Anoxic brain injury
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editors-In-Chief: Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.
Synonyms and keywords: Hypoxic brain injury, post cardiac arrest syndrome
Overview
Post-cardiac arrest: is defined as absence of pulses requiring chest compressions, regardless of location or presenting rhythm.
Post-cardiac arrest syndrome: is characterized by resumption of spontaneous systemic circulation after prolonged ischemia of whole body.[1] Anoxic or hypoxic brain injury is often seen after cardiac arrest as part of the post-cardiac arrest syndrome. Major efforts are underway to improve "The Chain of Survival" based upon early access to medical care, early defibrillation, early CPR and early hospital care. Therapeutic hypothermia may improve outcomes. Steroids, manitol, diuresis and hyperventilation have not been documented to meaningfully improve clinical outcomes.
Epidemiology
In a 1990s study from the UK, resuscitation for cardiac arrest was attempted in 10,081 patients. Of these only 1476 (14.6%) survived to be admitted to the hospital [2][3]. Of these small number of patients who survived to admission, 59.3% died during that admission, half of these within the first 24 hours. 46.1% survived to hospital discharge (this is 6.75% of those who had been resuscitated by ambulance staff). Of those who were successfully discharged from hospital, 70% were still alive 4 years after their discharge.
In a review of 68 studies through 1997, the incidence of survival to discharge was higher at 14% with a wide range of 0-28%.[4]
Pathophysiology
The underlying mechanism of post cardiac arrest syndrome is a combination of: [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15]
- Systemic response to ischemia and reperfusion
- Circulatory collapse
- Hyperglycemia
- Hypotension
- Infections
- Multiorgan failure
- Ongoing tissue hypoxia / ischemia
- Fever
- Myocardial dysfunction
- Circulatory collapse
- Dysrhythmias
- Hypotension
- Reduced cardiac output
- Brain death
- Cognitive dysfunction
- Coma
- Cortical stroke
- Myoclonus
- Persistent vegetative state
- Secondary parkinsonism
- Seizures
- Spinal stroke
- Effects of persistent precipitating pathologies
- Cardiovascular disease (Acute coronary syndromes, cardiomyopathy)
- Chronic obstructive pulmonary disease
- Central nervous system diseases (e.g. cerebrovascular accident)
- Thromboembolic disorders (e.g. pulmonary emboli)
- Drug / substance overdose, poisoning
- Infections (sepsis, pneumonia)
- Volume loss (Hypovolemia: e.g. hemorrhage, dehydration)
Natural History
Patients with anoxic injury due to cardiac arrest are at risk of death from a variety of causes including recurrent sudden cardiac death, congestive heart failure, pneumonia, sepsis from a variety of sources and pulmonary embolism.
Signs and Symptoms
Serial neurologic examinations are critical in the assessment of long term prognosis.
Assessment of the Brain Stem
The brainstem is more resistant to hypoperfusion that the cerebral cortex, and if the brainstem does not recover, the cerebral cortex is not likely to recover. The presence of brain stem reflexes is therefore critical to recovery. Preservation of brainstem function is indicated by the presence of blinking, coughing, gagging, sneezing, and yawning.
Pupillary Size
The presence of peristently dilated pupils is a poor prognostic sign [16]. It should be noted that both catecholamines and atropine, can affect pupillary size, and confound the assessment of pupillary size.
Laboratory Studies
(In alphabetical order)[17] [18] [19]
- Arterial blood gases
- Blood glucose. Elevated blood glucose is associated with a poorer prognosis.
- Cardiac output monitoring with noninvasive methods or pulmonary artery catheter
- Chest x-ray to evaluate for aspiration pneumonia
- Complete blood count
- Continuous ECG monitoring to prompltly shock any recurrent arrhythmia.
- Echocardiography to evaluate LV function and assess for the presence of hypertrophic obstructive cardiomyopathy (HOCM)
- EEG for early seizure detection and treatment
- Electrolytes to reat hyopkalemia and hypomagnesemia
- Cardiac enzymes to assess MI size
- Oxygen saturation by pulse oximetry
- Placement of an arterial catheter
- ScvO2
- Serum lactate
- Temperature
- Urine output
The Electroencephalogram (EEG)
Most often the EEGs of patients in coma after cardiac arrest shows diffuse slowing of both the theta and delta waves, and periodic epileptiform firing. Severe slowing or a flat line appearance is associated with a poor prognosis.
