Boceprevir: Difference between revisions
No edit summary |
No edit summary |
||
| Line 22: | Line 22: | ||
Coadministration with potent CYP3A4/5 inducers, where significantly reduced boceprevir plasma concentrations may be associated with reduced efficacy, including those in TABLE 2, is contraindicated. | Coadministration with potent CYP3A4/5 inducers, where significantly reduced boceprevir plasma concentrations may be associated with reduced efficacy, including those in TABLE 2, is contraindicated. | ||
[[file:Contraindications Boceprevir.png|none| | [[file:Contraindications Boceprevir.png|none|400px]] | ||
|warnings====Embryofetal Toxicity (Use with Ribavirin and Peginterferon Alfa)=== | |warnings====Embryofetal Toxicity (Use with Ribavirin and Peginterferon Alfa)=== | ||
[[Ribavirin]] may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. [[Ribavirin]] therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Refer to the prescribing information for ribavirin for additional information. | [[Ribavirin]] may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. [[Ribavirin]] therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Refer to the prescribing information for ribavirin for additional information. | ||
| Line 253: | Line 253: | ||
TABLE 11 presents sustained virologic response based on TW8 HCV-RNA results in previously untreated subjects. Fifty-seven percent (208/368) of subjects in the boceprevir-RGT arm and 56% (204/366) of subjects in the boceprevir-PR48 arm had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 17% (60/363) of subjects in the PR48 arm. | TABLE 11 presents sustained virologic response based on TW8 HCV-RNA results in previously untreated subjects. Fifty-seven percent (208/368) of subjects in the boceprevir-RGT arm and 56% (204/366) of subjects in the boceprevir-PR48 arm had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 17% (60/363) of subjects in the PR48 arm. | ||
[[file:Boceprevir CS2.png|none| | [[file:Boceprevir CS2.png|none|400px]] | ||
Among subjects with detectable HCV-RNA at TW8 who had attained undetectable HCV-RNA (Target Not Detected) at TW24 and completed at least 28 weeks of treatment, the SVR rates were 66% (45/68) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 24 weeks of VICTRELIS with PegIntron and REBETOL followed by 20 weeks of PegIntron and REBETOL alone) and 75% (55/73) in boceprevir-PR48 arms (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL). | Among subjects with detectable HCV-RNA at TW8 who had attained undetectable HCV-RNA (Target Not Detected) at TW24 and completed at least 28 weeks of treatment, the SVR rates were 66% (45/68) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 24 weeks of VICTRELIS with PegIntron and REBETOL followed by 20 weeks of PegIntron and REBETOL alone) and 75% (55/73) in boceprevir-PR48 arms (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL). | ||
| Line 272: | Line 272: | ||
The addition of VICTRELIS to the PegIntron and REBETOL therapy significantly increased the SVR rates compared to PegIntron/REBETOL alone (59% to 66% in arms containing VICTRELIS vs. 23% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population) (see TABLE 12). | The addition of VICTRELIS to the PegIntron and REBETOL therapy significantly increased the SVR rates compared to PegIntron/REBETOL alone (59% to 66% in arms containing VICTRELIS vs. 23% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population) (see TABLE 12). | ||
[[file:Boceprevir CS3.png|none| | [[file:Boceprevir CS3.png|none|400px]] | ||
In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of VICTRELIS with PegIntron and REBETOL for 44 weeks after 4 weeks of lead-in therapy with PegIntron and REBETOL (17/22, 77%) compared to those who received RGT (6/17, 35%). | In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of VICTRELIS with PegIntron and REBETOL for 44 weeks after 4 weeks of lead-in therapy with PegIntron and REBETOL (17/22, 77%) compared to those who received RGT (6/17, 35%). | ||
| Line 279: | Line 279: | ||
TABLE 13 presents sustained virologic response based on TW8 HCV-RNA results in subjects who were relapsers or partial responders to previous interferon and ribavirin therapy. Forty-six percent (74/162) of subjects in the boceprevir-RGT arm and 52% (84/161) in the boceprevir-PR48 had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 9% (7/80) in the PR48 arm. | TABLE 13 presents sustained virologic response based on TW8 HCV-RNA results in subjects who were relapsers or partial responders to previous interferon and ribavirin therapy. Forty-six percent (74/162) of subjects in the boceprevir-RGT arm and 52% (84/161) in the boceprevir-PR48 had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 9% (7/80) in the PR48 arm. | ||
[[file:Boceprevir CS4.png|none| | [[file:Boceprevir CS4.png|none|400px]] | ||
Among subjects with detectable HCV-RNA at TW8 who attained an undetectable HCV-RNA (Target Not Detected) at TW12 and completed at least 36 weeks of treatment, the SVR rates were 79% (27/34) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 32 weeks of VICTRELIS with PegIntron and REBETOL followed by 12 weeks of PegIntron and REBETOL alone) and 72% (29/40) in boceprevir-PR48 arm (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL). | Among subjects with detectable HCV-RNA at TW8 who attained an undetectable HCV-RNA (Target Not Detected) at TW12 and completed at least 36 weeks of treatment, the SVR rates were 79% (27/34) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 32 weeks of VICTRELIS with PegIntron and REBETOL followed by 12 weeks of PegIntron and REBETOL alone) and 72% (29/40) in boceprevir-PR48 arm (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL). | ||
Revision as of 19:01, 4 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Boceprevir is an antiviral and protease inhibitor that is FDA approved for the treatment of chronic hepatitis C genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders, partial responders, and relapsers. Common adverse reactions include fatigue, anemia, nausea, headache and dysgeusia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Boceprevir FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Boceprevir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Boceprevir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Boceprevir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Boceprevir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Boceprevir in pediatric patients.
Contraindications
Contraindications to peginterferon alfa and ribavirin also apply to VICTRELIS combination treatment. Refer to the respective prescribing information for a list of the contraindications for peginterferon alfa and ribavirin.
VICTRELIS in combination with peginterferon alfa and ribavirin is contraindicated in:
- Pregnant women and men whose female partners are pregnant because of the risks for birth defects and fetal death associated with ribavirin.
- Patients with a history of a hypersensitivity reaction to boceprevir.
- Coadministration with drugs that are highly dependent on CYP3A4/CYP3A5 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events, including those in TABLE 2, is contraindicated.
Coadministration with potent CYP3A4/5 inducers, where significantly reduced boceprevir plasma concentrations may be associated with reduced efficacy, including those in TABLE 2, is contraindicated.

Warnings
Embryofetal Toxicity (Use with Ribavirin and Peginterferon Alfa)
Ribavirin may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Ribavirin therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Refer to the prescribing information for ribavirin for additional information.
Women of childbearing potential and men must use at least two forms of effective contraception during treatment and for at least 6 months after treatment has concluded. One of these forms of contraception can be a combined oral contraceptive product containing at least 1 mg of norethindrone. Oral contraceptives containing lower doses of norethindrone and other forms of hormonal contraception have not been studied or are contraindicated. Routine monthly pregnancy tests must be performed during this time.
Anemia (Use with Ribavirin and Peginterferon Alfa)
Anemia has been reported with peginterferon alfa and ribavirin therapy. The addition of VICTRELIS to peginterferon alfa and [ribavirin]] is associated with an additional decrease in hemoglobin concentrations. Complete blood counts (with white blood cell differential counts) should be obtained pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. If hemoglobin is less than 10 g per dL, a decrease in dosage of ribavirin is recommended; and if hemoglobin is less than 8.5 g per dL, discontinuation of ribavirin is recommended. If ribavirin is permanently discontinued for management of anemia, then peginterferon alfa and VICTRELIS must also be discontinued. Refer to the prescribing information for ribavirin for additional information regarding dose reduction and/or discontinuation.
