Arachnoiditis: Difference between revisions
No edit summary |
|||
| Line 9: | Line 9: | ||
The phenomenon of adhesive arachnoiditis was first described by Quinke in 1893 in a [[case report]]. In 1897, Schwarz wrote about [[signs]] and [[symptoms]] of arachnoiditis caused by [[syphilis]]. Elkington wrote about the classic description of arachnoiditis in 1936 and termed it as [[meningitis]] serosa circumscripta spinalis. He also described various presentations of arachnoiditis in 1951. Foix and Alajouanine described a noninfectious spinal arachnoid scar reaction in 1926 and termed it as hypertrophic [[vascular]] ascending [[myelitis]]. Ransome and Monterio mentioned that [[tuberculous meningitis]] might present as extensive spinal arachnoiditis. The first case of arachnoiditis was associated with [[syphilis]].<ref>Elkington, J. St. C. (1951). Arachnoiditis. In Modern Trends in Neurology, ed. Anthony Feiling, ch. 5, pp. 149-161. Butterworths, London.</ref><ref>ELKINGTON, J. St. C. (1936). [https://doi.org/10.1093/brain/59.2.181 Meningitis serosa circumscripta spinalis (spinal arachnoiditis)]. Brain, 59, 181-203</ref><ref>Foix C, Alajouanine T . La myélite nécrotique subaigue. ''Rev Neurol (Paris)'' 1926; 2: 1–42.</ref><br /> | The phenomenon of adhesive arachnoiditis was first described by Quinke in 1893 in a [[case report]]. In 1897, Schwarz wrote about [[signs]] and [[symptoms]] of arachnoiditis caused by [[syphilis]]. Elkington wrote about the classic description of arachnoiditis in 1936 and termed it as [[meningitis]] serosa circumscripta spinalis. He also described various presentations of arachnoiditis in 1951. Foix and Alajouanine described a noninfectious spinal arachnoid scar reaction in 1926 and termed it as hypertrophic [[vascular]] ascending [[myelitis]]. Ransome and Monterio mentioned that [[tuberculous meningitis]] might present as extensive spinal arachnoiditis. The first case of arachnoiditis was associated with [[syphilis]].<ref>Elkington, J. St. C. (1951). Arachnoiditis. In Modern Trends in Neurology, ed. Anthony Feiling, ch. 5, pp. 149-161. Butterworths, London.</ref><ref>ELKINGTON, J. St. C. (1936). [https://doi.org/10.1093/brain/59.2.181 Meningitis serosa circumscripta spinalis (spinal arachnoiditis)]. Brain, 59, 181-203</ref><ref>Foix C, Alajouanine T . La myélite nécrotique subaigue. ''Rev Neurol (Paris)'' 1926; 2: 1–42.</ref><br /> | ||
==Classification== | ==Classification== | ||
Arachnoiditis may be classified according to National Organization for Rare Disorders (NORD) into 8 subtypes: | |||
*[[Adhesive]] Arachnoiditis | *[[Adhesive]] Arachnoiditis | ||
| Line 20: | Line 20: | ||
* Rhinosinusogenic Cerebral Arachnoiditis | * Rhinosinusogenic Cerebral Arachnoiditis | ||
Arachnoiditis may be classified according to Delamarter's MRI classification into 3 subtypes: | |||
• Type I—appears as an [[adhesive]] mass of adherent roots centrally in the thecal sac, considered mild arachnoiditis. | • Type I—appears as an [[adhesive]] mass of adherent roots centrally in the thecal sac, considered mild arachnoiditis. | ||
Revision as of 13:09, 21 July 2020
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Qasim Khurshid, M.B.B.S.[2]
Overview
Arachnoiditis is a term used to describe thickening, inflammation, and scaring of the arachnoid membrane, which is the middle layer surrounding the central nervous system. These abnormalities may be self-limited or may cause compression of the nerve roots and spinal cord. Patients may report a variety of clinical symptoms, including severe back pain that persists at rest, perineal/saddle numbness, neurological deficits, skin rashes, and sympathetic or vascular changes. The cause of arachnoiditis is unknown but may occur as a result of invasion (surgery), neuraxial injections, certain myelograms, infections, blood, a herniated disc, or tumors. Diagnosis is based on symptoms, and magnetic resonance imaging. Unfortunately, the treatment of arachnoiditis is difficult and limited and should focus on symptomatic management.
