is 86% bound to human plasma proteins in vitro. The blood-to-plasma concentration ratio is 0.58. The geometric mean (CV%) of apparent volume of distribution is 164 L (70%).
Elimination
- The mean (CV%) terminal half-life (t1/2) of encorafenib is 3.5 hours (17%), and the apparent clearance is 14 L/h (54%) at day 1, increasing to 32 L/h (59%) at steady-state.
Metabolism
- The primary metabolic pathway is N-dealkylation, with CYP3A4 as the main contributor (83%) to total oxidative clearance of encorafenib in human liver microsomes, followed by CYP2C19 (16%) and CYP2D6 (1%).
Excretion
- Following a single oral dose of 100 mg radiolabeled encorafenib, 47% (5% unchanged) of the administered dose was recovered in the feces and 47% (2% unchanged) was recovered in the urine.
Specific Populations
- Age (19 to 89 years), sex, body weight, mild hepatic impairment (Child-Pugh Class A), and mild or moderate renal impairment (CLcr 30 to < 90 mL/min) do not have a clinically meaningful effect on the pharmacokinetics of encorafenib. The effect of race or ethnicity, moderate or severe hepatic impairment (Child-Pugh Class B or C), and severe renal impairment (CLcr < 30 mL/min) on encorafenib pharmacokinetics have not been studied.
Drug Interaction Studies
Clinical Studies
- Effect of CYP3A4 Inhibitors on Encorafenib: Coadministration of a strong (posaconazole) or moderate (diltiazem) CYP3A4 inhibitor with encorafenib increased the AUC of encorafenib by 3- and 2-fold, respectively, and increased the Cmax by 68% and 45%, respectively, after a single encorafenib dose of 50 mg (0.1 times the recommended dose).
- Effect of CYP3A4 Inducers on Encorafenib: The effect of coadministration of a CYP3A4 inducer on encorafenib exposure has not been studied. In clinical trials, steady-state encorafenib exposures were lower than encorafenib exposures after the first dose, suggesting CYP3A4 auto-induction.
- Effect of Acid Reducing Agents on Encorafenib: Coadministration of a proton pump inhibitor, rabeprazole, had no effect on AUC and Cmax of encorafenib.
- Combination Treatment: Coadministration of encorafenib (UGT1A1 inhibitor) with binimetinib (UGT1A1 substrate) had no effect on binimetinib exposure.
In Vitro Studies
- Effect of Encorafenib on CYP/UGT Substrates: Encorafenib is a reversible inhibitor of UGT1A1, CYP1A2, CYP2B6, CYP2C8/9, CYP2D6, and CYP3A, and a time-dependent inhibitor of CYP3A4 at clinically relevant plasma concentrations. Encorafenib induced CYP2B6, CYP2C9, and CYP3A4 at clinically relevant plasma concentrations.
- Effect of Transporters on Encorafenib: Encorafenib is a substrate of P-glycoprotein (P-gp). Encorafenib is not a substrate of breast cancer resistance protein (BCRP), multidrug resistance-associated protein 2 (MRP2), organic anion transporting polypeptide (OATP1B1, OATP1B3) or organic cation transporter (OCT1) at clinically relevant plasma concentrations.
- Effect of Encorafenib on Transporters: Encorafenib inhibited P-gp, BCRP, OCT2, organic anion transporter (OAT1, OAT3), OATP1B1, and OATP1B3, but not OCT1 or MRP2 at clinically relevant plasma concentrations.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies with encorafenib have not been conducted. Encorafenib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, or micronuclei in bone marrow of rats.
- No dedicated fertility studies were performed with encorafenib in animals. In a general toxicology study in rats, decreased testes and epididymis weights, tubular degeneration in testes, and oligospermia in epididymides were observed at doses approximately 13 times the human exposure at the 450 mg clinical dose based on AUC. No effects on reproductive organs were observed in either sex in any of the non-human primate toxicity studies.
Animal Toxicology and/or Pharmacology
- Adverse histopathology findings of hyperplasia and hyperkeratosis occurred in the stomach of rats at encorafenib doses of 20 mg/kg/day (approximately 14 times the human exposure at the 450 mg clinical dose based on AUC) or greater, in both 4 and 13-week studies.