In clinical trials with VICTRELIS, the proportion of subjects who experienced hemoglobin values less than 10 g per dL and less than 8.5 g per dL was higher in subjects treated with the combination of VICTRELIS with PegIntron®/REBETOL® than in those treated with PegIntron/REBETOL alone (see TABLE 4). With the interventions used for anemia management in the clinical trials, the average additional decrease of hemoglobin was approximately 1 g per dL.
In clinical trials, the median time to onset of hemoglobin less than 10 g per dL from the initiation of therapy was similar among subjects treated with the combination of VICTRELIS and PegIntron/REBETOL (71 days with a range of 15-337 days), compared to those who received PegIntron/REBETOL (71 days with a range of 8-337 days). Certain adverse reactions consistent with symptoms of anemia, such as dyspnea, exertional dyspnea, dizziness and syncope were reported more frequently in subjects who received the combination of VICTRELIS with PegIntron/REBETOL than in those treated with PegIntron/REBETOL alone.
In clinical trials with VICTRELIS, dose modifications (generally of PegIntron/REBETOL) due to anemia occurred twice as often in subjects treated with the combination of VICTRELIS with PegIntron/REBETOL (26%) compared to PegIntron/REBETOL (13%). The proportion of subjects who discontinued study drug due to anemia was 1% in subjects treated with the combination of VICTRELIS with PegIntron/REBETOL and 1% in subjects who received PegIntron/REBETOL. The use of erythropoiesis stimulating agents (ESAs) was permitted for management of anemia, at the investigator's discretion, with or without ribavirin dose reduction in the Phase 2 and 3 clinical trials. The proportion of subjects who received an ESA was 43% in those treated with the combination of VICTRELIS with PegIntron/REBETOL compared to 24% in those treated with PegIntron/REBETOL alone. The proportion of subjects who received a transfusion for the management of anemia was 3% of subjects treated with the combination of VICTRELIS with PegIntron/REBETOL compared to less than 1% in subjects who received PegIntron/REBETOL alone.
Thromboembolic events have been associated with ESA use in other disease states; and have also been reported with peginterferon alfa use in hepatitis C patients. Thromboembolic events were reported in clinical trials with VICTRELIS among subjects receiving the combination of VICTRELIS with PegIntron/REBETOL, and among those receiving PegIntron/REBETOL alone, regardless of ESA use. No definite causality assessment or benefit risk assessment could be made for these events due to the presence of confounding factors and lack of randomization of ESA use.
A randomized, parallel-arm, open-label clinical trial was conducted in previously untreated CHC subjects with genotype 1 infection to compare use of an ESA versus ribavirin dose reduction for initial management of anemia during therapy with VICTRELIS in combination with peginterferon alfa-2b and ribavirin. Similar SVR rates were reported in subjects who were randomized to receive ribavirin dose reduction compared to subjects who were randomized to receive an ESA. In this trial, use of ESAs was associated with an increased risk of thromboembolic events including pulmonary embolism, acute myocardial infarction, cerebrovascular accident, and deep vein thrombosis compared to ribavirin dose reduction alone. The treatment discontinuation rate due to anemia was similar in subjects randomized to receive ribavirin dose reduction compared to subjects randomized to receive ESA (2% in each group). The transfusion rate was 4% in subjects randomized to receive ribavirin dose reduction and 2% in subjects randomized to receive ESA. Ribavirin dose reduction is recommended for the initial management of anemia.
Neutropenia (Use with Ribavirin and Peginterferon Alfa)
In Phase 2 and 3 clinical trials, seven percent of subjects receiving the combination of VICTRELIS with PegIntron/REBETOL had neutrophil counts of less than 0.5 × 109 per L compared to 4% of subjects receiving PegIntron/REBETOL alone (see TABLE 4). Three subjects experienced severe or life-threatening infections associated with neutropenia, and two subjects experienced life-threatening neutropenia while receiving the combination of VICTRELIS with PegIntron/REBETOL. Complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. Decreases in neutrophil counts may require dose reduction or discontinuation of peginterferon alfa and ribavirin. If peginterferon alfa and ribavirin are permanently discontinued, then VICTRELIS must also be discontinued. Refer to the prescribing information for peginterferon alfa and ribavirin for additional information regarding dose reduction or discontinuation.
Pancytopenia (Use with Ribavirin and Peginterferon Alfa)
Serious cases of pancytopenia have been reported postmarketing in patients receiving VICTRELIS in combination with peginterferon alfa and ribavirin. Complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. Refer to the prescribing information for ribavirin and peginterferon alfa for guidelines for discontinuation of therapy based on laboratory parameters.
Hypersensitivity
Serious acute hypersensitivity reactions (e.g., urticaria, angioedema) have been observed during combination therapy with VICTRELIS, peginterferon alfa and ribavirin. If such an acute reaction occurs, combination therapy should be discontinued and appropriate medical therapy immediately instituted.
Drug Interactions
See TABLE 2 for a listing of drugs that are contraindicated for use with VICTRELIS due to potentially life-threatening adverse events, significant drug interactions or loss of virologic activity. Please refer to TABLE 5 for established and other potentially significant drug interactions.
Laboratory Tests
HCV-RNA levels should be monitored at Treatment Weeks 4, 8, 12, and 24, at the end of treatment, during treatment follow-up, and for other time points as clinically indicated. Use of a sensitive real-time reverse-transcription polymerase chain reaction (RT-PCR) assay for monitoring HCV-RNA levels during treatment is recommended. The assay should have a lower limit of HCV-RNA quantification of equal to or less than 25 IU per mL, and a limit of HCV-RNA detection of approximately 10 to 15 IU per mL. For the purposes of assessing Response-Guided Therapy milestones, a confirmed "detectable but below limit of quantification" HCV-RNA result should not be considered equivalent to an "undetectable" HCV-RNA result (reported as "Target Not Detected" or "HCV-RNA Not Detected"). Complete blood count (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate.
Refer to the prescribing information for peginterferon alfa and ribavirin for pre-treatment, on-treatment and post-treatment laboratory testing recommendations including hematology, biochemistry (including hepatic function tests), and pregnancy testing requirements.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of VICTRELIS cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following serious and otherwise important adverse drug reactions (ADRs) are discussed in detail in another section of the labeling:
The most commonly reported adverse reactions (more than 35% of subjects regardless of investigator's causality assessment) in adult subjects were fatigue, anemia, nausea, headache, and dysgeusia when VICTRELIS was used in combination with PegIntron and REBETOL.
The safety of the combination of VICTRELIS 800 mg three times daily with PegIntron/REBETOL was assessed in 2095 subjects with chronic hepatitis C in one Phase 2, open-label trial and two Phase 3, randomized, double-blind, placebo-controlled clinical trials. SPRINT-1 (subjects who were previously untreated) evaluated the use of VICTRELIS in combination with PegIntron/REBETOL with or without a four-week lead-in period with PegIntron/REBETOL compared to PegIntron/REBETOL alone. SPRINT-2 (subjects who were previously untreated) and RESPOND-2 (subjects who had failed previous therapy) evaluated the use of VICTRELIS 800 mg three times daily in combination with PegIntron/REBETOL with a four-week lead-in period with PegIntron/REBETOL compared to PegIntron/REBETOL alone [see CLINICAL STUDIES (14)]. The population studied had a mean age of 49 years (3% of subjects were older than 65 years of age), 39% were female, 82% were white and 15% were black.