Historical Perspective
The phenomenon of adhesive arachnoiditis was first described by Quinke in 1893 in a case report. In 1897, Schwarz wrote about signs and symptoms of arachnoiditis caused by syphilis. Elkington wrote about the classic description of arachnoiditis in 1936 and termed it as meningitis serosa circumscripta spinalis. He also described various presentations of arachnoiditis in 1951. Foix and Alajouanine described a noninfectious spinal arachnoid scar reaction in 1926 and termed it as hypertrophic vascular ascending myelitis. Ransome and Monterio mentioned that tuberculous meningitis might present as extensive spinal arachnoiditis. The first case of arachnoiditis was associated with syphilis.[1][2][3]
Classification
Arachnoiditis may be classified according to National Organization for Rare Disorders (NORD) into 8 subtypes:
- Adhesive Arachnoiditis
- Arachnoiditis Ossificans
- Cerebral Arachnoiditis
- Hereditary Arachnoiditis
- Neoplastic Arachnoiditis
- Optochiasmatic Arachnoiditis
- Postmyelographic Arachnoiditis
- Rhinosinusogenic Cerebral Arachnoiditis
Arachnoiditis may be classified according to Delamarter's MRI classification into 3 subtypes:
• Type I—appears as an adhesive mass of adherent roots centrally in the thecal sac, considered mild arachnoiditis.
• Type II—the empty sac, the roots adhere peripherally to the meninges.
• Type III—a soft-tissue mass replaces the subarachnoid space.
Wilkinson developed another classification system that divided the myelographic appearances into four groups.
• Type I: A unilateral focal defect centered on the nerve root exit pouch adjacent to the intervertebral disc space.
• Type II: An annular or a circumferential defect with a bilateral notch and only filiform passage of the medium.
• Type III: Complete transverse obstruction with the picture of stalagmites, candle-guttering, or paintbrush defects.
• Type IV: An infundibuliform cul-de-sac with loss of radicular striation, a vitreous appearance, and cutting-off of the root sleeves.
Pathophysiology
{{#ev:youtube|XEBaA9LBZug}}Pathologically, an initial insult to the pia-arachnoid membrane precipitates a response to the injury leading to adhesion formation like other serous membranes. Initially,pia-arachnoid becomes inflamed, causing nerve root swelling that leads to radicular symptoms. Due to the lack of arachnoid vasculature, there are few enzymes bearing leukocytes and circulating CSF serves to dilute and carry off fibrinolytic enzymes and phagocytes. This situation allows the build‐up of fibrinous bands upon which collagen is deposited. This reduced blood supply, adhesions between the contents of the theca, encapsulation, and tethering of the nerve roots and cord result in recurrent microtrauma and atrophy. This process leads to the formation of adhesive arachnoiditis. Arachnoiditis also significantly reduces the flow of CSF, resulting in a reduced supply of nutrients to the neural elements.[4][5][6]
Causes
Common causes of arachnoiditis include the following.[7]
- Mechanical injury during spinal surgery ( about 60% cases)
- Spinal trauma
- neuroaxial (spinal and epidural) anesthesia ( about 22% cases)
- one or more spinal tabs (7% cases)
- myelography
- bacterial and viral spinal infections (7%)
- Spinal tumors
- Repeated subarachnoid injections of anticancer drugs or antimetabolites
- Spinal cord contamination
Differentiating arachnoiditis from other Diseases
Arachnoiditis is a clinical diagnosis that is supported by a combination of clinical history, physical examination, and radiography. Following pain syndromes may mimic arachnoiditis and should be considered in the differential diagnosis.[8][9][10]
- spinal cord tumors
- cauda equina syndrome
- arachnoiditis ossificans,
- failed back syndrome
- syringomyelia
- Spinal cord infections
| Onset | Disease | Symptoms | Gold Standard
Test |
CT/MRI Findings | Other Investigation Findings | |
|---|---|---|---|---|---|---|
| Headache
Characteristics |
Associated Features | |||||
| Sudden | Epidural hematoma [11][12][13][14] |
|
CT scan without contrast | Biconvex lens shaped hematoma which expand inward toward the brain rather than along the inside of the skull |
| |
| Meningitis[15][16]
|
Headache is associated with: | Lumbar puncture for CSF |
|
| ||