Clinical Studies
- Encorafenib in combination with binimetinib was evaluated in a randomized, active-controlled, open-label, multicenter trial (COLUMBUS; NCT01909453). Eligible patients were required to have BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma, as detected using the bioMerieux THxID™BRAF assay. Patients were permitted to have received immunotherapy in the adjuvant setting and one prior line of immunotherapy for unresectable locally advanced or metastatic disease. Prior use of BRAF inhibitors or MEK inhibitors was prohibited. Randomization was stratified by American Joint Committee on Cancer (AJCC) Stage (IIIB, IIIC, IVM1a or IVM1b, versus IVM1c), Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1), and prior immunotherapy for unresectable or metastatic disease (yes versus no).
- Patients were randomized (1:1:1) to receive encorafenib 450 mg once daily in combination with binimetinib 45 mg twice daily (encorafenib in combination with binimetinib), encorafenib 300 mg once daily, or vemurafenib 960 mg twice daily. Treatment continued until disease progression or unacceptable toxicity. Only the results of the approved dosing (encorafenib 450 mg in combination with binimetinib 45 mg) are described below.
- The major efficacy outcome measure was progression-free survival (PFS), as assessed by a blinded independent central review, to compare encorafenib in combination with binimetinib with vemurafenib. Additional efficacy outcome measures included overall survival (OS), as well as objective response rate (ORR) and duration of response (DoR) which were assessed by central review.
- A total of 577 patients were randomized, 192 to the encorafenib in combination with binimetinib arm, 194 to the encorafenib arm, and 191 to the vemurafenib arm. Of the 383 patients randomized to either the encorafenib in combination with binimetinib or the vemurafenib arms, the median age was 56 years (20 to 89 years), 59% were male, 91% were White, and 72% had baseline ECOG performance status of 0. Ninety-five percent (95%) had metastatic disease, 65% were Stage IVM1c, and 4% received prior CTLA-4, PD-1, or PD-L1 directed antibodies. Twenty-eight percent (28%) had elevated baseline serum lactate dehydrogenase (LDH), 45% had ≥ 3 organs with tumor involvement at baseline, and 3% had brain metastases. Based on centralized testing, 100% of patients' tumors tested positive for BRAF mutations; BRAF V600E (88%), BRAF V600K (11%), or both (<1%).
- Encorafenib in combination with binimetinib demonstrated a statistically significant improvement in PFS compared to vemurafenib. Efficacy results are summarized in Table 6 and Figure 1.


How Supplied
- Encorafenib is supplied as 75 mg hard gelatin capsules.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Do not use if safety seal under cap is broken or missing. Dispense in original bottle. Do not remove desiccant. Protect from moisture. Keep container tightly closed.
Images
Drug Images
{{#ask: Page Name::Encorafenib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Encorafenib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
- Inform patients of the following:
New Primary Cutaneous Malignancies
- Advise patients to contact their healthcare provider immediately for change in or development of new skin lesions.
Hemorrhage
- Advise patients to notify their healthcare provider immediately with any symptoms suggestive of hemorrhage, such as unusual bleeding.
Uveitis
- Advise patients to contact their healthcare provider if they experience any changes in their vision.
QT Prolongation
- Advise patients that encorafenib can cause QTc interval prolongation and to inform their physician if they have any QTc interval prolongation symptoms, such as syncope.
Females and Males of Reproductive Potential
- Embryo-Fetal Toxicity: Advise females with reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective non-hormonal contraception during treatment with encorafenib and for 2 weeks after the final dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with encorafenib.
- Lactation: Advise women not to breastfeed during treatment with encorafenib and for 2 weeks after the final dose.
- Infertility: Advise males of reproductive potential that encorafenib may impair fertility.
Strong or Moderate CYP3A Inducers or Inhibitors
- Coadministration of encorafenib with a strong or moderate CYP3A inhibitor may increase encorafenib concentrations; while coadministration of encorafenib with a strong or moderate CYP3A inducer may decrease encorafenib concentrations. Advise patients that they need to avoid certain medications while taking encorafenib and to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Advise patients to avoid grapefruit or grapefruit juice while taking encorafenib.
Storage
- Encorafenib is moisture sensitive. Advise patients to store encorafenib in the original bottle with desiccant and to keep the cap of the bottle tightly closed. Do not remove the desiccants from the bottle.
Medication Guide

Precautions with Alcohol
Alcohol-Encorafenib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Encorafenib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.