During the four week lead-in period with PegIntron/REBETOL in subjects treated with the combination of VICTRELIS with PegIntron/REBETOL, 28/1263 (2%) subjects experienced adverse reactions leading to discontinuation of treatment. During the entire course of treatment, the proportion of subjects who discontinued treatment due to adverse reactions was 13% for subjects receiving the combination of VICTRELIS with PegIntron/REBETOL and 12% for subjects receiving PegIntron/REBETOL alone. Events resulting in discontinuation were similar to those seen in previous studies with PegIntron/REBETOL. Only anemia and fatigue were reported as events that led to discontinuation in more than 1% of subjects in any arm.
Adverse reactions that led to dose modifications of any drug (primarily PegIntron and REBETOL) occurred in 39% of subjects receiving the combination of VICTRELIS with PegIntron/REBETOL compared to 24% of subjects receiving PegIntron/REBETOL alone. The most common reason for dose reduction was anemia, which occurred more frequently in subjects receiving the combination of VICTRELIS with PegIntron/REBETOL than in subjects receiving PegIntron/REBETOL alone.
Serious adverse events were reported in 11% of subjects receiving the combination of VICTRELIS with PegIntron/REBETOL and in 8% of subjects receiving PegIntron/REBETOL.
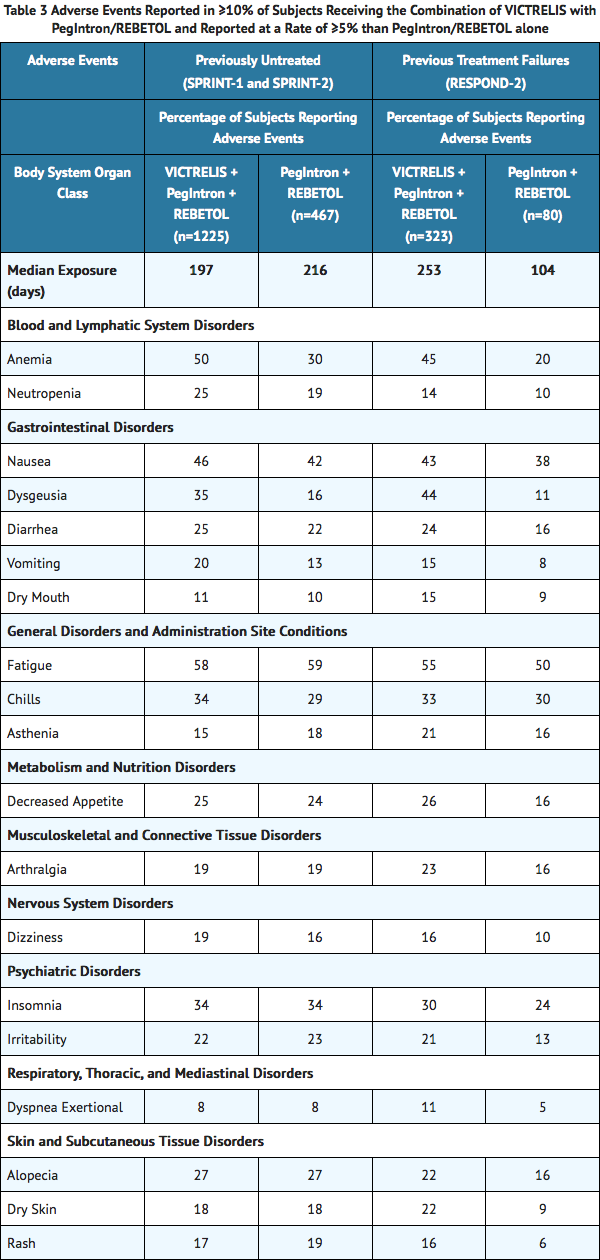
Adverse events (regardless of investigator's causality assessment) reported in greater than or equal to 10% of subjects receiving the combination of VICTRELIS with PegIntron/REBETOL and reported at a rate of greater than or equal to 5% than PegIntron/REBETOL alone in SPRINT-1, SPRINT-2, and RESPOND-2 are presented in TABLE 3.
Other Important Adverse Reactions Reported in Clinical Trials
Among subjects (previously untreated subjects or those who failed previous therapy) who received VICTRELIS in combination with peginterferon alfa and ribavirin, the following adverse drug reactions were reported. These events are notable because of their seriousness, severity, or increased frequency in subjects who received VICTRELIS in combination with peginterferon alfa and ribavirin compared with subjects who received only peginterferon alfa and ribavirin.
Gastrointestinal Disorders
Dysgeusia (alteration of taste) was an adverse event reported at an increased frequency in subjects receiving VICTRELIS in combination with peginterferon alfa and ribavirin compared with subjects receiving peginterferon alfa and ribavirin alone. Adverse events such as dry mouth, nausea, vomiting and diarrhea were also reported at an increased frequency in subjects receiving VICTRELIS in combination with peginterferon alfa and ribavirin.
Laboratory Values
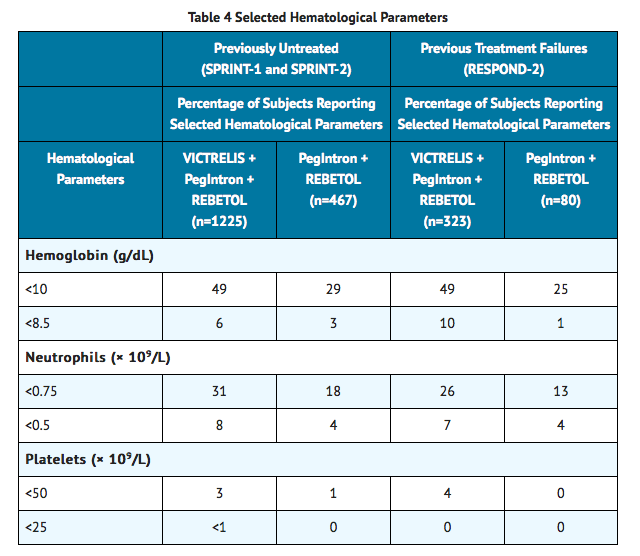
Changes in selected hematological parameters during treatment of adult subjects with the combination of VICTRELIS with PegIntron and REBETOL are described in TABLE 4.
Hemoglobin
Decreases in hemoglobin may require a decrease in dosage or discontinuation of ribavirin. If ribavirin is permanently discontinued, then peginterferon alfa and VICTRELIS must also be discontinued.
Neutrophils and Platelets
The proportion of subjects with decreased neutrophil and platelet counts was higher in subjects treated with VICTRELIS in combination with PegIntron/REBETOL compared to subjects receiving PegIntron/REBETOL alone. Three percent of subjects receiving the combination of VICTRELIS with PegIntron/REBETOL had platelet counts of less than 50 × 109 per L compared to 1% of subjects receiving PegIntron/REBETOL alone. Decreases in neutrophils or platelets may require a decrease in dosage or interruption of peginterferon alfa, or discontinuation of therapy. If peginterferon alfa is permanently discontinued, then ribavirin and VICTRELIS must also be discontinued.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VICTRELIS in combination with peginterferon alfa and ribavirin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: agranulocytosis, pancytopenia, thrombocytopenia.