| Gradual | Intracranial mass[17][18] | Morning headache | MRI |
|
||
| Spinal disc herniation[19][20][21][22] | Soft central nucleus pulposus bulging out, lower back pain, leg pain, tingling, numbness, and reflex changes | |||||
| Vertebral osteomyelitis[23][24] | ||||||
| Arachnoiditis .[25][26][27] |
|
MRI | MRI findings include adherent nerve roots located centrally in the thecal sac, empty sac( roots are adherent to the walls of the thecal wall), and a mass of soft tissue replacing the subarachnoid space |
| ||
Epidemiology and Demographics
Arachnoiditis is a rare disorder. Its exact incidence is hard to determine as its difficult-to-diagnose condition with a wide range of clinical presentations. Some cases of arachnoiditis may go undiagnosed or misdiagnosed. Therefore the exact incidence of arachnoiditis remains not only unknown but also likely significantly underestimated. However, recently the number of lumbar arachnoiditis cases are increasing due to lumbar supine surgeries and spinal anesthesia.[28] [29]
Arachnoiditis affects more females than males because two-thirds of pregnant females receive spinal or epidural anesthesia during child delivery.
Risk Factors
The major risk-factors of arachnoiditis are briefly highlighted below:
- Bacterial, viral or fungal infections that spread to the brain and spinal cord
- Diseases that weaknen the immune system e.g AIDS, diabetes or organ transplantation
- Surgical or needle neural trauma
- Intrathecal corticosteroids
- Unintentional injection of subarachnoid blood
- Injection of neurotoxic or neuroirritant substances into the subarachnoid space
- Cancers such as melanoma, non-Hodgkin’s lymphoma, breast cancer, lung cancer as well as other cancers specific to the brain
Screening
There is insufficient evidence to recommend routine screening for arachnoiditis.
Natural History, Complications, and Prognosis
Symptoms of arachnoiditis often start after many years of the suspected inciting event. Chronic arachnoiditis can vary in severity from mild to moderate or may progress to severe and catastrophic, disrupting quality of life. There may be remissions and relapses; however: symptoms may resolve with treatment. The course of chronic arachnoiditis is typically irregular, but it is progressive in up to 33% of patients and non-progressive in 50-59%.
Aside from the chronic pain and various possible neurologic deficits, common complications of arachnoiditis may include hydrocephalus, syringomyelia, and arachnoid cysts. A rare complication is internal malabsorptive hydrocephalus.[30][31]
Supportive care and symptomatic management is the mainstay of treatment. Although this is not a life-threatening condition, the quality of life can be significantly compromised due to pain and neurological deficits. The course of the disease is very unpredictable. The majority of the patients have a stable neurological disability that does not progress over time.
Diagnosis
Diagnostic Study of Choice
There is no single best test for the definitive diagnosis of arachnoiditis. Diagnosis is based on clinical presentation, signs, and the supporting MRI or CT or myelography. The gold standard is MRI.
History and Symptoms
Patients suggestive of arachnoiditis should be asked about any history of spinal trauma, spinal cord infections, spinal surgery, or spinal anesthesia. Sometimes an inciting event can not be identified. Arachnoiditis symptoms vary from subclinical to advance and serve. Symptoms of arachnoiditis are challenging to distinguish from other types of compressive neurological diseases because many of the symptoms are similar, and the disease onset can be months to years from the initial injury. Patients will complain about pain, numbness, paraesthesia, and tingling in affected nerve root or roots.
- Chronic and persistent pain in the lower back that may radiate down the legs
- Muscle cramps, spasms, and uncontrolled twitching
- Burning in the ankles and feet
- Unexplained skin rashes and itching
- Loss of sensation below the area afflicted
- Partial or complete paralysis of the lower extremities
- Neurologic deficits
Physical Examination
The findings of the physical examination depend on which nerves are affected by the disease. A physical examination can reveal changes in reflexes, sensation, proprioception, or weakness.