Evoked-Response Testing
If there is absence of bilateral somatosensory evoked potentials, then it is unlikely that the patient will survive.[20][21][22] In particular, if there is no N20 response, there is a very highly likelihood of a vegetative state or death, with only 1 patient of 21 surviving in one study compared with survival in 11 of 26 patients surviving if the N20 response was positive.[23]
Imaging Findings
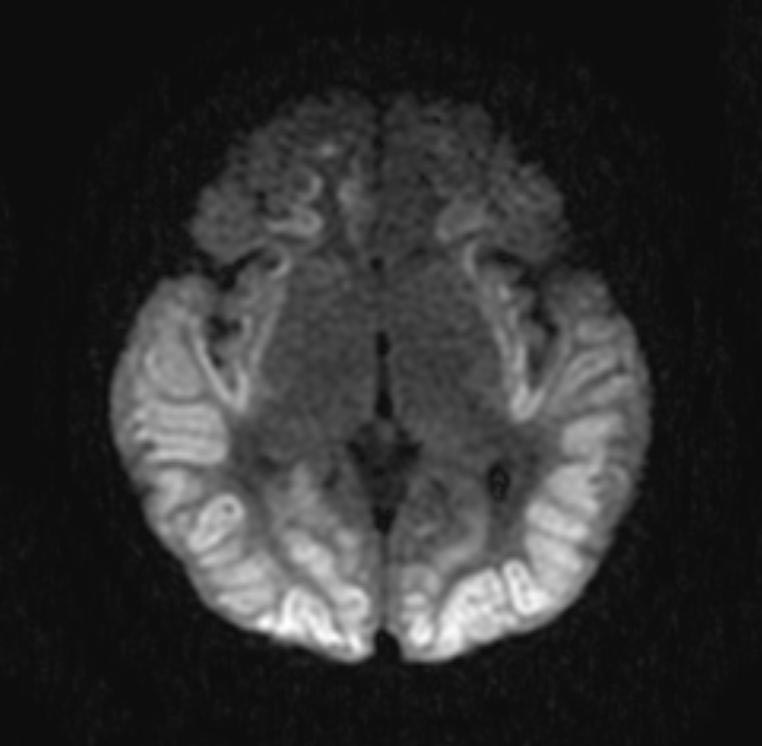
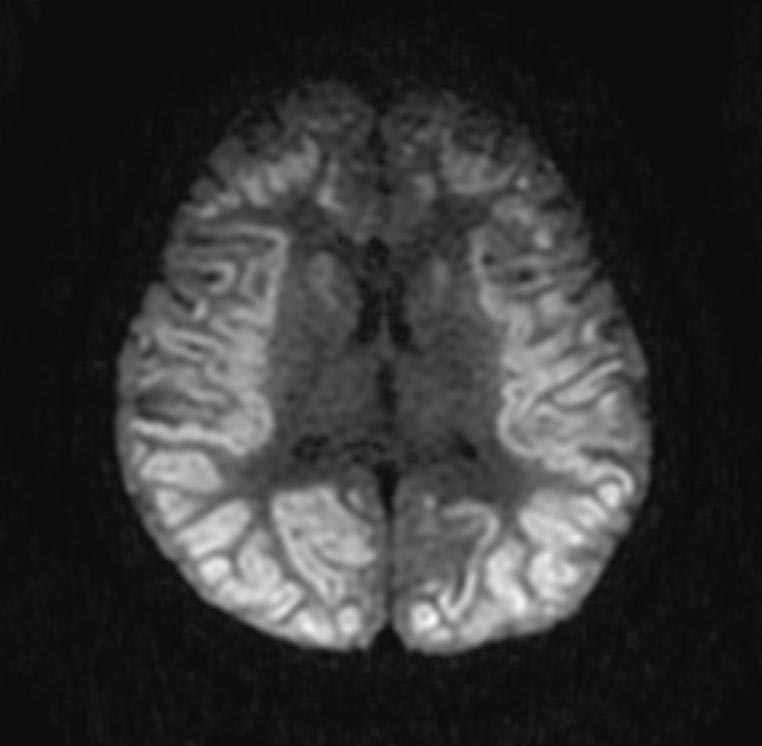
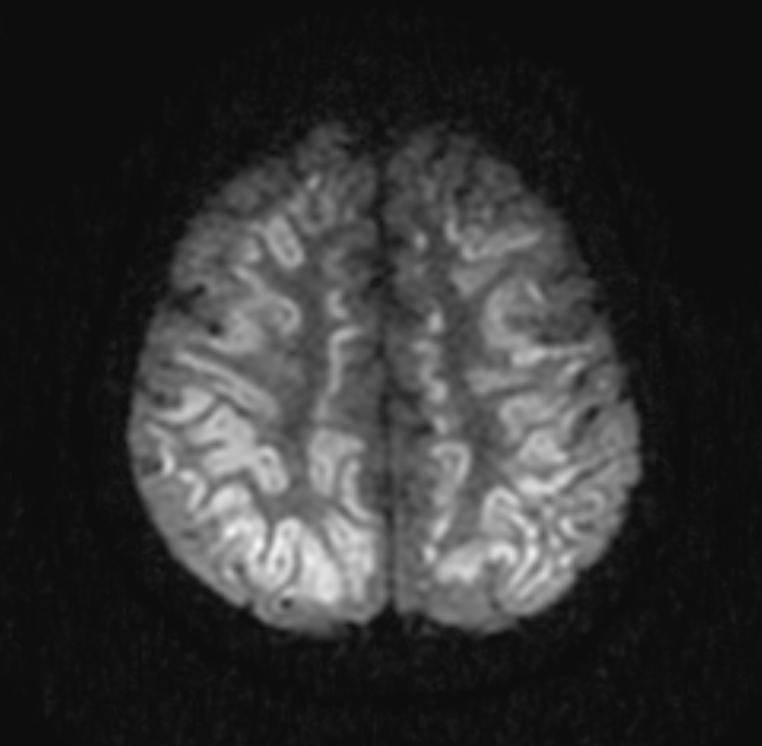
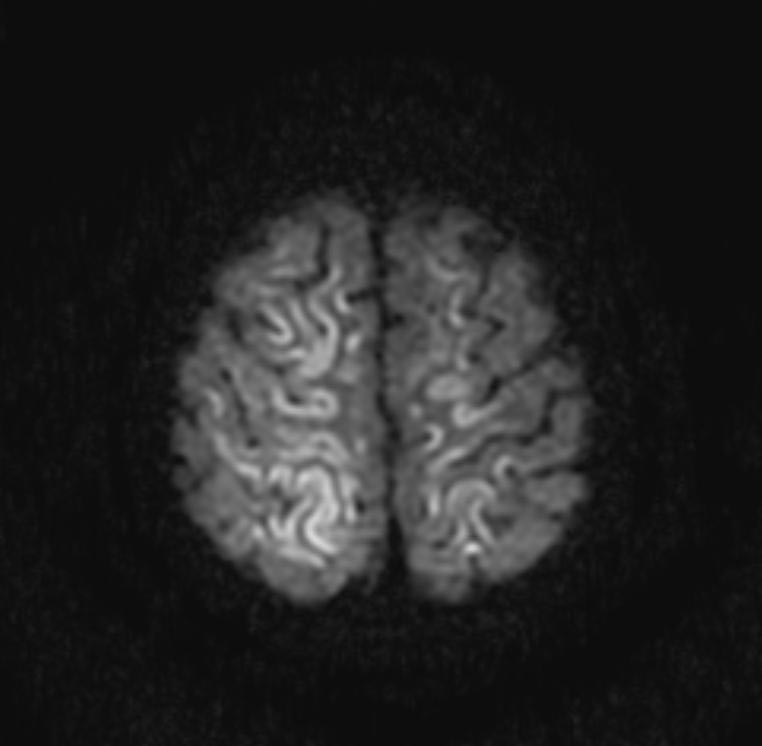
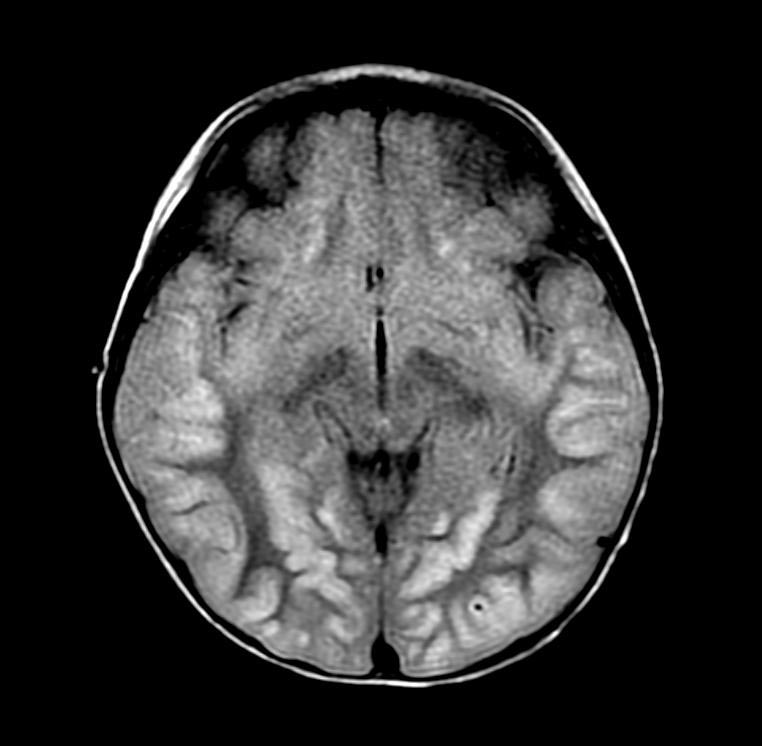
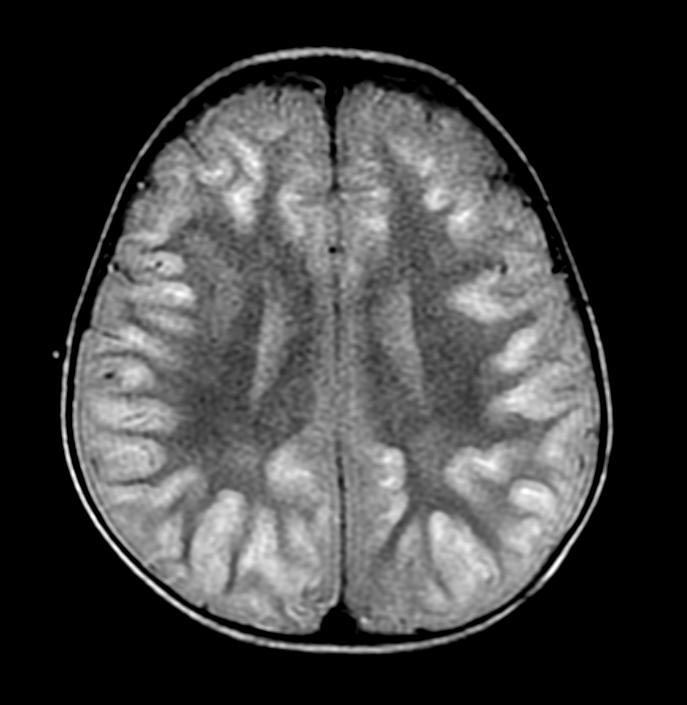
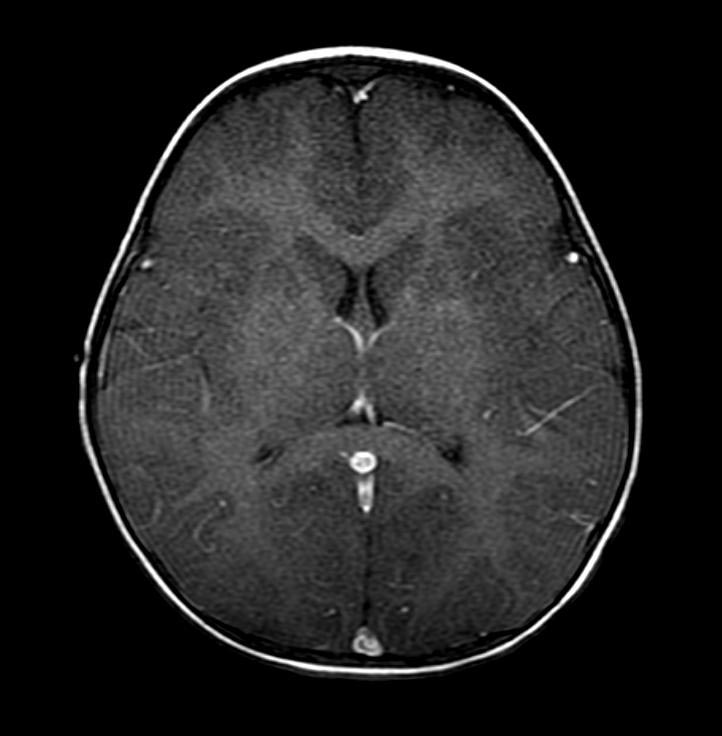
In the early hours and days after anoxic brain injury, there is often diffuse cerebral edema and blurring of the border between the grey and white matter. In some patients there may be discrete infarcts after a few days.
If there is irreversible bilateral medial tegmental brainstem injury, then patients do not survive.
Patient #1
Patient #2
-
DWI
-
DWI
-
DWI
-
DWI
-
FLAIR
-
FLAIR
-
T1 with GAD