- Gastrointestinal Disorders: mouth ulceration, stomatitis.
- Infections and Infestations: pneumonia, sepsis.
- Skin and Subcutaneous Tissue Disorders: angioedema, urticaria; drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, exfoliative rash, exfoliative dermatitis, Stevens-Johnson syndrome, toxic skin eruption, toxicoderma.
Drug Interactions
Potential for VICTRELIS to Affect Other Drugs
Boceprevir is a strong inhibitor of CYP3A4/CYP3A5. Drugs metabolized primarily by CYP3A4/CYP3A5 may have increased exposure when administered with VICTRELIS, which could increase or prolong their therapeutic and adverse effects. Boceprevir does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1 in vitro. In addition, boceprevir does not induce CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP3A4/5 in vitro.
Boceprevir is a potential inhibitor of p-glycoprotein (P-gp) based on in vitro studies. In a drug interaction trial conducted with digoxin, VICTRELIS had limited p-glycoprotein inhibitory potential at clinically relevant concentrations.
Potential for Other Drugs to Affect VICTRELIS
Boceprevir is primarily metabolized by aldo-ketoreductase (AKR). In drug interaction trials conducted with AKR inhibitors diflunisal and ibuprofen, boceprevir exposure did not increase to a clinically significant extent. VICTRELIS may be coadministered with AKR inhibitors.
Boceprevir is partly metabolized by CYP3A4/CYP3A5. It is also a substrate for p-glycoprotein. Coadministration of VICTRELIS with drugs that induce or inhibit CYP3A4/CYP3A5 could decrease or increase exposure to boceprevir.
Established and Other Potential Significant Drug Interactions
TABLE 5 provides recommendations based on established or potentially clinically significant drug interactions. VICTRELIS is contraindicated with drugs that are potent inducers of CYP3A4/CYP3A5 and drugs that are highly dependent on CYP3A4/CYP3A5 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events.

Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B VICTRELIS must be administered in combination with peginterferon alfa and ribavirin
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin; and therefore ribavirin is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Interferons have abortifacient effects in animals and should be assumed to have abortifacient potential in humans.
Extreme caution must be taken to avoid pregnancy in female patients and female partners of male patients while taking this combination. Women of childbearing potential and their male partners should not receive ribavirin unless they are using effective contraception (two reliable forms) during treatment with ribavirin and for 6 months after treatment. One of these reliable forms of contraception can be a combined oral contraceptive product containing at least 1 mg of norethindrone. Oral contraceptives containing lower doses of norethindrone and other forms of hormonal contraception have not been studied or are contraindicated.
In case of exposure during pregnancy, a Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Physicians and patients are encouraged to report such cases by calling 1-800-593-2214.
VICTRELIS must not be used as a monotherapy. There are no adequate and well-controlled studies with VICTRELIS in pregnant women. No effects on fetal development have been observed in rats and rabbits at boceprevir AUC exposures approximately 11.8- and 2.0-fold higher, respectively, than those in humans at the recommended dose of 800 mg three times daily.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Boceprevir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Boceprevir during labor and delivery.
Nursing Mothers
It is not known whether VICTRELIS is excreted into human breast milk. Levels of boceprevir and/or metabolites in the milk of lactating rats were slightly higher than levels observed in maternal blood. Peak blood concentrations of boceprevir and/or metabolites in nursing pups were less than 1% of those of maternal blood concentrations. Because of the potential for adverse reactions from the drug in nursing infants, a decision must be made whether to discontinue nursing or discontinue treatment with VICTRELIS, taking into account the importance of the therapy to the mother.
Pediatric Use
The safety, efficacy, and pharmacokinetic profile of VICTRELIS in pediatric patients have not been studied.
Geriatic Use
Clinical studies of VICTRELIS did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration and monitoring of VICTRELIS in geriatric patients due to the greater frequency of decreased hepatic function, concomitant diseases and other drug therapy.
Gender
There is no FDA guidance on the use of Boceprevir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Boceprevir with respect to specific racial populations.
Renal Impairment
No dosage adjustment of VICTRELIS is required for patients with any degree of renal impairment.
Hepatic Impairment
No dose adjustment of VICTRELIS is required for patients with mild, moderate or severe hepatic impairment. Safety and efficacy of VICTRELIS have not been studied in patients with decompensated cirrhosis.
In published observational studies of patients with compensated cirrhosis treated with first generation HCV protease inhibitors, including boceprevir, in combination with peginterferon alfa and ribavirin, platelet count < 100,000/mm3 and serum albumin < 3.5 g/dL were baseline characteristics that were identified as predictors of death or serious complications (severe infection or hepatic decompensation) during therapy.
The potential risks and benefits of VICTRELIS in combination with peginterferon alfa and ribavirin should be carefully considered before initiating therapy in patients with compensated cirrhosis who have platelet count < 100,000/mm3 and serum albumin < 3.5 g/dL at baseline. If therapy is initiated, close monitoring for signs of infections and worsening liver function is warranted.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Boceprevir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Boceprevir in patients who are immunocompromised.
Organ Transplantation
The safety and efficacy of VICTRELIS alone or in combination with peginterferon alfa and ribavirin for the treatment of chronic hepatitis C genotype 1 infection in liver or other organ transplant recipients have not been studied.
Administration and Monitoring
Administration
There is limited information regarding Boceprevir Administration in the drug label.
Monitoring
There is limited information regarding Boceprevir Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Boceprevir and IV administrations.
Overdosage
Daily doses of 3600 mg have been taken by healthy volunteers for 5 days without untoward symptomatic effects.
There is no specific antidote for overdose with VICTRELIS. Treatment of overdosage with VICTRELIS should consist of general supportive measures, including monitoring of vital signs, and observation of the patient's clinical status.
Pharmacology
Mechanism of Action
VICTRELIS is a direct acting antiviral drug against the hepatitis C virus
Structure
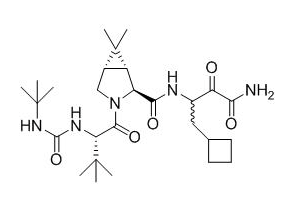
Boceprevir has the following chemical name: (1R,5S)-N-3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl-3-[2(S)-(1,1-dimethylethyl)amino]carbonylamino-3,3-dimethyl-1-oxobutyl-6,6-dimethyl-3-azabicyclo3.1.0hexan-2(S)-carboxamide. The molecular formula is C27H45N5O5 and its molecular weight is 519.7. Boceprevir has the following structural formula:
Pharmacodynamics
The effect of boceprevir 800 mg and 1200 mg on QTc interval was evaluated in a randomized, multiple-dose, placebo-, and active-controlled (moxifloxacin 400 mg) 4-way crossover thorough QT study in 36 healthy subjects. In the study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo-adjusted, baseline-corrected QTc based on individual correction method (QTcI) was below 10 ms, the threshold for regulatory concern. The dose of 1200 mg yields a boceprevir maximum exposure increase of approximately 15% which may not cover exposures due to coadministration with strong CYP3A4 inhibitors or use in patients with severe hepatic impairment. However, at the doses studied in the thorough QT study, no apparent concentration-QT relationship was identified. Thus, there is no expectation of a QTc effect under a higher exposure scenario.
