Blinatumomab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
|
Overview
Blinatumomab is an antineoplastic agent that is FDA approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).. There is a Black Box Warning for this drug as shown here. Common adverse reactions include pyrexia, headache , peripheral edema , febrile neutropenia , nausea , hypokalemia , and constipation and the most common serious adverse reactions included febrile neutropenia, pyrexia, pneumonia, sepsis, neutropenia, device-related infection, tremor, encephalopathy, infection, overdose, confusion, Staphylococcal bacteremia, and headache..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute lymphoblastic leukemia
- Blinatumomab is indicated for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
- This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verification of clinical benefit in subsequent trials
Dosing Information
- Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and reinitiation (eg, if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Do not flush the Blinatumomab infusion line especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. Preparation and administration errors resulting in overdose have occurred .
Dosage
- A single cycle of treatment of Blinatumomab consists of 4 weeks of continuous intravenous infusion followed by a 2-week treatment-free interval.
- For patients at least 45 kg in weight:
- In Cycle 1, administer Blinatumomab at 9 mcg/day on Days 1–7 and at 28 mcg/day on Days 8-28.
- For subsequent cycles, administer Blinatumomab at 28 mcg/day on Days 1–28.
- Allow for at least 2 weeks treatment-free between cycles of Blinatumomab .
- A treatment course consists of up to 2 cycles of Blinatumomab for induction followed by 3 additional cycles for consolidation treatment (up to a total of 5 cycles).
Administration
- Premedicate with dexamethasone 20 mg intravenously 1 hour prior to the first dose of Blinatumomab of each cycle, prior to a step dose (such as Cycle 1 day 8), or when restarting an infusion after an interruption of 4 or more hours.
- Administer Blinatumomab as a continuous intravenous infusion at a constant flow rate using an infusion pump. The pump should be programmable, lockable, non-elastomeric, and have an alarm.
- Blinatumomab infusion bags should be infused over 24 hours or 48 hours . Infuse the total 240 mL Blinatumomab solution according to the instructions on the pharmacy label on the bag at one of the following constant infusion rates:
- Infusion rate of 10 mL/h for a duration of 24 hours, OR
- Infusion rate of 5 mL/h for a duration of 48 hours
- The Blinatumomab solution for infusion must be administered using IV tubing that contains a sterile, non-pyrogenic, low protein-binding, 0.2 micron in-line filter.
Important Note: Do not flush the infusion line, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage. Blinatumomab should be infused through a dedicated lumen.
- At the end of the infusion, any unused Blinatumomab solution in the IV bag and IV lines should be disposed of in accordance with local requirements.
Dosage Adjustments
- If the interruption after an adverse event is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse event is longer than 7 days, start a new cycle.

Reconstitution and Preparation of Solution for Infusion
- It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose) .
Gather Supplies
NOTE: 1 package Blinatumomab includes 1 vial of Blinatumomab and 1 vial of IV Solution Stabilizer.
- Before preparation, ensure you have the following supplies ready:
- 1 package of Blinatumomab for preparation of 9 mcg/day dose infused over 24 hours at a rate of 10 mL/h, 9 mcg/day dose infused over 48 hours at a rate of 5 mL/h, and 28 mcg/day dose infused over 24 hours at a rate of 10 mL/h
- 2 packages of Blinatumomab for preparation of 28 mcg/day dose infused over 48 hours at a rate of 5 mL/h
- The following supplies are also required, but not included in the package:
- Sterile, single-use disposable syringes
- 21- to 23- gauge needle(s) (recommended)
- Preservative-free Sterile Water for Injection, USP
- 250 mL 0.9% Sodium Chloride IV bag
- To minimize the number of aseptic transfers, it is recommended to use a 250 mL-prefilled IV bag. 250 mL-prefilled IV bags typically contain overfill with a total volume of 265 to 275 mL. Blinatumomab dose calculations provided in section 2.4.4 are based on a starting volume of 265 mL to 275 mL 0.9% Sodium Chloride.
- Use only polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.
- Polyolefin, PVC non-DEHP, or EVA IV tubing with a sterile, non-pyrogenic, low protein-binding 0.2 micron in-line filter
- Ensure that the IV tubing is compatible with the infusion pump.
