Cefotetan microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Microbiology
The bactericidal action of cefotetan results from inhibition of cell wall synthesis. Cefotetan has in vitro activity against a wide range of aerobic and anaerobic gram-positive and gram-negative organisms. The methoxy group in the 7-alpha position provides cefotetan with a high degree of stability in the presence of beta-lactamases including both penicillinases and cephalosporinases of gram-negative bacteria.
Cefotetan has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE).
Gram-Negative Aerobes
Haemophilus influenzae (including ampicillin-resistant strains)
Klebsiella species (including K. pneumoniae)
Neisseria gonorrhoeae (nonpenicillinase-producing strains)
NOTE: Approximately one-half of the usually clinically significant strains of Enterobacter species (eg, E. aerogenes and E. cloacae) are resistant to cefotetan. Most strains of Pseudomonas aeruginosa and Acinetobacter species are resistant to cefotetan.
Gram-Positive Aerobes
Staphylococcus aureus (including penicillinase- and
nonpenicillinase-producing strains)
Streptococcus agalactiae (group B beta-hemolytic streptococcus)
NOTE: Methicillin-resistant staphylococci are resistant to cephalosporins. Some strains of Staphylococcus epidermidis and most strains of enterococci, eg,Enterococcus faecalis (formerly Streptococcus faecalis) are resistant to cefotetan
Anaerobes
Prevotella bivia (formerly Bacteroides bivius)
Prevotella disiens (formerly Bacteroides disiens)
Prevotella melaninogenica (formerly Bacteroides
melaninogenicus)
Gram-positive bacilli (including Clostridium species; seeWARNINGS)
NOTE: Most strains of C. difficile are resistant (see WARNINGS).
Peptococcus niger
Peptostreptococcus species
NOTE: Many strains of B. distasonis, B. ovatus and B. thetaiotaomicron are resistant to cefotetan in vitro. However, the therapeutic utility of cefotetan against these organisms cannot be accurately predicted on the basis of in vitro susceptibility tests alone.
The following in vitro data are available but their clinical significance is unknown. Cefotetan has been shown to be active in vitro against most strains of the following organisms:
Gram-Negative Aerobes
Citrobacter species (including C. diversus and C. freundii)
Klebsiella oxytoca
Moraxella (Branhamella) catarrhalis
Neisseria gonorrhoeae (penicillinase-producing strains)
Salmonella species
Shigella species
Yersinia enterocolitica Anaerobes
Porphyromonas asaccharolytica (formerly Bacteroides
asaccharolyticus)
Prevotella oralis (formerly Bacteroides oralis)
Bacteroides splanchnicus
Clostridium difficile (see WARNINGS)
Propionibacterium species
Veillonella species
Susceptibility Tests
Dilution Techniques: Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MIC’s). These MIC’s provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations or cefotetan powder. The MIC values should be interpreted according to the following criteria:
A report of ‘Susceptible’ indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of ‘Intermediate’ indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of ‘Resistant’ indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard cefotetan powder should provide the following MIC values:
Diffusion Techniques: Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of the standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 μg cefotetan to test the susceptibility of microorganisms to cefotetan.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 µg cefotetan disk should be interpreted according to the following criteria:
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefotetan.
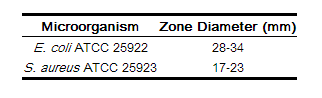
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30 μg cefotetan disk should provide the following zone diameters in these laboratory test quality control strains.
Anaerobic Techniques: For anaerobic bacteria, the susceptibility to cefotetan as MIC’s can be determined by standardized test methods3. The MIC values obtained should be interpreted according to the following criteria:
Interpretation is identical to that stated above for results using dilution techniques.
As with other susceptibility techniques, the use of laboratory control microorganisms is required to control the technical aspects of the laboratory standardized procedures. Standardized cefotetan powder should provide the following MIC values:
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/50588slr027_cefotan_lbl.pdf