Pharmacokinetics
VICTRELIS capsules contain a 1:1 mixture of two diastereomers, SCH534128 and SCH534129. In plasma the diastereomer ratio changes to 2:1, favoring the active diastereomer, SCH534128. Plasma concentrations of boceprevir described below consist of both diastereomers SCH534128 and SCH534129, unless otherwise specified.
In healthy subjects who received 800 mg three times daily alone, boceprevir drug exposure was characterized by AUC(т) of 5408 ng × hr per mL (n=71), Cmax of 1723 ng per mL (n=71), and Cmin of 88 ng per mL (n=71). Pharmacokinetic results were similar between healthy subjects and HCV-infected subjects.
Absorption
Boceprevir was absorbed following oral administration with a median Tmax of 2 hours. Steady state AUC, Cmax, and Cmin increased in a less-than-dose-proportional manner and individual exposures overlapped substantially at 800 mg and 1200 mg, suggesting diminished absorption at higher doses. Accumulation is minimal (0.8- to 1.5-fold) and pharmacokinetic steady state is achieved after approximately 1 day of three times daily dosing. The absolute bioavailability of boceprevir has not been studied.
- Effects of Food on Oral Absorption: VICTRELIS should be administered with food. Food enhanced the exposure of boceprevir by up to 65% at the 800 mg three times daily dose, relative to the fasting state. The bioavailability of boceprevir was similar regardless of meal type (e.g., high-fat vs. low-fat) or whether taken 5 minutes prior to eating, during a meal, or immediately following completion of the meal. Therefore, VICTRELIS may be taken without regard to either meal type or timing of the meal.
Distribution
Boceprevir has a mean apparent volume of distribution (Vd/F) of approximately 772 L at steady state in healthy subjects. Human plasma protein binding is approximately 75% following a single dose of boceprevir 800 mg. Boceprevir is administered as an approximately equal mixture of two diastereomers, SCH534128 and SCH534129, which rapidly interconvert in plasma. The predominant diastereomer, SCH534128, is pharmacologically active and the other diastereomer is inactive.
Metabolism
Studies in vitro indicate that boceprevir primarily undergoes metabolism through the aldo-keto reductase (AKR)-mediated pathway to ketone-reduced metabolites that are inactive against HCV. After a single 800-mg oral dose of 14C-boceprevir, the most abundant circulating metabolites were a diastereomeric mixture of ketone-reduced metabolites with a mean exposure approximately 4-fold greater than that of boceprevir. Boceprevir also undergoes, to a lesser extent, oxidative metabolism mediated by CYP3A4/5.
Drug Interactions
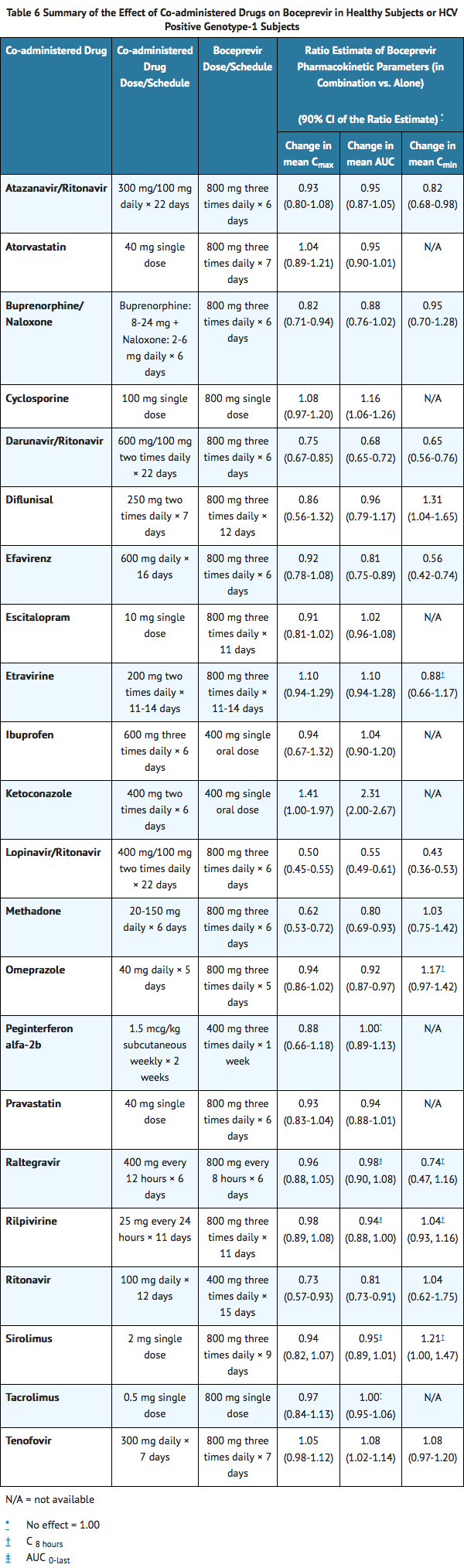
Drug interaction studies were performed with boceprevir and drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration of boceprevir on AUC, Cmax and Cmin are summarized in TABLE 6 (effects of coadministered drugs on boceprevir) and TABLE 7 (effects of boceprevir on coadministered drugs).
Elimination
Boceprevir is eliminated with a mean plasma half-life (t½) of approximately 3.4 hours. Boceprevir has a mean total body clearance (CL/F) of approximately 161 L per hr. Following a single 800 mg oral dose of 14C-boceprevir, approximately 79% and 9% of the dose was excreted in feces and urine, respectively, with approximately 8% and 3% of the dosed radiocarbon eliminated as boceprevir in feces and urine. The data indicate that boceprevir is eliminated primarily by the liver.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
- Use with Ribavirin and Peginterferon alfa: Ribavirin is genotoxic in in vitro and in vivo assays. Ribavirin was not oncogenic in mouse and rat carcinogenicity studies at doses less than the maximum recommended daily human dose. Please refer to the prescribing information for ribavirin for additional information.
- Two-year carcinogenicity studies in mice and rats were conducted with boceprevir. Mice were administered doses of up to 500 mg per kg in males and 650 mg per kg in females, and rats were administered doses of up to 125 mg per kg in males and 100 mg per kg in females. In mice, no significant increases in the incidence of drug-related neoplasms were observed at the highest doses tested resulting in boceprevir AUC exposures approximately 2.3- and 6.0-fold higher in males and females, respectively, than those in humans at the recommended dose of 800 mg three times daily. In rats, no increases in the incidence of drug-related neoplasms were observed at the highest doses tested resulting in boceprevir AUC exposures similar to those in humans at the recommended dose of 800 mg three times daily.
- Boceprevir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosomal aberration in human peripheral blood lymphocytes and mouse micronucleus assays.
Impairment of Fertility
- Use with Ribavirin and Peginterferon alfa: In fertility studies in male animals, ribavirin induced reversible testicular toxicity; while peginterferon alfa may impair fertility in females. Please refer to the prescribing information for ribavirin and peginterferon alfa for additional information.
- Boceprevir-induced reversible effects on fertility and early embryonic development in female rats, with no effects observed at a 75 mg per kg dose level. At this dose, boceprevir AUC exposures are approximately 1.3-fold higher than those in humans at the recommended dose of 800 mg three times daily. Decreased fertility was also observed in male rats, most likely as a consequence of testicular degeneration. No testicular degeneration was observed at a 15 mg per kg dose level resulting in boceprevir AUC exposures of less than those in humans at the recommended dose of 800 mg three times daily. Testicular degeneration was not observed in mice or monkeys administered boceprevir for 3 months at doses of up to 900 or 1000 mg per kg, respectively. At these doses, boceprevir AUC exposures are approximately 6.8- and 4.4-fold higher in mice and monkeys, respectively, than those in humans at the recommended dose of 800 mg three times daily. Additionally, limited clinical monitoring has revealed no evidence of testicular toxicity in human subjects.