Diagnosis of Brain Death
If there is no longer brain activity, and there is 0% chance of the recovery of the patient, a patient is declared "Brain Dead". Brain death is diagnosed when all four of the following criteria are met:[24][25][26]
1. There is coma with a loss of cerebral reactivity.
2. There is absence of spontaneous respiration.
3. There is a loss of brainstem reflexes (pupillary, corneal, oculovestibular, and oculocephalic). In some definitions this is qualified by a requirement that the loss of reflexes exceeds 24 hours in duration.
4. There is no activity on the electroencephalogram ("electrocerebral silence", a "flat electroencephalogram") for > than 12 hours. This last criteria requires that the patient is not hypothermic or on sedative drugs. In some definitions > 24 hours is required.
Treatment
A more detailed care pathway to care for patients with anoxic brain injury can be found here.
Therapeutic Hypothermia (TMH)
Hypothermia has been associated with a reduction in ischemic brain injury in animal models [27][28][29]. The initial data supporting the efficacy of therapeutic hypothermia was collected in patients with coma following VF[30][31][32]. In the Bernard study, the number of people discharged to home or a rehabilitation facility was 49% of patients (21/43) treated with hypothermia versus 26% (9/34) treated with normothermia (univariate p=0.046, multivariate p=0.011). While patients with a shockable rhythm such as VT/VF derive significant benefits from therapeautic hypothermia, patients with non-shockable rhythms such as PEA and asystole may not derive the same benefits[33]. While 39% (274/708) of patients with VT/VF treated with TMH achieved an acceptable level of neurologic outcome (cerebral performance categories level 1 or 2) at discharge, only 16% (68/437) of patients with PEA/asystole treated with TMH achieved an acceptable outcome. In multivariate analyses, TMH was associated with increased odds of an acceptable neurological outcome (multivariate odds ratio = 1.90) in patients with VT/VF, while in contrast TMH was not associated with acceptable neurological outcome (multivariate odds ratio = 0.71) in patients with PEA/asystole.