Aseptic Preparation
- Aseptic technique must be strictly observed when preparing the solution for infusion since Blinatumomab vials do not contain antimicrobial preservatives. To prevent accidental contamination, prepare Blinatumomab according to aseptic standards, including but not limited to:
- Preparation must be done in a USP <797> compliant facility.
- Preparation must be done in an ISO Class 5 laminar flow hood or better.
- The admixing area should have appropriate environmental specifications, confirmed by periodic monitoring.
- Personnel should be appropriately trained in aseptic manipulations and admixing of oncology drugs.
- Personnel should wear appropriate protective clothing and gloves.
- Gloves and surfaces should be disinfected.
SPECIAL CONSIDERATIONS TO SUPPORT ACCURATE PREPARATION
- IV Solution Stabilizer is provided with the Blinatumomab package and is used to coat the prefilled IV bag prior to addition of reconstituted Blinatumomab to prevent adhesion of Blinatumomab to IV bags and IV lines. Therefore, add IV Solution Stabilizer to the IV bag containing 0.9% Sodium Chloride. Do not use IV Solution Stabilizer for reconstitution of Blinatumomab .
- The entire volume of the admixed Blinatumomab will be more than the volume administered to the patient (240 mL) to account for the priming of the IV line and to ensure that the patient will receive the full dose of Blinatumomab .
- When preparing an IV bag, remove air from IV bag. This is particularly important for use with an ambulatory infusion pump.
- Use the specific volumes described in the admixing instructions to minimize errors in calculation.
Preparation of Blinatumomab Solution for Infusion Using a Prefilled 250 mL 0.9% Sodium Chloride IV Bag
- Specific admixing instructions are provided for each dose and infusion time. Verify the prescribed dose and infusion time of Blinatumomab and identify the appropriate dosing preparation section listed below. Follow the steps for reconstituting Blinatumomab and preparing the IV bag.
- 9 mcg/day infused over 24 hours at a rate of 10 mL/h.
- 9 mcg/day infused over 48 hours at a rate of 5 mL/h.
- 28 mcg/day infused over 24 hours at a rate of 10 mL/h.
- 28 mcg/day infused over 48 hours at a rate of 5 mL/h.
Preparation of Blinatumomab 9 mcg/day infused over 24 hours at a rate of 10 mL/h
- Use a prefilled 250 mL 0.9% Sodium Chloride IV bag. 250 mL-prefilled bags typically contain overfill to a total volume of 265 to 275 mL. If necessary adjust the IV bag volume by adding or removing 0.9% Sodium Chloride to achieve a starting volume between 265 and 275 mL.
- Using a 10 mL syringe, aseptically transfer 5.5 mL of IV Solution Stabilizer to the IV bag with 0.9% Sodium Chloride. Gently mix the contents of the bag to avoid foaming. Discard remaining IV Solution Stabilizer vial.
- Using a 5 mL syringe, reconstitute one vial of Blinatumomab using 3 mL of preservative-free Sterile Water for Injection, USP. Direct preservative-free Sterile Water for Injection, USP, toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.
- Do not reconstitute Blinatumomab with IV Solution Stabilizer.
- The addition of preservative-free Sterile Water for Injection, USP, to the lyophilized powder results in a final Blinatumomab concentration of 12.5 mcg/mL.
- Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow. Do not use if solution is cloudy or has precipitated.
- Using a 1 mL syringe, aseptically transfer 0.83 mL of reconstituted Blinatumomab into the IV bag. Gently mix the contents of the bag to avoid foaming.
- Under aseptic conditions, attach the IV tubing to the IV bag with the sterile 0.2 micron in-line filter.
- Remove air from the IV bag and prime the IV line only with the prepared solution for infusion. Do not prime with 0.9% Sodium Chloride.
- Store at 2°C to 8°C if not used immediately.
Preparation of Blinatumomab 9 mcg/day infused over 48 hours at a rate of 5 mL/h
- Use a prefilled 250 mL 0.9% Sodium Chloride IV bag. 250 mL-prefilled bags typically contain overfill to a total volume of 265 to 275 mL. If necessary adjust the IV bag volume by adding or removing 0.9% Sodium Chloride to achieve a starting volume between 265 and 275 mL.
- Using a 10 mL syringe, aseptically transfer 5.5 mL of IV Solution Stabilizer to the IV bag with 0.9% Sodium Chloride. Gently mix the contents of the bag to avoid foaming. Discard remaining IV Solution Stabilizer vial.