Clinical Studies
The efficacy of VICTRELIS as a treatment for chronic hepatitis C (genotype 1) infection was assessed in approximately 1500 adult subjects who were previously untreated (SPRINT-2) or who had failed previous peginterferon alfa and ribavirin therapy (RESPOND-2) in Phase 3 clinical studies.
Previously Untreated Subjects
SPRINT-2 was a randomized, double-blind, placebo-controlled study comparing two therapeutic regimens of VICTRELIS 800 mg orally three times daily in combination with PR [PegIntron 1.5 micrograms per kg per week subcutaneously and weight-based dosing with REBETOL (600вАУ1400 mg per day orally divided twice daily)] to PR alone in adult subjects who had chronic hepatitis C (HCV genotype 1) infection with detectable levels of HCV-RNA and were not previously treated with interferon alfa therapy. Subjects were randomized in a 1:1:1 ratio within two separate cohorts (Cohort 1/non-Black and Cohort 2/Black) and were stratified by HCV genotype (1a or 1b) and by HCV-RNA viral load (less than or equal to 400,000 IU per mL vs. more than 400,000 IU per mL) to one of the following three treatment arms:
- PegIntron + REBETOL for 48 weeks (PR48).
- PegIntron + REBETOL for four weeks followed by VICTRELIS 800 mg three times daily + PegIntron + REBETOL for 24 weeks. The subjects were then continued on different regimens based on Treatment Week (TW) 8 through TW24 response-guided therapy (boceprevir-RGT). All subjects in this treatment arm were limited to 24 weeks of therapy with VICTRELIS.
- Subjects with undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) and remained undetectable through TW24 discontinued therapy and entered follow-up at the TW28 visit.
- Subjects with detectable HCV-RNA at TW8 or any subsequent treatment week but subsequently achieving undetectable HCV-RNA (Target Not Detected) at TW24 (late responders) were changed in a blinded fashion to placebo at the TW28 visit and continued therapy with PegIntron + REBETOL for an additional 20 weeks, for a total treatment duration of 48 weeks.
- PegIntron + REBETOL for four weeks followed by VICTRELIS 800 mg three times daily + PegIntron + REBETOL for 44 weeks (boceprevir-PR48).
All subjects with detectable HCV-RNA in plasma at TW24 were discontinued from treatment. Sustained Virologic Response (SVR) was defined as plasma HCV-RNA less than 25 IU/mL at Follow-up Week 24. Plasma HCV-RNA results at Follow-up Week 12 were used if plasma HCV-RNA results at Follow-up Week 24 were missing.
Mean age of subjects randomized was 49 years. The racial distribution of subjects was as follows: 82% White, 14% Black, and 4% others. The distribution of subjects by gender was 60% men and 40% women.
The addition of VICTRELIS to PegIntron and REBETOL significantly increased the SVR rates compared to PegIntron and REBETOL alone in the combined cohort (63% to 66% in arms containing VICTRELIS vs. 38% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population). SVR rates for Blacks who received the combination of VICTRELIS with PegIntron and REBETOL were 42% to 53% in a predefined analysis (see TABLE 10).
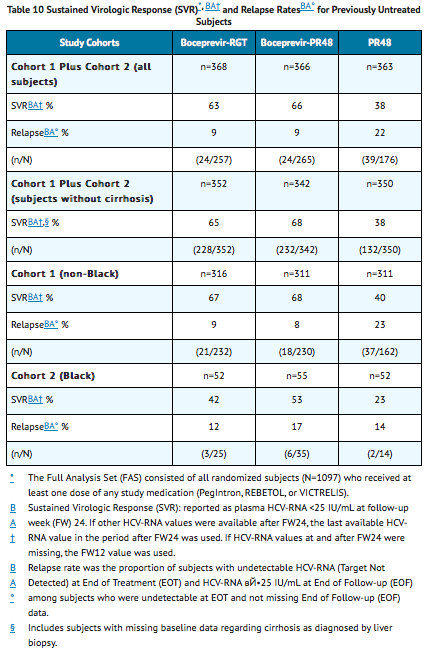
In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of VICTRELIS with PegIntron and REBETOL for 44 weeks after lead-in therapy with PegIntron and REBETOL (10/24, 42%) compared to those who received RGT (5/16 , 31%).
Sustained Virologic Response (SVR) Based on TW8 HCV-RNA Results
TABLE 11 presents sustained virologic response based on TW8 HCV-RNA results in previously untreated subjects. Fifty-seven percent (208/368) of subjects in the boceprevir-RGT arm and 56% (204/366) of subjects in the boceprevir-PR48 arm had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 17% (60/363) of subjects in the PR48 arm.
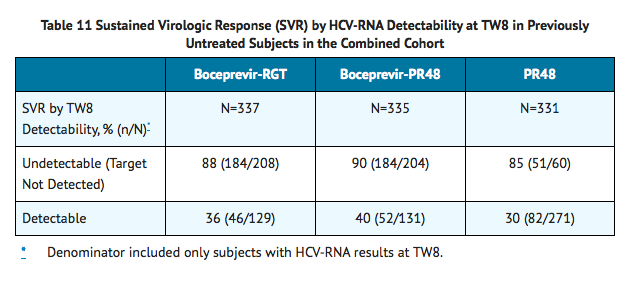
Among subjects with detectable HCV-RNA at TW8 who had attained undetectable HCV-RNA (Target Not Detected) at TW24 and completed at least 28 weeks of treatment, the SVR rates were 66% (45/68) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 24 weeks of VICTRELIS with PegIntron and REBETOL followed by 20 weeks of PegIntron and REBETOL alone) and 75% (55/73) in boceprevir-PR48 arms (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL).
Previous Partial Responders and Relapsers to Interferon and Ribavirin Therapy
RESPOND-2 was a randomized, parallel-group, double-blind study comparing two therapeutic regimens of VICTRELIS 800 mg orally three times daily in combination with PR [PegIntron 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600вАУ1400 mg per day orally divided twice daily)] compared to PR alone in adult subjects with chronic hepatitis C (HCV genotype 1) infection with demonstrated interferon responsiveness (as defined historically by a decrease in HCV-RNA viral load greater than or equal to 2-log10 by Week 12, but never achieved SVR [partial responders] or undetectable HCV-RNA at end of prior treatment with a subsequent detectable HCV-RNA in plasma [relapsers]). Subjects with less than 2-log10 decrease in HCV-RNA by week 12 of previous treatment (prior null responders) were not eligible for enrollment in this trial. Subjects were randomized in a 1:2:2 ratio and stratified based on response to their previous qualifying regimen (relapsers vs. partial responders) and by HCV subtype (1a vs. 1b) to one of the following treatment arms:
- PegIntron + REBETOL for 48 weeks (PR48)
- PegIntron + REBETOL for 4 weeks followed by VICTRELIS 800 mg three times daily + PegIntron + REBETOL for 32 weeks. The subjects were then continued on different treatment regimens based on TW8 and TW12 response-guided therapy (boceprevir-RGT). All subjects in this treatment arm were limited to 32 weeks of VICTRELIS.
- Subjects with undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) and TW12 completed therapy at TW36 visit.