Abnormal findings on the physical exam may include the following.
- Reduced reflexes in extremities which are often asymmetrical
- Loss of touch or vibration sensation
- Pain on arms extension
- Weakness of the extremities which is often asymmetrical
- Restriction of range of extremities
- Asymmetry of paraspinal muscle groups
- Indentation of lower supine
Laboratory Findings
While the definitive diagnosis of arachnoiditis requires imaging, other tests contribute to understanding the risks of disease and may provide clues to the selection of treatment options. These tests include those that access the underlying inflammation and other hormonal levels that may be altered in chronic pain conditions. Addressing these conditions may improve the quality of life.
Electrocardiogram
X-ray
Echocardiography or Ultrasound
Routine plain radiographs can not be used to evaluate spinal cord and nerve roots. However, the contents of the thecal sac can be evaluated with myelography with intrathecal administration of iodinated contrast. Conus medullaris usually terminates between T12-L1 and L1-L2 levels in adults. Below the level of conus medullaris termination, nerve roots float freely within the thecal sac. Meningeal inflammation can lead to thickened nerve roots, the formation of CSF loculations, and blockage of CSF flow. Radiographic findings can aid in the diagnosis.
CT scan
MRI is superior to CT scanning for the diagnosis of arachnoiditis because of poor contrast resolution in CT scans. However, CT myelography is useful in demonstrating the classic imaging findings of arachnoiditis. The classic image findings include narrowing or blockage of the subarachnoid space, thickened or matted nerve roots, soft tissue mass within the arachnoid space, as well as intrathecal calcification, irregular collections of contrast material, and absent filling of nerve root sleeves. The degree of confidence is high with myelography as compared to conventional CT. [32]
MRI
Presently, the diagnosis of arachnoiditis is often made through the use of magnetic resonance imaging (MRI). Historically, myelography was used to diagnose arachnoiditis. The prominent radiological findings included observance of various patterns of filling defects and prominent nerve roots. The advent of CT and MRI has made the diagnosis easier. MRI is preferred over conventional myelography because of its noninvasive nature. Typical MRI findings include adherent nerve roots located centrally in the thecal sac(shown in figure), empty sac( roots are adherent to the walls of the thecal wall), and a mass of soft tissue replacing the subarachnoid space. These findings are well seen on T-2 weighted images. Contrast administration with MRI can be used to differentiate arachnoiditis from other diseases such as infections and tumors, but the contract is not needed to visualize the appearance of the characteristic of arachnoiditis.MRI has a high specificity of 100%, high sensitivity of 92 % as well as high accuracy of 99%.[33][34]