- Using a 5 mL syringe, reconstitute one vial of Blinatumomab using 3 mL of preservative-free Sterile Water for Injection, USP. Direct preservative-free Sterile Water for Injection, USP, toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.
- Do not reconstitute Blinatumomab with IV Solution Stabilizer.
- The addition of preservative-free Sterile Water for Injection, USP, to the lyophilized powder results in a final Blinatumomab concentration of 12.5 mcg/mL.
- Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow. Do not use if solution is cloudy or has precipitated.
- Using a 3 mL syringe, aseptically transfer 1.7 mL of reconstituted Blinatumomab into the IV bag. Gently mix the contents of the bag to avoid foaming.
- Under aseptic conditions, attach the IV tubing to the IV bag with the sterile 0.2 micron in-line filter.
- Remove air from the IV bag and prime the IV line only with the prepared solution for infusion. Do not prime with 0.9% Sodium Chloride.
- Store at 2°C to 8°C if not used immediately.
Preparation of Blinatumomab 28 mcg/day infused over 24 hours at a rate of 10 mL/h
- Use a prefilled 250 mL 0.9% Sodium Chloride IV bag. 250 mL-prefilled bags typically contain overfill to a total volume of 265 to 275 mL. If necessary adjust the IV bag volume by adding or removing 0.9% Sodium Chloride to achieve a starting volume between 265 and 275 mL.
- Using a 10 mL syringe, aseptically transfer 5.6 mL of IV Solution Stabilizer to the IV bag with 0.9% Sodium Chloride. Gently mix the contents of the bag to avoid foaming. Discard remaining IV Solution Stabilizer vial.
- Using a 5 mL syringe, reconstitute one vial of Blinatumomab using 3 mL of preservative-free Sterile Water for Injection, USP. Direct preservative-free Sterile Water for Injection, USP, toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.
- Do not reconstitute Blinatumomab with IV Solution Stabilizer.
- The addition of preservative-free Sterile Water for Injection, USP, to the lyophilized powder results in a final Blinatumomab concentration of 12.5 mcg/mL.
- Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow. Do not use if solution is cloudy or has precipitated.
- Using a 3 mL syringe, aseptically transfer 2.6 mL of reconstituted Blinatumomab into the IV bag. Gently mix the contents of the bag to avoid foaming.
- Under aseptic conditions, attach the IV tubing to the IV bag with the sterile 0.2 micron in-line filter.
- Remove air from the IV bag and prime the IV line only with the prepared solution for infusion. Do not prime with 0.9% Sodium Chloride.
- Store at 2°C to 8°C if not used immediately.
Preparation of Blinatumomab 28 mcg/day infused over 48 hours at a rate of 5 mL/h
- Use a prefilled 250 mL 0.9% Sodium Chloride IV bag. 250 mL-prefilled bags typically contain overfill to a total volume of 265 to 275 mL. If necessary adjust the IV bag volume by adding or removing 0.9% Sodium Chloride to achieve a starting volume between 265 and 275 mL.
- Using a 10 mL syringe, aseptically transfer 5.6 mL of IV Solution Stabilizer to the IV bag with 0.9% Sodium Chloride. Gently mix the contents of the bag to avoid foaming. Discard remaining IV Solution Stabilizer vials.
- Use two vials of Blinatumomab . Using a 5 mL syringe, reconstitute each vial of Blinatumomab using 3 mL of preservative-free Sterile Water for Injection, USP. Direct preservative-free Sterile Water for Injection, USP, toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.
- Do not reconstitute Blinatumomab with IV Solution Stabilizer.
- The addition of preservative-free Sterile Water for Injection, USP, to the lyophilized powder results in a final Blinatumomab concentration of 12.5 mcg/mL.
- Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow. Do not use if solution is cloudy or has precipitated.
- Using a 3 mL syringe, aseptically transfer 5.2 mL of reconstituted Blinatumomab into the IV bag (2.7 mL from one vial and the remaining 2.5 mL from the second vial). Gently mix the contents of the bag to avoid foaming.
- Under aseptic conditions, attach the IV tubing to the IV bag with the sterile 0.2 micron in-line filter.
- Remove air from the IV bag and prime the IV line only with the prepared solution for infusion. Do not prime with 0.9% Sodium Chloride.