Complications and Side Effects Associated with Hypothermia
- Hypothermia activates the sympathetic nervous system causing vasoconstriction and shivering. Shivering increases O2 consumption by 40-100%. Sedatives, opiates, and neuromuscular blockers can counteract these responses and enhance the effectiveness of active cooling. However, initiating paralysis in a patient that is already hypothermic should be avoided because it can result in a precipitous drop in core body temperature. Elderly patients will cool more quickly than younger or obese patients. [34]
- Hypothermia shifts the oxyhemoglobin curve to the left may result in decreased O2 delivery. However, the metabolic rate is also lowered, decreasing O2 consumption / CO2 production, cardiac output and cerebral blood flow. Ventilator settings may need to be adjusted due to decreased CO2 production, using temperature corrected blood gases. [35]
- Hypothermia initially causes sinus tachycardia, then bradycardia. With temp <30º C there is an increased risk for arrhythmias. With temp <28º C there is an increased risk for ventricular fibrillation. The severely hypothermic myocardium (<30°C) is less responsive to defibrillation and medications. Therefore it is extremely important to keep temp >30ºC.
- Hypothermia can induce coagulopathy which is treatable with platelets and FFP.
- Hypothermia-induced diuresis is to be expected and should be treated aggressively with fluid and electrolyte repletion. Magnesium, phosphorus and potassium should be monitored closely and maintained in the normal (because it will rebound to very high) range.
- Decreased insulin secretion and sensitivity leads to hyperglycemia, which should be treated aggressively.
- Re-warming too rapidly can cause vasodilation, hypotension, and rapid electrolyte shifts.
Eligibility Criteria for Post-Cardiac Arrest Therapeutic Hypothermia
- Meets eligibility criteria for Post-Cardiac Arrest Care Pathway
- Comatose at enrollment with a Glasgow Coma Motor Score <6 pre-sedation (i.e., patient doesn’t follow commands)
- No other obvious reasons for coma
- No uncontrolled bleeding
- Hemodynamically stable with no evidence of:
- Uncontrollable dysrhythmias
- Cardiogenic shock
- Refractory hypotension (MAP <60 mm Hg) despite preload optimization and use of vasoactive medications
- No existing, multi-organ dysfunction syndrome, severe sepsis, or comorbidities with minimal chance of meaningful survival independent of neurological status
Relative Contraindications for Therapeutic Hypothermia:
- Prolonged arrest time (> 60 minutes)
- Thrombocytopenia or other coagulopathies
- Pregnancy (Therapeutic hypothermia can potentially be performed on pregnant female in consultation with OB/Gyn)
Guidelines for Therapeutic Hypothermia
Preparation:
If criteria are met, the patient is cooled using the induced hypothermia protocol for 24 hours to a goal temperature of 32-34° C (89-93° F). The patient should be cooled to the target temperature as quickly as possible. The 24-hour time period is from the time of initiation of cooling
- Place arterial line for blood pressure monitoring.
- A continuous temperature monitor with bladder probe or esophageal catheter will aid in cooling process and prevents overcooling.
- Use of secondary temperature device (Exergen) is also recommended to monitor temperature as bladder probe is accurate only if there is adequate urine output. This alternative temperature probe can be any core temperature monitor that is compatible with the Arctic Sun console.
Methods:
External cooling with cooling blankets and ice:
- Eligibility should be confirmed and materials should be gathered.
- Obtain two cooling blankets and cables (one machine) to sandwich the patient between them. Each blanket should be covered with a sheet to protect the patient’s skin.
- Cisatracurium (Nimbex) should be administered via microinfusion for paralysis. Bolus of 150mcg/kg and a maintenance dose of 2mcg/kg/min is used. Use of BIS or train of four are not recommended.
- Propofol (Diprivan) or Midazolam (Versed) to be administered for sedation. Propofol- Bolus (optional) 0.3-0.5mg/kg followed by infusion of 1mg/kg/hour while patient is paralyzed. Midazolam- Bolus (optional) 0.05mg/kg followed by infusion of 0.125mg/kg/hour.
- Pack the patient in ice (groin, axilla, side of neck and chest) and additional measures can also be used as needed to achieve the target temperature. Packing ice on top of chest should be avoided as ventilation may be impaired.
- Cold saline infusion via a peripheral line or femoral venous catheter (NOT via jugular or subclavian line) can be performed to assist in achieving target temperature. 30cc/kg of 4°C normal saline over 30minutes..
- Monitor vitals with attention towards arrhythmia detection.
- Ice bags should be removed once target temperature is reached and the temperature should be maintained using cooling blankets.
External cooling with Arctic Sun Vest Device:
- Eligibility should be confirmed and materials should be gathered.
- Patient’s temperature should be noted and cooling pads should be placed on patient as per manufacturer’s guidelines.
- Set target temperature after applying pads.
- Sedate and paralyze the patient with agents mentioned above to control shivering.
- External pacing pads can also be used with these pads. Place external pacing pads on the chest and cover with Arctic Sun pads.
- Rewarming strategies as mentioned below.
Supportive Therapy
- A mean arterial pressure (MAP) of more than 90mm of Hg is preferred for cerebral perfusion. In addition to hypothermia, hypertension improves neuroprotection. Target MAP should be determined by the treating physician taking into account the cardiac safety and advantage of higher cerebral perfusion pressures.
- Monitor the patient for arrhythmias. Active cooling should be discontinued and actively re-warmed when significant dysrhythmias, hemodynamic instability or bleeding develops.