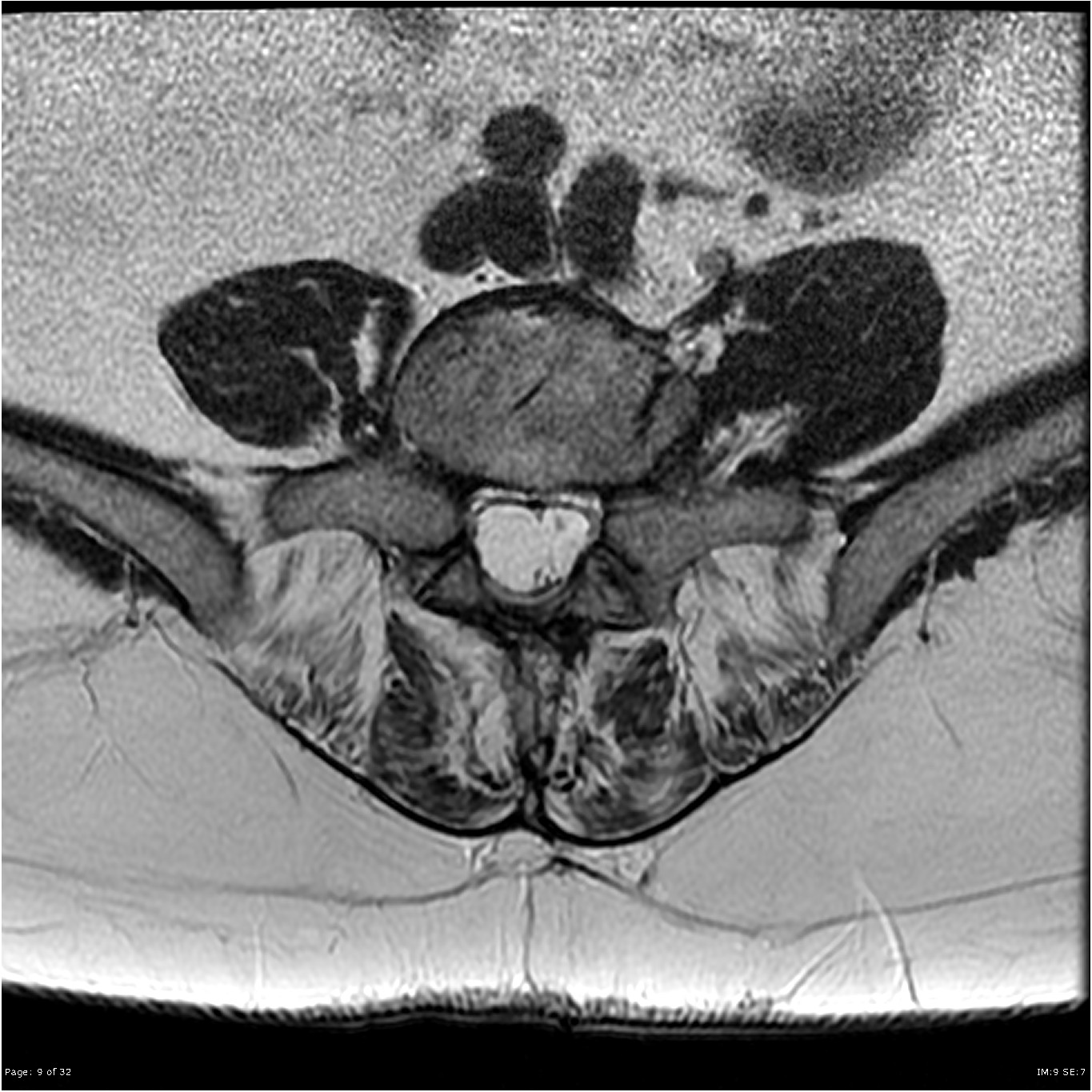
Other Imaging Findings
There are no other image findings associated with arachnoiditis.
Other Diagnostic Studies
Treatment
Arachnoiditis is a difficult condition to treat. Treatment is limited to alleviation of pain and other symptoms. Surgical intervention generally has a poor outcome and only provides temporary relief. Steroid injection is generally discouraged and may worsen the condition.
Medical Therapy
Treatment of arachnoiditis is palliative, and mostly medications are used for this purpose. Antidepressant and anticonvulsant analgesics are the mainstays of treatment. However, other classes of medications can also be beneficial. The U.S. Food and Drug Administration has approved several medications for neuropathic pain syndromes: pregabalin and duloxetine for diabetic neuropathy and gabapentin and pregabalin for postherpetic neuralgia. These medications are often used for the treatment of arachnoiditis with variable efficacy. These medications are often started at a low dosage and titrated upward as tolerated. Most of these medications take a few weeks to take analgesic effects to be reached. Some examples of these medications dosage are given in the table.[35]
| Medication | Starting dose | Dose increase and interval | Maximal dose |
|---|---|---|---|
| Tricyclic anti depressant | 10-25mg at night | 10-25 mg per week | 150 mg /day |
| Duloxetine | 20-30mg per day | 20-30 mg per week | 60mg /day |
| Pregabalin | 50-75mg BID | 50-75 mg per week | 600mg /day |
| Gabapentine | 100-300mg BID | 100-300 mg per week | 1800-3600mg /day |
Anti-inflammatory medications (NSAIDs) are commonly used with modest efficacy. The majority of the patients with arachnoiditis have a concomitant lumbar disc herniation or osteoarthritis of the facet joints that can respond to anti-inflammatory medications. Likewise, back pain in these patients can also be treated with trials of muscle relaxants or antispasticity agents such as baclofen and tizanidine. Opiates are often prescribed with varying degrees of usefulness. In general, neuropathic pain is less responsive to opiates. One of the significant limitations of opiate use is the development of tolerance that requires dose escalation. Medications side effects are common. Antidepressant and anticonvulsant analgesics are often causing sedation or altered mental status. Opiates can produce sedation as well as constipation. NSAIDs that are often used to treat musculoskeletal pain associated with arachnoiditis can cause gastrointestinal side effects, can affect kidneys and can aggravate hypertension. Rehabilitation modalities are generally considered safe, but the inappropriate application of superficial modalities can cause thermal injury. Spinal nerve stimulation is contraindicated in patients having cardiac pacemakers. Therapeutic exercises can aggravate the pain.