- Store at 2°C to 8°C if not used immediately.
Storage Requirements
- The information in Table 1 indicates the storage time for the reconstituted Blinatumomab vial and prepared IV bag containing Blinatumomab solution for infusion. Lyophilized Blinatumomab vial and IV Solution Stabilizer may be stored for a maximum of 8 hours at room temperature.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Blinatumomab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Blinatumomab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Limited experience in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Blinatumomab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Blinatumomab in pediatric patients.
Contraindications
- Blinatumomab is contraindicated in patients with known hypersensitivity to blinatumomab or to any component of the product formulation.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
|
Cytokine Release Syndrome
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving Blinatumomab .
- Infusion reactions have occurred with the Blinatumomab infusion and may be clinically indistinguishable from manifestations of CRS.
- Serious adverse events that may be associated with CRS included pyrexia, headache, nausea, asthenia, hypotension, increased alanine aminotransferase, increased aspartate aminotransferase, and increased total bilirubin; these events infrequently led to Blinatumomab discontinuation. Life-threatening or fatal CRS was infrequently reported in patients receiving Blinatumomab . In some cases, disseminated intravascular coagulation (DIC), capillary leak syndrome (CLS), and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) have been reported in the setting of CRS.
- Patients should be closely monitored for signs or symptoms of these events. Management of these events may require either temporary interruption or discontinuation of Blinatumomab .
Neurological Toxicities
- In patients receiving Blinatumomab in clinical trials, neurological toxicities have occurred in approximately 50% of patients. The median time to onset of any neurological toxicity was 7 days. Grade 3 or higher (severe, life-threatening, or fatal) neurological toxicities following initiation of Blinatumomab administration occurred in approximately 15% of patients and included encephalopathy, convulsions, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders. The majority of events resolved following interruption of Blinatumomab , but some resulted in treatment discontinuation.
- Monitor patients receiving Blinatumomab for signs and symptoms of neurological toxicities, and interrupt or discontinue Blinatumomab as recommended.
Infections
- In patients receiving Blinatumomab in clinical trials, serious infections such as sepsis, pneumonia, bacteremia, opportunistic infections, and catheter-site infections were observed in approximately 25% of patients, some of which were life-threatening or fatal. As appropriate, administer prophylactic antibiotics and employ surveillance testing during treatment with Blinatumomab . Monitor patients for signs and symptoms of infection and treat appropriately.
Tumor Lysis Syndrome
- Tumor lysis syndrome (TLS), which may be life-threatening or fatal, has been observed in patients receiving Blinatumomab . Appropriate prophylactic measures, including pretreatment nontoxic cytoreduction and on-treatment hydration, should be used for the prevention of TLS during Blinatumomab treatment. Monitor for signs or symptoms of TLS. Management of these events may require either temporary interruption or discontinuation of Blinatumomab .
Neutropenia and Febrile Neutropenia
- Neutropenia and febrile neutropenia, including life-threatening cases, have been observed in patients receiving Blinatumomab . Monitor laboratory parameters (including, but not limited to, white blood cell count and absolute neutrophil count) during Blinatumomab infusion. Interrupt Blinatumomab if prolonged neutropenia occurs.
Effects on Ability to Drive and Use Machines
- Due to the potential for neurologic events, including seizures, patients receiving Blinatumomab are at risk for loss of consciousness . Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while Blinatumomab is being administered.
Elevated Liver Enzymes
- Treatment with Blinatumomab was associated with transient elevations in liver enzymes. Although the majority of these events were observed in the setting of CRS, some were observed outside of this setting. For these events, the median time to onset was 15 days. *In patients receiving Blinatumomab in clinical trials, Grade 3 or greater elevations in liver enzymes occurred in approximately 6% of patients outside the setting of CRS and resulted in treatment discontinuation in less than 1% of patients.
- Monitor alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during Blinatumomab treatment. Interrupt Blinatumomab if the transaminases rise to greater than 5 times the upper limit of normal or if bilirubin rises to more than 3 times the upper limit of normal.
Leukoencephalopathy
- Cranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving Blinatumomab , especially in patients with prior treatment with cranial irradiation and antileukemic chemotherapy (including systemic high-dose methotrexate or intrathecal cytarabine). The clinical significance of these imaging changes is unknown.
