Leukocyte extravasation
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
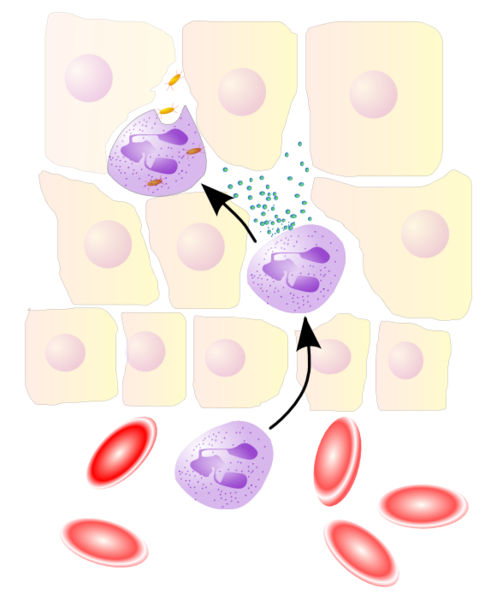
Leukocyte extravasation is the movement of leukocytes out of the circulatory system, towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.
Overview
Leukocyte extravasation occurs mainly in post-capillary venules, where haemodynamic shear forces are minimised. This process can be understood in several steps, outlined below as "chemoattraction", "rolling adhesion", "tight adhesion" and "(endothelial) transmigration". It has been demonstrated that leukocyte recruitment is halted whenever any of these steps is suppressed.
Chemoattraction
Upon recognition of and activation by pathogens, resident macrophages in the affected tissue release cytokines such as IL-1, TNFα and chemokines. IL-1 and TNFα cause the endothelial cells of blood vessels near the site of infection to express cellular adhesion molecules, including selectins. Circulating leukocytes are localised towards the site of injury or infection due to the presence of chemokines.
Rolling adhesion
Like velcro, selectin ligands on circulating leukocytes bind selectins on the inner wall of the vessel, with marginal affinity. This causes the leukocytes to slow down and begin rolling along the inner surface of the vessel wall. During this rolling motion, transitory bonds are formed and broken between selectins and their ligands.
Tight adhesion
At the same time, chemokines released by macrophages activate the rolling leukocytes and cause surface integrin molecules to switch from the default low-affinity state to a high-affinity state. This is assisted through juxtacrine activation of integrins by chemokines and soluble factors released by endothelial cells. In the activated state, integrins bind tightly to complementary receptors expressed on endothelial cells, with high affinity. This effects the immobilisation of the leukocytes, despite the shear forces of the ongoing blood flow.
Transmigration
The cytoskeletons of the leukocytes are reorganised in such a way that the leukocytes are spread out over the endothelial cells. In this form, leukocytes extend pseudopodia and pass through gaps between endothelial cells. Transmigration of the leukocyte occurs as PECAM proteins, found on the leukocyte and endothelial cell surfaces, interact and effectively pull the cell through the endothelium. The leukocytes secrete proteases that degrade the basement membrane, allowing them to escape the blood vessel – a process known as diapedesis. Once in the interstitial fluid, leukocytes migrate along a chemotactic gradient towards the site of injury or infection.
Molecular biology
Selectins
Selectins are expressed shortly after cytokine activation of endothelial cells by tissue macrophages. Activated endothelial cells initially express P-selecin molecules, but within two hours after activation E-selectin expression is favoured. Endothelial selectins bind carbohydrates on leukocyte transmembrane glycoproteins, including sialyl-LewisX.
- P-selectins: P-selectin is expressed on activated endothelial cells and platelets. Synthesis of P-selectin can be induced by thrombin, leukotriene B4, complement fragment C5a, histamine, TNFα or LPS. These cytokines induce the externalisation of Weibel-Palade bodies in endothelial cells, presenting pre-formed P-selectins on the endothelial cell surface. P-selectins bind PSGL-1 as a ligand.
- E-selectins: E-selectin is expressed on activated endothelial cells. Synthesis of E-selectin follows shortly after P-selectin synthesis, induced by cytokines such as IL-1 and TNFα. E-selectins bind PSGL-1 and ESL-1.