Surgery
Surgical intervention has a limited role in the treatment of arachnoiditis. There is no surgical method to untangle the nerve roots. Indications for surgery include rapidly progressive neurological deterioration such as myelopathy due to syringomyelia or cauda equina syndrome from arachnoiditis ossificans. In these cases, surgical intervention such as stent placement or removal of calcified mass can be done. These procedures are done to halt the progression of neurological deterioration.
Rehabilitation
Rehabilitation intervention can be divided into modalities for therapeutic exercises to improve functional status and for pain management. Modalities such as ice application and heat application are useful for mechanical back pain that is often associated with arachnoiditis. Electrical nerve stimulation is primarily used to treat neuropathic pain, but it can also treat associated musculoskeletal pain. Unfortunately, exercises provide little benefit for symptomatic improvement, but exercise is still the part of treatment. Therapeutic exercises such as stretching and aerobic exercises can improve musculoskeletal pain in a subset of patients. Patients with intractable pain often avoid exercise due to fear of pain aggravation. Because of this they often become deconditioned and benefit from progressive exercise regimens.[35]
Procedures
The most effective treatment for intractable pain that is associated with arachnoiditis is spinal cord nerve stimulation. This procedure involves the placement of the stimulating electrode over the dorsal aspect of the spinal cord that can be placed either subcutaneously or through a laminectomy. The exact location of electrode placement depends on the location of the patient's pain. Typically, the patient undergoes a percutaneous trial to test efficacy before placing permanent implantation. Spinal cord stimulation is more effective for neuropathic pain as compared to musculoskeletal pain. Only limited data is available for the support of neuraxial corticostriatal injections such as nerve blocks and epidural steroids. These epidural injections may provide short term symptom-free intervals.
Primary Prevention
Following strategies can be used to prevent the occurrence of arachnoiditis:
- The necessity for invasive surgery should be avoided unless required, and there is no other alternative.
- Women could select natural childbirth that usually does not require epidural administration of anesthesia.
- Physicians could avoid unnecessary invasive investigative techniques such as intrathecal injections of drugs , dyes, or chemicals.
Secondary Prevention
Future or Investigational Therapies
According to recent research, cytokines produced by various cells are in the body may be responsible for generating pain. Medications that block the action or release of cytokines may be useful for reducing pain. Various kinds of these medications are being used to treat painful chronic diseases such as Crohn,s disease, and Rheumatoid arthritis. Thalidomide, an anti-cytokine medication is being evaluated for its effectiveness in treating the pain associated with Arachnoiditis.
External links
- Circle Of Friends With Arachnoiditis (COFWA)
- American Chronic Pain Association (ACPA)
- National Chronic Pain Outreach Association (NCPOA)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- National Institute of Dental and Craniofacial Research (NIDCR)
- Arachnoiditis
- Arachnoiditis, if you have ever suffered from back pain or know anyone who has then this is where to find help and information.
- Support for those with disorders of the Arachnoid Membrane
- http://www.burtonreport.com/InfSpine/AdhesArachAnatomy.htm
References
- ↑ Elkington, J. St. C. (1951). Arachnoiditis. In Modern Trends in Neurology, ed. Anthony Feiling, ch. 5, pp. 149-161. Butterworths, London.
- ↑ ELKINGTON, J. St. C. (1936). Meningitis serosa circumscripta spinalis (spinal arachnoiditis). Brain, 59, 181-203
- ↑ Foix C, Alajouanine T . La myélite nécrotique subaigue. Rev Neurol (Paris) 1926; 2: 1–42.