Preparation and Administration Errors
- Preparation and administration errors have occurred with Blinatumomab treatment. Follow instructions for preparation (including admixing) and administration strictly to minimize medication errors (including underdose and overdose)
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the label:
- Cytokine release syndrome
- Neurological Toxicities
- Infections
- Tumor Lysis Syndrome
- Neutropenia and Febrile Neutropenia
- Effects on Ability to Drive and Use Machines
- Elevated Liver Enzymes
- Leukoencephalopathy
- Preparation and Administration Errors
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described in this section reflect exposure to Blinatumomab in clinical trials in which 212 patients with relapsed or refractory ALL received up to 28 mcg/day. All patients received at least one dose of Blinatumomab . The median age of the study population was 37 years (range: 18 to 79 years), 63% were male, 79% were White, 3% were Asian, and 3% were Black or African American.
- The most common adverse reactions (≥ 20%) were pyrexia (62%), headache (36%), peripheral edema (25%), febrile neutropenia (25%), nausea (25%), hypokalemia (23%), and constipation (20%).
- Serious adverse reactions were reported in 65% of patients. The most common serious adverse reactions (≥ 2%) included febrile neutropenia, pyrexia, pneumonia, sepsis, neutropenia, device-related infection, tremor, encephalopathy, infection, overdose, confusion, Staphylococcal bacteremia, and headache.
- Adverse reactions of Grade 3 or higher were reported in 80% of patients. Discontinuation of therapy due to adverse reactions occurred in 18% of patients treated with Blinatumomab . The adverse reactions reported most frequently as the reason for discontinuation of treatment included encephalopathy and sepsis. Fatal adverse events occurred in 15% of patients. The majority of these events were infections. No fatal adverse events occurred on treatment among patients in remission.
- The adverse reactions with ≥ 10% incidence for any grade or ≥ 5% incidence for Grade 3 or higher are summarized in Table 2.

- Additional important adverse reactions that did not meet the threshold criteria for inclusion in Table 2 were:
- Blood and lymphatic system disorders: leukocytosis (2%), lymphopenia (1%)
- Cardiac disorders: tachycardia (8%)
- General disorders and administration site conditions: edema (5%)
- Immune system disorders: cytokine storm (1%)
- Investigations: decreased immunoglobulins (9%), increased blood bilirubin (8%), increased gamma-glutamyl-transferase (6%), increased liver enzymes (1%)
- Metabolism and nutrition disorders: tumor lysis syndrome (4%), hypoalbuminemia (4%)
- Nervous system disorders: encephalopathy (5%), paresthesia (5%), aphasia (4%), convulsion (2%), memory impairment (2%), cognitive disorder (1%), speech disorder (< 1%)
- Psychiatric disorders: confusion (7%), disorientation (3%)
- Vascular disorders: capillary leak syndrome (< 1%).
- Hypersensitivity reactions related to Blinatumomab treatment were hypersensitivity (1%) and bronchospasm (< 1%).
Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity. The immunogenicity of Blinatumomab has been evaluated using either an electrochemiluminescence detection technology (ECL) or an enzyme-linked immunosorbent assay (ELISA) screening immunoassay for the detection of binding anti-blinatumomab antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
- In clinical studies, less than 1% of patients treated with Blinatumomab tested positive for binding anti-blinatumomab antibodies. All patients who tested positive for binding antibodies also tested positive for neutralizing anti-blinatumomab antibodies.
- Anti-blinatumomab antibody formation may affect pharmacokinetics of Blinatumomab . No association was seen between antibody development and development of adverse events.
- The detection of anti-blinatumomab antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to blinatumomab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Blinatumomab Postmarketing Experience in the drug label.
Drug Interactions
- No formal drug interaction studies have been conducted with Blinatumomab . Initiation of Blinatumomab treatment causes transient release of cytokines that may suppress CYP450 enzymes. The highest drug- drug interaction risk is during the first 9 days of the first cycle and the first 2 days of the second cycle in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index. In these patients, monitor for toxicity (eg, warfarin) or drug concentrations (eg, cyclosporine). Adjust the dose of the concomitant drug as needed
Use in Specific Populations
Pregnancy
Risk Summary
- There are no adequate and well-controlled studies of Blinatumomab in pregnant women. Based on its mechanism of action, Blinatumomab may cause fetal toxicity including B-cell lymphocytopenia when administered to a pregnant woman. Blinatumomab should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
- Animal reproduction studies have not been conducted with blinatumomab. In embryo-fetal developmental toxicity studies, a murine surrogate molecule was administered intravenously to pregnant mice during the period of organogenesis. The surrogate molecule crossed the placental barrier and did not cause embryo-fetal toxicity or teratogenicity. The expected depletions of B and T cells were observed in the pregnant mice, but hematological effects were not assessed in fetuses.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Blinatumomab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Blinatumomab during labor and delivery.