- L-selectins: L-selectins are constitutively expressed on some leukocytes, and are known to bind GlyCAM-1, MadCAM-1 and CD34 as ligands.
Suppressed expression of some selectins results in a slower immune response. If L-selectin is not produced, the immune response may be ten times slower, as P-selectins (which can also be produced by leukocytes) bind to each other. P-selectins can bind each other with high affinity, but occur less frequently because the receptor-site density is lower than with the smaller E-selectin molecules. This increases the initial leukocyte rolling speed, prolonging the slow rolling phase.
Integrins
Integrins involved in cellular adhesion are primarily expressed on leukocytes. β2 integrins on rolling leukocytes bind endothelial cellular adhesion molecules, arresting cell movement.
- LFA-1 is found on circulating leukocytes, and binds ICAM-1 and ICAM-2 on endothelial cells
- Mac-1 is found on circulating leukocytes, and binds ICAM-1 on endothelial cells
- VLA-4 is found on leukocytes and endothelial cells, and facilitates chemotaxis; it also binds VCAM-1
Cellular activation via and extracellular chemokines causes pre-formed β2 integrins to be released from cellular stores. Integrin molecules migrate to the cell surface and congregate in high-avidity patches. Intracellular integrin domains associate with the leukocyte cytoskeleton, via mediation with cytosolic factors such as talin, α-actinin and vinculin. This association causes a conformational shift in the integrin's tertiary structure, allowing ligand access to the binding site. Divalent cations (e.g. Mg2+) are also required for integrin-ligand binding.
Integrin ligands ICAM-1 and VCAM-1 are activated by inflammatory cytokines, while ICAM-2 is constitutively expressed by some endothelial cells but downregulated by inflammatory cytokines. ICAM-1 and ICAM-2 share two homologous N-terminal domains; both can bind LFA-1.
During chemotaxis, cell movement is facilitated by the binding of β1 integrins to components of the extracellular matrix: VLA-3, VLA-4 and VLA-5 to fibronectin and VLA-2 and VLA-3 to collagen and other extracellular matrix components.
Cytokines
Extravasation is regulated by the background cytokine environment produced by the inflammatory response, and is independent of specific cellular antigens. Cytokines released in the initial immune response induce vasodilation and lower the electrical charge along the vessel's surface. Blood flow is slowed, facilitating intermolecular binding.
- IL-1 activates resident lymphocytes and vascular endothelia
- TNFα increases vascular permeability and activates vascular endothelia
- CXCL8 (IL-8) forms a chemotactic gradient that directs leukocytes towards site of tissue injury/infection (CCL2 has a similar function to CXCL8, inducing monocyte extravasation and development into macrophages); also activates leukocyte integrins
Leukocyte adhesion deficiency
Leukocyte adhesion deficiency (LAD) is a genetic disease associated with a defect in the leukocyte extravasation process, caused by a defective integrin β2 chain (found in LFA-1 and Mac-1). This impairs the ability of the leukocytes to stop and undergo diapedesis. People with LAD suffer from recurrent bacterial infections and impaired wound healing. Neutrophilia is a hallmark of LAD.
References
- Aplin AE, Howe A, Alahari SK and Juliano RL (1998). "Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins". Pharmacological Reviews. 50 (2): 197–264.
- Dept of Biomedical Engineering, University of Virginia. "Inflammation: The Leukocyte Adhesion Cascade".
- Janeway CA, Travers P, Walport M and Shlomick MJ (2005). Immunobiology: The immune system in health and disease (6th edition ed.). New York: Garland Science Publishing. pp. 76–84. ISBN 0-8153-4101-6.
- Kindt TJ, Osborne BA and Goldsby RA (2007). "Overview of the Immune System". Kuby Immunology (6th edition ed.).
- Mak TW and Saunders ME (2006). The Immune Response: Basic and Clinical Principles. Oxford, UK: Elsevier Inc. pp. 61–2. Unknown parameter
|coauthors=ignored (help) - Male D, Brostoff J, Roth DB and Roitt I (2006). Immunology (7th edition ed.). Philadelphia: Elsevier Limited. pp. 130–8.
- Nairn R and Helbert M (2002). Immunology for Medical Students. Edinburgh: Harcourt Publishers Ltd. pp. 106–7.