- ↑ Quiles M, Marchisello PJ, Tsairis P. Lumbar adhesive arachnoiditis. Etiologic and pathologic aspects. Spine (Phila Pa 1976). 1978;3(1):45-50. doi:10.1097/00007632-197803000-00010
- ↑ Burton CV. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):24-30. doi:10.1097/00007632-197803000-00006
- ↑ Miaki K, Matsui H, Nakano M, Tsuji H. Nutritional supply to the cauda equina in lumbar adhesive arachnoiditis in rats. Eur Spine J. 1999;8(4):310-316. doi:10.1007/s005860050179
- ↑ "Antonio Aldrete. Arachnoiditis. Orphanet. March, 2010".
- ↑ Vloeberghs M, Herregodts P, Stadnik T, Goossens A, D'Haens J. Spinal arachnoiditis mimicking a spinal cord tumor: a case report and review of the literature. Surg Neurol. 1992;37(3):211-215. doi:10.1016/0090-3019(92)90233-d
- ↑ Wright MH, Denney LC. A comprehensive review of spinal arachnoiditis. Orthop Nurs. 2003;22(3):215-221. doi:10.1097/00006416-200305000-00010
- ↑ Martin RJ, Yuan HA. Neurosurgical care of spinal epidural, subdural, and intramedullary abscesses and arachnoiditis. Orthop Clin North Am. 1996;27(1):125-136.
- ↑ Scheibl A, Calderón EM, Borau MJ, Prieto RM, González PF, Galiana GG (2012). "Epidural hematoma". J Pediatr Surg. 47 (2): e19–21. doi:10.1016/j.jpedsurg.2011.10.078. PMID 22325415.
- ↑ Yanagawa Y, Sakamoto T, Okada Y (2007). "Clinical features of temporal tip epidural hematomas". J Neurosurg. 107 (1): 18–20. doi:10.3171/JNS-07/07/0018. PMID 17639868.
- ↑ Maugeri R, Anderson DG, Graziano F, Meccio F, Visocchi M, Iacopino DG (2015). "Conservative vs. Surgical Management of Post-Traumatic Epidural Hematoma: A Case and Review of Literature". Am J Case Rep. 16: 811–7. PMC 4652627. PMID 26567227.
- ↑ Mitsuyama T, Ide M, Kawamura H (2004). "Acute epidural hematoma caused by contrecoup head injury--case report". Neurol Med Chir (Tokyo). 44 (11): 584–6. PMID 15686177.
- ↑ Schwermer, Benedikt; Eschle, Daniel; Bloch-Infanger, Constantine (2017). "Fieber und Kopfschmerzen nach Thailandurlaub". DMW - Deutsche Medizinische Wochenschrift. 142 (14): 1063–1066. doi:10.1055/s-0043-106282. ISSN 0012-0472.
- ↑ Rapalino, Otto; Mullins, Mark E. (2017). "Intracranial Infectious and Inflammatory Diseases Presenting as Neurosurgical Pathologies". Neurosurgery. 81 (1): 10–28. doi:10.1093/neuros/nyx201. ISSN 0148-396X.
- ↑ Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S (2017). "Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours". Nat Rev Neurol. 13 (1): 52–64. doi:10.1038/nrneurol.2016.185. PMID 27982041.
- ↑ Sato N, Sze G, Endo K (1998). "Hypophysitis: endocrinologic and dynamic MR findings". AJNR Am J Neuroradiol. 19 (3): 439–44. PMID 9541295.
- ↑ Stern, Scott D. (2006). "Back Pain". In Janet Foltin, Harriet Lebowitz, Karen Davis. Symptom to Diagnosis: An Evidence-Based Guide. New York: Lange Medical Books/McGraw-Hill. pp. 67–81. ISBN 0071463895. Unknown parameter
|coauthors=ignored (help) - ↑ Weinstein JN, Tosteson TD, Lurie JD; et al. (2006). "Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial". JAMA. 296 (20): 2441–50. doi:10.1001/jama.296.20.2441. PMID 17119140.