- Subjects with a detectable HCV-RNA at TW8 but subsequently undetectable (Target Not Detected) at TW12 (late responders) were changed in a blinded fashion to placebo at the TW36 visit and continued treatment with PegIntron + REBETOL for an additional 12 weeks, for a total treatment duration of 48 weeks.
- PegIntron + REBETOL for 4 weeks followed by VICTRELIS 800 mg three times daily + PegIntron + REBETOL for 44 weeks (boceprevir-PR48).
All subjects with detectable HCV-RNA in plasma at TW12 were discontinued from treatment. Sustained Virologic Response (SVR) was defined as plasma HCV-RNA less than 25 IU/mL at Follow-up Week 24. Plasma HCV-RNA results at Follow-up Week 12 were used if plasma HCV-RNA results at Follow-up Week 24 were missing.
Mean age of subjects randomized was 53 years. The racial distribution of subjects was as follows: 85% White, 12% Black, and 3% others. The distribution of subjects by gender was 67% men and 33% women.
The addition of VICTRELIS to the PegIntron and REBETOL therapy significantly increased the SVR rates compared to PegIntron/REBETOL alone (59% to 66% in arms containing VICTRELIS vs. 23% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population) (see TABLE 12).
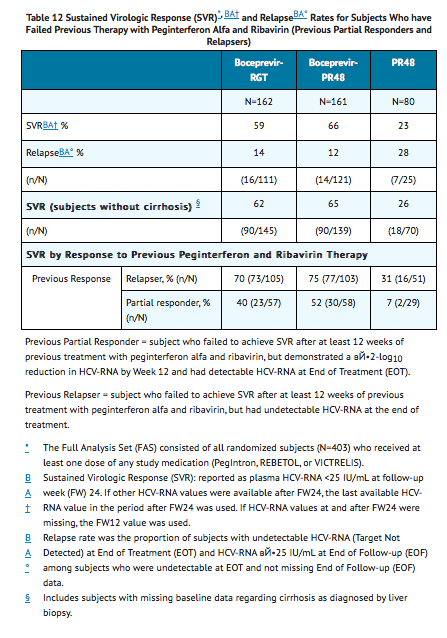
In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of VICTRELIS with PegIntron and REBETOL for 44 weeks after 4 weeks of lead-in therapy with PegIntron and REBETOL (17/22, 77%) compared to those who received RGT (6/17, 35%).
Sustained Virologic Response (SVR) Based on TW8 HCV-RNA Results
TABLE 13 presents sustained virologic response based on TW8 HCV-RNA results in subjects who were relapsers or partial responders to previous interferon and ribavirin therapy. Forty-six percent (74/162) of subjects in the boceprevir-RGT arm and 52% (84/161) in the boceprevir-PR48 had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 9% (7/80) in the PR48 arm.
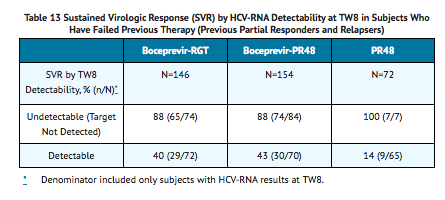
Among subjects with detectable HCV-RNA at TW8 who attained an undetectable HCV-RNA (Target Not Detected) at TW12 and completed at least 36 weeks of treatment, the SVR rates were 79% (27/34) in boceprevir-RGT arm (4 weeks of PegIntron and REBETOL then 32 weeks of VICTRELIS with PegIntron and REBETOL followed by 12 weeks of PegIntron and REBETOL alone) and 72% (29/40) in boceprevir-PR48 arm (4 weeks of PegIntron and REBETOL then 44 weeks of VICTRELIS with PegIntron and REBETOL).
Interferon Responsiveness during Lead-In Therapy with Peginterferon alfa and Ribavirin
Previously Untreated Subjects
In previously untreated subjects evaluated in SPRINT-2, interferon-responsiveness (defined as greater than or equal to 1-log10 decline in viral load at TW4) was predictive of SVR. Subjects treated with VICTRELIS who demonstrated interferon responsiveness at TW4 achieved SVR rates of 81% (203/252) in boceprevir-RGT arm and 79% (200/254) in boceprevir-PR48 arm, compared to 52% (134/260) in subjects treated with PegIntron/REBETOL.
Subjects treated with VICTRELIS who demonstrated poor interferon responsiveness (defined as less than 1-log10 decline in viral load at TW4), achieved SVR rates of 28% (27/97) in boceprevir-RGT arm and 38% (36/95) in boceprevir-PR48 arm, compared to 4% (3/83) in subjects treated with PegIntron/REBETOL. Subjects with less than a 0.5-log10 decline in viral load at TW4 achieved SVR rates of 28% (13/47) in boceprevir-RGT arm and 30% (11/37) in boceprevir-PR48 arm, compared to 0% (0/25) in subjects treated with PegIntron/REBETOL. Subjects with less than a 0.5-log10 decline in viral load at TW4 with peginterferon alfa plus ribavirin therapy alone are predicted to have a null response (less than 2-log10 viral load decline at TW12) to peginterferon alfa and ribavirin.
Previous Partial Responders and Relapsers to Interferon and Ribavirin Therapy
In subjects who were previous relapsers and partial responders evaluated in RESPOND-2, interferon-responsiveness (defined as greater than or equal to 1-log10 decline in viral load at TW4) was predictive of SVR. Subjects treated with VICTRELIS who demonstrated interferon responsiveness at TW4 achieved SVR rates of 74% (81/110) in boceprevir-RGT arm and 79% (90/114) in boceprevir-PR48 arm, compared to 27% (18/67) in subjects treated with PegIntron/REBETOL. Subjects treated with VICTRELIS who demonstrated poor interferon responsiveness (defined as less than 1-log10 decline in viral load at TW4) achieved SVR rates of 33% (15/46) in boceprevir-RGT arm and 34% (15/44) in boceprevir-PR48 arm, compared to 0% (0/12) in subjects treated with PegIntron/REBETOL.
Prior Null Responders to Interferon and Ribavirin Therapy
PROVIDE was an open-label, single-arm trial of VICTRELIS 800 mg orally three times daily in combination with peginterferon alfa-2b 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600 вАУ 1,400 mg per day orally divided twice daily) in adult subjects with chronic hepatitis C (HCV) genotype 1 infection who did not achieve SVR while in the peginterferon alfa/ribavirin control arms of previous Phase 2 and 3 trials of combination therapy with VICTRELIS. Subjects who enrolled in PROVIDE within 2 weeks after the last dose of peginterferon alfa/ribavirin in the prior trial received VICTRELIS 800 mg three times daily + peginterferon alfa-2b + ribavirin for 44 weeks. Subjects who were not able to enroll in this trial within 2 weeks received PegIntron/REBETOL lead-in for 4 weeks followed by VICTRELIS 800 mg three times daily + peginterferon alfa-2b + ribavirin for 44 weeks.
Among subjects who were null responders in the peginterferon alfa/ribavirin control arm of the prior trial, SVR (reported as plasma HCV-RNA <25 IU/mL at follow-up week 24) was 38% (20/52) and the relapse rate was 13% (3/23).