Nursing Mothers
- It is not known whether blinatumomab is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from blinatumomab, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- There is limited experience in pediatric patients. Blinatumomab was evaluated in a dose-escalation study of 41 pediatric patients with relapsed or refractory B-precursor ALL. The median age was 6 years (range: 2 to 17 years). Blinatumomab was administered at doses of 5 to 30 mcg/m2/day. The recommended phase 2 regimen was 5 mcg/m2/day on Days 1-7 and 15 mcg/m2/day on Days 8-28 for cycle 1, and 15 mcg/m2/day on Days 1-28 for subsequent cycles. At a higher dose, a fatal cardiac failure event occurred in the setting of life-threatening cytokine release syndrome (CRS) .
- The steady-state concentrations of blinatumomab were comparable in adult and pediatric patients at the equivalent dose levels based on body surface area (BSA)-based regimens.
Geriatic Use
- Of the total number of patients with relapsed or refractory ALL, approximately 13% were 65 years of age and over. Generally, safety and efficacy were similar between elderly patients (≥ 65 years of age) and patients less than 65 years of age treated with Blinatumomab . Elderly patients experienced a higher rate of neurological toxicities, including cognitive disorder, encephalopathy, confusion, and serious infections
Gender
There is no FDA guidance on the use of Blinatumomab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Blinatumomab with respect to specific racial populations.
Renal Impairment
- No formal pharmacokinetic studies using Blinatumomab have been conducted in patients with renal impairment. No dose adjustment is needed for patients with baseline creatinine clearance (CrCL) equal to or greater than 30 mL/min. There is no information available in patients with CrCL less than 30 mL/min or patients on hemodialysis
Hepatic Impairment
- No formal pharmacokinetic studies using Blinatumomab have been conducted in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Blinatumomab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Blinatumomab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- Monitor patients receiving Blinatumomab for signs and symptoms of neurological toxicities, and interrupt or discontinue Blinatumomab as recommended
- Monitor for signs or symptoms of TLS.
- Monitor laboratory parameters (including, but not limited to, white blood cell count and absolute neutrophil count) during Blinatumomab infusion. Interrupt Blinatumomab if prolonged neutropenia occurs.
- Monitor alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during Blinatumomab treatment.
IV Compatibility
There is limited information regarding the compatibility of Blinatumomab and IV administrations.
Overdosage
- Overdoses have been observed, including one patient who received 133-fold the recommended therapeutic dose of Blinatumomab delivered over a short duration. Overdoses resulted in adverse reactions which were consistent with the reactions observed at the recommended therapeutic dose and included fever, tremors, and headache. In the event of overdose, interrupt the infusion, monitor the patient for signs of toxicity, and provide supportive care . Consider reinitiation of Blinatumomab at the correct therapeutic dose when all toxicities have resolved and no earlier than 12 hours after interruption of the infusion
Pharmacology
Mechanism of Action
- Mechanism of Action
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells. It activates endogenous T cells by connecting CD3 in the T-cell receptor (TCR) complex with CD19 on benign and malignant B cells. Blinatumomab mediates the formation of a synapse between the T cell and the tumor cell, upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T cells, which result in redirected lysis of CD19+ cells.
Structure
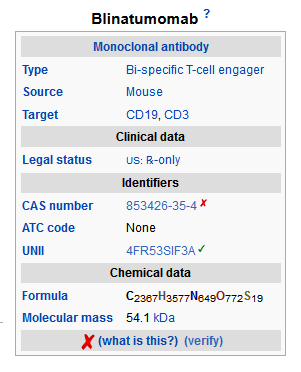
- Blinatumomab (blinatumomab) is a bispecific CD19-directed CD3 T-cell engager that binds to CD19 (expressed on cells of B-lineage origin) and CD3 (expressed on T cells). Blinatumomab is produced in Chinese hamster ovary cells. It consists of 504 amino acids and has a molecular weight of approximately 54 kilodaltons.