- Electrolyte panel, glucose and complete blood count should be measured at 12hours and 24hours.
- Arterial blood gases should be mkeasured at the patient's actual body temperature. CO2 should be maintained in the normal range (35-45).
- Blood cultures should be drawn at 12 hours after the initiation of cooling as infections will be masked during the cooling phase.
- Skin should be checked every 2 hours for burns caused by cold blankets. If the Arctic Sun device is utilized, skin should be checked every 6 hours.
- Using a secondary temperature monitoring device when using the Arctic Sun is recommended. The patient temperature on the Arctic Sun, the secondary temperature source and the water temperature of the Arctic Sun are recorded. The water temp will help to determine the work of the machine in trying to maintain target temperature.
Re-warming
This is the most critical phase, as the previously constricted peripheral beds start to dilate with resultant hypotension as mentioned above.
Re-warming of the patient is begun 24hours after the initiation of cooling. It is recommended that the body be re-warmed at the rate of 0.5-1ºC every hour, thereby approximately 8-12hrs to passively re-warm up to a target temperature of 36ºC (96.8ºF).
Re-warming phase is a total of 72 hours, with passive re-warming for 24hours and controlled re-warming for 48hours.
Passive Re-warming:
At 24 hours (after the initiation of cooling) -
- Remove cooling blankets (and ice if still in use).
- Paralysis and sedation must be maintained until target temperature of 36ºC is reached: paralysis is discontinued first followed by midazolam once train of 4 is achieved.
- Monitor patient for hypotension related to re-warming.
- Monitor patient for hyperkalemia during re-warming.
Controlled Re-warming: If the Arctic Sun cooling vest is used, program the machine for controlled rewarming over 6-8hours. Dial the desired warming on the machine to maintain a target temperature for the next 48 hours.
The patient should be on constant follow-up with the stroke service to reassess the neurological status after the discontinuation of hypothermia.
Prognosis
Predictors of Survival
Improved Prognosis with In-Hospital versus Out-of-Hospital Cardiac Arrest
Out-of-hospital cardiac arrest (OHCA) has a worse survival rate (2-8% survival at discharge) than in-hospital cardiac arrest (15% survival at discharge).
Improved Prognosis with VT/VF versus PEA or Asystole
A major determining factor in survival is the initially documented electrocardiographic rhythm. Patients with ventricular fibrilation (VF) or ventricual tachycardia (VT) (aka VT/VF) have a 10-15 fold greater chance of survival than patients with pulseless electrical activity (PEA) or asystole. VT and VF are responsive to defibrillation, whereas asystole and PEA are not.
Rapid Defibrillation is Associated with Imporved Survival
Rapid intervention with a defibrillator increases survival rates.[36][37]
Incidence and Predictors of Entering Into a Vegetative State versus Making a Full Neurologic Recovery
Cardiac arrest is the third leading cause of coma. Approximately 80% of patients who suffered a cardiac arrest who survived to be admitted to the hospital will be in coma for varying lengths of time. Of these patients, approximately 40% will enter into a persistent vegetative state and 80% die within 1 year. In contrast, those rare patients who survive until discharge without significant neurological impairment can expect a fair to good quality of life.
The duration of hypoxia/ischemia determines the extent of neuronal injury i.e. in patients who suffer hypoxia for less than 5 minutes, are less likely to have permanent neurologic deficits, while with prolonged, global hypoxia, patients may develop myoclonus or a persistent vegetative state.[38]
The duration of coma is an important predictor of the recovery of neurologic function. In a 1979 study of 181 cardiac arrest patients who survived to hospital admission, 84% were comatose for more than 1 hour and 56% were comatose for more than 24 hours[39]. There was minimal neurologic deficit if coma lasted less than 24 hours. However, among the 85 patients who were comatose for more than 24 hours, only 7 of them were discharged alive. The severity of neurological impairment increased with increased duration of coma. Of the patients who were in coma for more than 7 days, none regained consciousness. It should be noted that 80 patients died in a coma.
A JAMA article in 1985 attempted to identify the multivariate predictors neurologic prognosis in 210 patients with coma due to cerebral hypoxia. A total of 13% of patients regained neurologic function and independent function at some time during the first year.
Initial Neurologic Findings:
- Patients who had the initial absence of pupillary light reflexes did not recover independent functioning (52 patients, 25% of patients)[39].
- In contrast, patients who had the initial presence of pupillary light reflexes, the development of spontaneous eye movements that were roving conjugate or better, and the presence of either extensor, flexor, or withdrawal responses to pain had a 41% chance of regaining independent function (of the 27 patients in this group, 11 (41%) regained independence).[39].
- In a study by Snyder et al, the absence of corneal or pupillary light reflexes at 3 hours after cardiac arrest was associated with death in all patients [40][41]. By 6 hours, all the patients who survived had the presence of three brainstem reflexes: pupillary light response, corneal reflex, and reflex eye movements.
- The absence of spontaneous limb movements and the absence of withdrawal to pain in the early hours is a poor prognostic sign.
- The presence of either decorticate or decerebrate posturing is a poor prognostic sign.
- Frequent myoclonic jerking is associated with a poor prognosis.