Use of Ribavirin Dose Reduction versus Erythropoiesis Stimulating Agent (ESA) in the Management of Anemia in Previously Untreated Subjects
A randomized, parallel-arm, open-label study was conducted to compare two strategies for the management of anemia (use of ESA versus ribavirin dose reduction) in 687 subjects with previously untreated CHC genotype 1 infection who became anemic during therapy with VICTRELIS 800 mg orally three times daily plus peginterferon alfa-2b 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600 вАУ 1,400 mg orally per day divided twice daily). The study enrolled subjects with serum hemoglobin concentrations of less than 15 g per dL. Subjects were treated for 4 weeks with peginterferon alfa-2b and ribavirin followed by up to 44 weeks of VICTRELIS plus peginterferon alfa-2b and ribavirin. If a subject became anemic (serum hemoglobin of approximately less than or equal to 10 g per dL within the treatment period), the subject was randomized in a 1:1 ratio to either ribavirin dose reduction (N=249) or use of erythropoietin 40,000 units subcutaneously once weekly for the management of the anemia (N=251). If serum hemoglobin concentrations continued to decrease to less than or equal to 8.5 g per dL, subjects could be treated with additional anemia interventions, including the addition of erythropoietin (18% of those in the ribavirin dose reduction arm) or ribavirin dose reduction (37% of those in the ESA arm).
Mean age of subjects randomized was 49 years. The racial distribution of subjects was as follows: 77% White, 19% Black, and 4% other. The distribution of subjects by gender was 37% men and 63% women.
The overall intent-to-treat SVR rate for all enrolled subjects (including those subjects who were not randomized to RBV dose reduction or ESA for the management of anemia) was 63% (431/687). The SVR rate in subjects randomized who received ribavirin dose reduction was 71% (178/249), similar to the SVR rate of 71% (178/251) in subjects randomized to receive an ESA. The relapse rates in subjects randomized to receive ribavirin dose reduction or an ESA were 10% (19/196) and 10% (19/197), respectively.
How Supplied
There is limited information regarding Boceprevir How Supplied in the drug label.
Storage
There is limited information regarding Boceprevir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Boceprevir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Boceprevir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Boceprevir Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Boceprevir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Boceprevir Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Boceprevir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Kiser JJ, Burton JR, Anderson PL, Everson GT (May 2012). "Review and Management of Drug Interactions with Boceprevir and Telaprevir". Hepatology. 55 (5): 1620–8. doi:10.1002/hep.25653. PMC 3345276.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Boceprevir (INN) is a protease inhibitor being studied as a treatment for hepatitis C.[1][2]It is being developed by Schering-Plough.[3] As of 2008, it is in phase II clinical trials.[3]The hepatitis C virus, often described as the “silent epidemic,” affects more than 170-180 million people around the world and as the most common blood borne infection worldwide it has become a serious global health crisis.2 [4] [5][6] It is currently the leading cause of chronic liver diseases which include cirrhosis, carcinoma and liver failure, and approximately 130 million patients with the disease are at high risk of developing one of these conditions.4 HCV is an enveloped virus with a 9.6 kb single-stranded RNA genome that serves as a template for viral replication that is translated into a polyprotein and cleaved by proteases to allow for viral assembly.5
Before the development of new, more successful drug therapies such as boceprevir, the leading standard treatment therapy included the combination of pegylated interferon and ribavirin over a prolonged period of 24 to 48 weeks.2 4 However, for the genotype 1 strain of the virus, which is the most prevalent, this regimen achieves the goal of sustained virologic response (SVR), in only about 50% of treated patients, and it tends to be poorly tolerated and requires injection.[7] [8] Boceprevir is part of a treatment regimen that has been found to easier to administer, less toxic, and overall more effective. It is expected that the development of boceprevir and other new treatment will significantly broaden the treatment options for infected individuals.4
The FDA has recently approved the drug boceprevir as a new and improved HCV therapy to be used in combination with peginterferon and ribavirin, the previous standard of treatment.[9] It is widely agreed that boceprevir seems to be a safe and effective treatment innovation. The drug will treat patients with hepatitis C genotype 1.1 Boceprevir, marketed as Victrelis by Merck, is the first HCV protease inhibitor to reach market and it expected to be a major advance in HCV treatment. Throughout its phases of study, boceprevir has been shown to provide more effective treatment than just the combination of peginterferon and ribavirin alone, and will offer a greater chance of cure than the previous standards of therapy.
Category
Antiviral
US Brand Names
VICTRELIS®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Boceprevir is an inhibitor of the HCV NS3/4A protease that is necessary for the proteolytic cleavage of the HCV encoded polyprotein into mature forms of the NS4A, NS4B, NS5A and NS5B proteins. Boceprevir covalently, yet reversibly, binds to the NS3 protease active site serine (S139) through an (alpha)-ketoamide functional group to inhibit viral replication in HCV-infected host cells. In a biochemical assay, boceprevir inhibited the activity of recombinant HCV genotype 1a and 1b NS3/4A protease enzymes, with Ki values of 14 nM for each subtype.
References
- ↑ Degertekin B, Lok AS (2008). "Update on viral hepatitis: 2007". Curr. Opin. Gastroenterol. 24 (3): 306–11. doi:10.1097/MOG.0b013e3282f70285. PMID 18408458. Unknown parameter
|month=ignored (help) - ↑ Njoroge FG, Chen KX, Shih NY, Piwinski JJ (2008). "Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection". Acc. Chem. Res. 41 (1): 50–9. doi:10.1021/ar700109k. PMID 18193821. Unknown parameter
|month=ignored (help) - ↑ 3.0 3.1 "Interim Results from Boceprevir Phase II Study in Genotype 1 Treatment-Naive Hepatitis C Patients Presented At EASL - Forbes.com" (Press release). Forbes.com. Retrieved 2008-05-19.
- ↑ Susser, Simone, Christoph Welsch, Yalan Wang, Markus Zettler, Franciso S. Domingues, Ursula Karey, Eric Hughes, Robert Ralston, Xiao Tong, Eva Herrmann, Stefan Zeuzem, and Christoph Sarrazin. "Characterization of Resistance to the Protease Inhibitor Boceprevir in Hepatitis C Virus–infected Patients." Hepatology 50.6 (2009): 1709-718.
- ↑ Asselah, Tarik, and Patrick Marcellin. "New Direct-acting Antivirals' Combination for the Treatment of Chronic Hepatitis C." Liver International 31.S1 (2011): 68-22.
- ↑ Kwo, Paul Y., and Rakesh Vinayek. "The Therapeutic Approaches for Hepatitis C Virus: Protease Inhibitors and Polymerase Inhibitors." Gut and Liver 5.4 (2011): 406-17.
- ↑ DeNoon, Daniel J. "Boceprevir Boosts Hepatitis C Treatment Success." WebMD. WebMD Health News, 9 Aug. 2010. Web. <http://www.webmd.com/hepatitis/news/20100809/boceprevir-ups-hepatitis-c-treatment-success>.
- ↑ Flint, Mike, Stanley Mullen, Anne M. Deatly, Wei Chen, Lynn Z. Miller, Robert Ralston, Colin Broom, Emilio A. Emini, and Anita Y. M. Howe. "Selection and Characterization of Hepaitis C Virus Replicons Dually Resistant to the Polymerase and Protease Inhibitors HCV-769 and Boceprevir." Antimicrobial Agents and Chemotherapy 53.2 (2009): 401-11.
- ↑ Walker, Emily P. "Boceprevir Wins FDA Approval to Treat Hepatitis C." MedPage Today. N.p., 13 May 2011. Web.<http://www.medpagetoday.com/InfectiousDisease/Hepatitis/26469>.