- Each Blinatumomab package contains 1 vial Blinatumomab and 1 vial IV Solution Stabilizer.
- Blinatumomab is supplied in a single-use vial as a sterile, preservative-free, white to off-white lyophilized powder for intravenous administration. Each single-use vial of Blinatumomab contains 35 mcg blinatumomab, citric acid monohydrate (3.35 mg), lysine hydrochloride (23.23 mg), polysorbate 80 (0.64 mg), trehalose dihydrate (95.5 mg), and sodium hydroxide to adjust pH to 7.0. After reconstitution with 3 mL of preservative-free Sterile Water for Injection, USP, the resulting concentration is 12.5 mcg/mL blinatumomab.
- IV Solution Stabilizer is supplied in a single-use vial as a sterile, preservative-free, colorless to slightly yellow, clear solution. Each single-use vial of IV Solution Stabilizer contains citric acid monohydrate (52.5 mg), lysine hydrochloride (2283.8 mg), polysorbate 80 (10 mg), sodium hydroxide to adjust pH to 7.0, and water for injection.
Pharmacodynamics
- During the continuous intravenous infusion over 4 weeks, the pharmacodynamic response was characterized by T-cell activation and initial redistribution, reduction in peripheral B cells, and transient cytokine elevation.
- Peripheral T cell redistribution (ie, T cell adhesion to blood vessel endothelium and/or transmigration into tissue) occurred after start of Blinatumomab infusion or dose escalation. T cell counts initially declined within 1 to 2 days and then returned to baseline levels within 7 to 14 days in majority patients. Increase of T cell counts above baseline (T cell expansion) was observed in few patients.
- Peripheral B cell counts decreased to less than or equal to 10 cells/microliter during the first treatment cycle at doses ≥ 5 mcg/m2/day or ≥ 9 mcg/day in the majority of patients. No recovery of peripheral B-cell counts was observed during the 2-week Blinatumomab -free period between treatment cycles. Incomplete depletion of B cells occurred at doses of 0.5 mcg/m2/day and 1.5 mcg/m2/day and in a few patients at higher doses.
Cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, and IFN-γ were measured, and IL-6, IL-10, and IFN-γ were elevated. The highest elevation of cytokines was observed in the first 2 days following start of Blinatumomab infusion. The elevated cytokine levels returned to baseline within 24 to 48 hours during the infusion. In subsequent treatment cycles, cytokine elevation occurred in fewer patients with lesser intensity compared to the initial 48 hours of the first treatment cycle.
Pharmacokinetics
- The pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 90 mcg/m2/day (approximately equivalent to 9 to 162 mcg/day) in adult patients. Following continuous intravenous infusion, the steady-state serum concentration (Css) was achieved within a day and remained stable over time. The increase in mean Css values was approximately proportional to the dose in the range tested. At the clinical doses of 9 mcg/day and 28 mcg/day for the treatment of relapsed/refractory ALL, the mean (SD) Css was 211 (258) pg/mL and 621 (502) pg/mL, respectively.
Distribution
- The estimated mean (SD) volume of distribution based on terminal phase (Vz) was 4.52 (2.89) L with continuous intravenous infusion of blinatumomab.
Metabolism
- The metabolic pathway of blinatumomab has not been characterized. Like other protein therapeutics, Blinatumomab is expected to be degraded into small peptides and amino acids via catabolic pathways.
Elimination
- The estimated mean (SD) systemic clearance with continuous intravenous infusion in patients receiving blinatumomab in clinical studies was 2.92 (2.83) L/hour. The mean (SD) half-life was 2.11 (1.42) hours. Negligible amounts of blinatumomab were excreted in the urine at the tested clinical doses.
Body Weight, Body Surface Area, Gender, and Age
- Results of population pharmacokinetic analyses indicate that age (18 to 80 years of age), gender, body weight (44 to 134 kg), and body surface area (1.39 to 2.57 m2) do not influence the pharmacokinetics of blinatumomab.
Renal Impairment
- No formal pharmacokinetic studies of blinatumomab have been conducted in patients with renal impairment.