- The presence of seizures in the initial 24 hours is modestly associated with outcomes: 53% of patients who seize survive compared to 70% of those who do not seize during the first day[42].
24 Hour Neurologic Findings:
- Most patients who survive become alert by 24-48 hours. In one series, of those patients who were in a coma through day 2, only 2 of the 27 (7%) survived.[43] In a second series, no patient who remained in a coma by the third day sirvived.[44]
- Absent motor responses, the presence of posturing (extensor / flexor motor responses) and the lack of spontaneous eye movements that were either orienting or roving conjugate was associated with a lack of independent recovery in 92 of 93 patients. [39].
- In contrast, of the 30 patients who showed improvement in their eye-opening responses, obeyed commands or had withdraw to pain, 19 (63%) regained independent function.[39].
- Seizures that occur after the initial 24 hours are associated with a poorer outcomes. In one study only 3 of 15 patients who seized recovered consciousness, and only one patient lived a year[45]. The presence of status epilepticus at any time following cardiac arrest is associated with a very poor prognosis as all nine patients with status epilepticus died in one series.[46]
- The absence of spontaneous eye opening and intermittent visual fixation by the end of the first day is associated with a poor prognosis. Although eye opening is necessary for a good outcomes, it alone is not sufficient, as many patients who have spontaneous eye opening still go on to have a poor prognosis. Roving eye movements in the absence of visual fixation is often indicative of extensive bilateral cerebral hemispheral damage and portends a poor prognosis. If the gaze is sustained in an upeard direction, this carries a poor prognosis as well.[47]
References
- ↑ Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post– cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118 DOI:10.1161/ CirculationAHA.108.190652 Published online on 27.10.2008
- ↑ Lyon RM, Cobbe SM, Bradley JM, Grubb NR (2004). "Surviving out of hospital cardiac arrest at home: a postcode lottery?". Emerg Med J. 21 (5): 619–24. doi:10.1136/emj.2003.010363. PMC 1726412. PMID 15333549. Unknown parameter
|month=ignored (help) - ↑ Cobbe SM, Dalziel K, Ford I, Marsden AK (1996). "Survival of 1476 patients initially resuscitated from out of hospital cardiac arrest". BMJ. 312 (7047): 1633–7. PMC 2351362. PMID 8664715. Unknown parameter
|month=ignored (help) - ↑ Ballew KA (1997). "Cardiopulmonary resuscitation". BMJ. 314 (7092): 1462–5. PMC 2126720. PMID 9167565. Unknown parameter
|month=ignored (help) - ↑ Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. Sep 10 2001; 161(16): 2007-2012.
- ↑ van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. New England Journal of Medicine. Nov 8 2001;345(19): 1359-1367.
- ↑ Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. Feb 2003;31(2):359-366.
- ↑ Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862-871.
- ↑ Zandbergen EG, de Haan RJ, Stoutenbeek CP, et al. Systematic review of early prediction of poor outcome in anoxic-ischaemic coma. Lancet. Dec 5 1998; 352(9143): 1808-1812.
- ↑ Rello J. Risk factors for developing pneumonia within 48 hours of intubation. Am J Respir Crit Care Med. 1999;159:1742-1746.
- ↑ Spaulding CM, Joly LM, Rosenberg A, et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. New England Journal of Medicine. Jun 5 1997;336(23):1629-1633.
- ↑ Adrie C, Laurent I, Monchi M, et al. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. Jun 2004;10(3):208-212.
- ↑ Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine. 2001;345(19):1368-1377.
- ↑ Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of hospital cardiac arrest with induced hypothermia. New England Journal of Medicine. Feb 21 2002;346(8):557-563.
- ↑ Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. Erratum appears in N Engl J Med 2002 May 30;346(22):1756]. New England Journal of Medicine. Feb 21 2002;346(8):549-556.
- ↑ Steen-Hansen JE, Hansen NN, Vaagenes P, Schreiner B: Pupil size and light reactivity during cardiopulmonary resuscitation. A clinical study. Crit Care Med 1988;16:69-70.
- ↑ Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post– cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication: a consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118 DOI:10.1161/ CirculationAHA.108.190652 Published online on 27.10.2008
- ↑ Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 [published correction appears in Crit Care Med. 2008;36: 1394–1396]. Crit Care Med. 2008;36:296 –327.
- ↑ Gazmuri RJ, Nolan JP, Nadkarni VM, Arntz HR, Billi JE, Bossaert L, Deakin CD, Finn J, Hammill WW, Handley AJ, Hazinski MF, Hickey RW, Jacobs I, Jauch EC, Kloeck WG, Mattes MH, Montgomery WH, Morley P, Morrison LJ, Nichol G, O’Connor RE, Perlman J, Richmond S, Sayre M, Shuster M, Timerman S, Weil MH, Weisfeldt ML, Zaritsky A, Zideman DA. Scientific knowledge gaps and clinical research priorities for cardiopulmonary resuscitation and emergency cardiovascular care identified during the 2005 International Consensus Conference on ECC and CPR Science with Treatment Recommendations: a consensus statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Stroke Council; and the Cardiovascular Nursing Council. Resuscitation. 2007;75:400–411.