- ↑ Weinstein JN, Lurie JD, Tosteson TD; et al. (2006). "Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort". JAMA. 296 (20): 2451–9. doi:10.1001/jama.296.20.2451. PMID 17119141.
- ↑ Peul WC, van Houwelingen HC, van den Hout WB; et al. (2007). "Surgery versus prolonged conservative treatment for sciatica". N. Engl. J. Med. 356 (22): 2245–56. doi:10.1056/NEJMoa064039. PMID 17538084.
- ↑ Carek, M.D., Peter (15). "Diagnosis and Management of Osteomyelitis". American Family Physician. 12 (63): 2413–2421. Retrieved March 27, 2012. Unknown parameter
|month=ignored (help); Unknown parameter|coauthors=ignored (help); Check date values in:|date=, |year= / |date= mismatch(help) - ↑ National Center for Biotechnology Information (2000). "Discitis versus Vertebral Osteomyelitis". Archives of Disease in Childhood. 4 (83): 368. PMC 1718514. PMID 10999882. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Quiles M, Marchisello PJ, Tsairis P. Lumbar adhesive arachnoiditis. Etiologic and pathologic aspects. Spine (Phila Pa 1976). 1978;3(1):45-50. doi:10.1097/00007632-197803000-00010
- ↑ Burton CV. Lumbosacral arachnoiditis. Spine (Phila Pa 1976). 1978;3(1):24-30. doi:10.1097/00007632-197803000-00006
- ↑ Miaki K, Matsui H, Nakano M, Tsuji H. Nutritional supply to the cauda equina in lumbar adhesive arachnoiditis in rats. Eur Spine J. 1999;8(4):310-316. doi:10.1007/s005860050179
- ↑ Anderson TL, Morris JM, Wald JT, Kotsenas AL. Imaging Appearance of Advanced Chronic Adhesive Arachnoiditis: A Retrospective Review. AJR Am J Roentgenol. 2017;209(3):648-655. doi:10.2214/AJR.16.16704
- ↑ Todeschi J, Chibbaro S, Gubian A, Pop R, Proust F, Cebula H. Spinal adhesive arachnoiditis following the rupture of an Adamkiewicz aneurysm: Literature review and a case illustration. Neurochirurgie. 2018;64(3):177-182. doi:10.1016/j.neuchi.2017.11.003
- ↑ Di Ieva A, Barolat G, Tschabitscher M, et al. Lumbar arachnoiditis and thecaloscopy: brief review and proposed treatment algorithm. Cent Eur Neurosurg. 2010;71(4):207-212. doi:10.1055/s-0029-1243201
- ↑ Anderson TL, Morris JM, Wald JT, Kotsenas AL. Imaging Appearance of Advanced Chronic Adhesive Arachnoiditis: A Retrospective Review. AJR Am J Roentgenol. 2017;209(3):648-655. doi:10.2214/AJR.16.16704
- ↑ APA Dong, Aisheng, MD*; Zuo, Changjing, MD*; Zhang, Ping, MSc†; Lu, Jianping, MD‡; Bai, Yushu, MD§ MRI and FDG PET/CT Findings in 3 Cases of Spinal Infectious Arachnoiditis, Clinical Nuclear Medicine: October 2014 - Volume 39 - Issue 10 - p 900-903
- ↑ Delamarter RB, Ross JS, Masaryk TJ, Modic MT, Bohlman HH. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine (Phila Pa 1976). 1990;15(4):304-310. doi:10.1097/00007632-199004000-00011
- ↑ Johnson CE, Sze G. Benign lumbar arachnoiditis: .MR imaging with gadopentetate dimeglumine AJNR Am J Neuroradiol. 1990;11(4):763-770.
- ↑ 35.0 35.1 Frontera, Walter R., J. K. Silver, and Thomas D. Rizzo. Essentials of physical medicine and rehabilitation : musculoskeletal disorders, pain, and rehabilitation. Philadelphia, PA: Elsevier Saunders, 2015. Print. Frontera, Walter R., J. K. Silver, and Thomas D. Rizzo. Essentials of physical medicine and rehabilitation : musculoskeletal disorders, pain, and rehabilitation. Philadelphia, PA: Elsevier Saunders, 2015. Print.