- Pharmacokinetic analyses showed an approximately 2-fold difference in mean blinatumomab clearance values between patients with moderate renal impairment (CrCL ranging from 30 to 59 mL/min, N = 21) and normal renal function (CrCL more than 90 mL/min, N = 215). However, high interpatient variability was discerned (CV% up to 95.6%), and clearance values in renal impaired patients were essentially within the range observed in patients with normal renal function. There is no information available in patients with severe renal impairment (CrCL less than 30 mL/min) or patients on hemodialysis.
Drug Interactions
- Transient elevation of cytokines may suppress CYP450 enzyme activities
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity or genotoxicity studies have been conducted with blinatumomab.
- No studies have been conducted to evaluate the effects of blinatumomab on fertility. A murine surrogate molecule had no adverse effects on male and female reproductive organs in a 13-week repeat-dose toxicity study in mice.
Clinical Studies
Acute Lymphoblastic Leukemia
- The safety and efficacy of Blinatumomab were evaluated in an open-label, multicenter, single-arm study. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B‑precursor ALL (relapsed with first remission duration of ≤ 12 months in first salvage or relapsed or refractory after first salvage therapy or relapsed within 12 months of allogeneic hematopoietic stem cell transplantation [HSCT], and had ≥ 10% blasts in bone marrow).
- Blinatumomab was administered as a continuous intravenous infusion. In the first cycle, the initial dose was 9 mcg/day for week 1, then 28 mcg/day for the remaining 3 weeks. The target dose of 28 mcg/day was administered in cycle 2 and subsequent cycles starting on day 1 of each cycle. Dose adjustment was possible in case of adverse events. The treated population included 185 patients who received at least 1 infusion of Blinatumomab ; the median number of treatment cycles was 2 (range: 1 to 5). Patients who responded to Blinatumomab but later relapsed had the option to be retreated with Blinatumomab . Among treated patients, the median age was 39 years (range: 18 to 79 years), 63 out of 185 (34.1%) had undergone HSCT prior to receiving Blinatumomab , and 32 out of 185 (17.3%) had received more than 2 prior salvage therapies.
- The primary endpoint was the complete remission/complete remission with partial hematological recovery (CR/CRh*) rate within 2 cycles of treatment with Blinatumomab . Seventy-seven out of 185 (41.6%) evaluable patients achieved CR/CRh* within the first 2 treatment cycles, with the majority of responses (81%, 62 out of 77) occurring within cycle 1 of treatment. See Table 3 for efficacy results from this study. The HSCT rate among those who achieved CR/CRh* was 39% (30 out of 77).

How Supplied
- Each Blinatumomab package (NDC 55513-160-01) contains:
- One Blinatumomab 35 mcg single-use vial containing a sterile, preservative-free, white to off-white lyophilized powder and
- One IV Solution Stabilizer 10 mL single-use glass vial containing a sterile, preservative-free, colorless to slightly yellow, clear solution. Do not use the IV Solution Stabilizer to reconstitute Blinatumomab .
Storage
- Store Blinatumomab and IV Solution Stabilizer vials in the original package refrigerated at 2°C to 8°C (36°F to 46°F) and protect from light until time of use. Do not freeze.
- Store and transport the prepared IV bag containing Blinatumomab solution for infusion at 2°C to 8°C (36°F to 46°F) conditions. Ship in packaging that has been validated to maintain temperature of the contents at 2°C to 8°C (36°F to 46°F). Do not freeze.
Images
Drug Images
{{#ask: Page Name::Blinatumomab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Blinatumomab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients to contact a healthcare professional for any of the following:
- Signs and symptoms that may be associated with cytokine release syndrome and infusion reactions including pyrexia, fatigue, nausea, vomiting, chills, hypotension, rash, and wheezing
- Signs and symptoms of neurological toxicities including convulsions, speech disorders, and confusion
- Signs and symptoms of infections including pneumonia
- Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while Blinatumomab is being administered. Patients should be advised that they may experience neurological events .
- Inform patients that:
- It is very important to keep the area around the intravenous catheter clean to reduce the risk of infection.
- They should not adjust the setting on the infusion pump. Any changes to pump function may result in dosing errors. If there is a problem with the infusion pump or the pump alarms, patients should contact their doctor or nurse immediately.
Precautions with Alcohol
Alcohol-Blinatumomab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Blincyto
Look-Alike Drug Names
There is limited information regarding Blinatumomab Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.