- ↑ Chen R, Bolton CF, Young B: Prediction of outcome in patients with anoxic coma: A clinical and electrophysiological study. Crit Care Med 1996;24:672-678.
- ↑ Kaplan PW: Electrophysiological prognostication and brain injury from cardiac arrest. Semin Neurol 2006;26:403-412.
- ↑ Young GB, Doig G, Ragazzoni A: Anoxic-ischemic encephalopathy: Clinical and electrophysiological associations with outcome. Neurocrit Care 2005;2:159-164.
- ↑ Young GB, Doig G, Ragazzoni A: Anoxic-ischemic encephalopathy: Clinical and electrophysiological associations with outcome. Neurocrit Care 2005;2:159-164.
- ↑ Ad Hoc Committee of the Harvard Medical School: A defi nition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to examine the defi nition of brain death. JAMA 1968;205:337-340.
- ↑ Walker A: An appraisal of the criteria of cerebral death. JAMA 1977;237:982-986.
- ↑ Shemie SD, Pollack MM, Morioka M, Bonner S: Diagnosis of brain death in children. Lancet Neurol 2007;6:87-92.
- ↑ Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H (1993). "Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study". Crit. Care Med. 21 (9): 1348–58. PMID 8370299. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Ginsberg MD, Sternau LL, Globus MY, Dietrich WD, Busto R (1992). "Therapeutic modulation of brain temperature: relevance to ischemic brain injury". Cerebrovasc Brain Metab Rev. 4 (3): 189–225. PMID 1389956.
|access-date=requires|url=(help) - ↑ Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A (1992). "Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs". Stroke. 23 (10): 1454–62. PMID 1412583. Retrieved 2011-03-02. Unknown parameter
|month=ignored (help) - ↑ Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K (2002). "Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia". N. Engl. J. Med. 346 (8): 557–63. doi:10.1056/NEJMoa003289. PMID 11856794. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ "Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest". N. Engl. J. Med. 346 (8): 549–56. 2002. doi:10.1056/NEJMoa012689. PMID 11856793. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Müllner M (2005). "Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis". Crit. Care Med. 33 (2): 414–8. PMID 15699847. Retrieved 2011-03-02. Unknown parameter
|month=ignored (help) - ↑ Dumas F, Grimaldi D, Zuber B, Fichet J, Charpentier J, Pène F, Vivien B, Varenne O, Carli P, Jouven X, Empana JP, Cariou A (2011). "Is Hypothermia After Cardiac Arrest Effective in Both Shockable and Nonshockable Patients?: Insights From a Large Registry". Circulation. doi:10.1161/CIRCULATIONAHA.110.987347. PMID 21321156. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation 2007;73:29-39
- ↑ Kim F, Olsufka M, Longstreth WT Jr, Maynard C, Carlbom D, Deem S, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007;115:3064-70
- ↑ Eisenberg MS, Mengert TJ (2001). "Cardiac resuscitation". N. Engl. J. Med. 344 (17): 1304–13. PMID 11320390. Unknown parameter
|month=ignored (help) - ↑ Bunch TJ, White RD, Gersh BJ; et al. (2003). "Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation". N. Engl. J. Med. 348 (26): 2626–33. doi:10.1056/NEJMoa023053. PMID 12826637. Unknown parameter
|month=ignored (help) - ↑ Mellion ML (2005). "Neurologic consequences of cardiac arrest and preventive strategies". Medicine and Health, Rhode Island. 88 (11): 382–5. PMID 16363390. Unknown parameter
|month=ignored (help) - ↑ 39.0 39.1 39.2 39.3 39.4 Thomassen A, Wernberg M (1979). "Prevalence and prognostic significance of coma after cardiac arrest outside intensive care and coronary units". Acta Anaesthesiologica Scandinavica. 23 (2): 143–8. PMID 442945. Unknown parameter
|month=ignored (help) - ↑ Snyder BD, Loewenson RB, Gumnit RJ, et al: Neurologic prognosis after cardiopulmonary arrest: II. Level of consciousness. Neurology 1980;30:52-58.
- ↑ Snyder BD, Gumnit RJ, Leppik IE, et al: Neurologic prognosis after cardiopulmonary arrest: IV. Brainstem refl exes. Neurology 1981;31: 1092-1097
- ↑ Roine RO: Neurological Outcome of Out-of-Hospital Cardiac Arrest [dissertation]. University of Helsinki, 1993.
- ↑ Snyder BD, Loewenson RB, Gumnit RJ, et al: Neurologic prognosis after cardiopulmonary arrest: II. Level of consciousness. Neurology 1980;30:52-58.
- ↑ Bell JA, Hodgson HJF: Coma after cardiac arrest. Brain 1974;97:361-372.
- ↑ Roine RO: Neurological Outcome of Out-of-Hospital Cardiac Arrest [dissertation]. University of Helsinki, 1993.
- ↑ Roine RO: Neurological Outcome of Out-of-Hospital Cardiac Arrest [dissertation]. University of Helsinki, 1993.
- ↑ Keane JR: Sustained upgaze in coma. Annals of Neurolology 1981;9:409-412.