Anencephaly
|
WikiDoc Resources for Anencephaly |
|
Articles |
|---|
|
Most recent articles on Anencephaly Most cited articles on Anencephaly |
|
Media |
|
Powerpoint slides on Anencephaly |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Anencephaly at Clinical Trials.gov Clinical Trials on Anencephaly at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Anencephaly
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Anencephaly Discussion groups on Anencephaly Patient Handouts on Anencephaly Directions to Hospitals Treating Anencephaly Risk calculators and risk factors for Anencephaly
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Anencephaly |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ayesha Javid, MBBS[2]
For patient information click here
| Anencephaly | ||
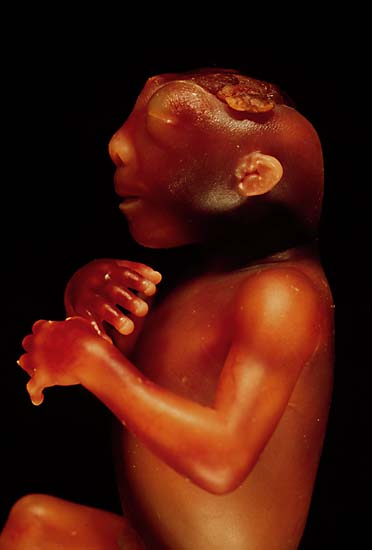 | ||
|---|---|---|
| The side view of the head of an anencephalic fetus | ||
| ICD-10 | Q00.0 | |
| ICD-9 | 740.0 | |
| OMIM | 206500 | |
| DiseasesDB | 705 | |
| MeSH | C10.500.680.196 | |
Overview
Anencephaly is a cephalic disorder that results from a neural tube defect that occurs when the cephalic (head) end of the neural tube fails to close, usually between the 23rd and 26th day of pregnancy, resulting in the absence of a major portion of the brain, skull, and scalp. Children with this disorder are born without a forebrain, the largest part of the brain consisting mainly of the cerebral hemispheres (which include the isocortex, which is responsible for higher level cognition, i.e., thinking). The remaining brain tissue is often exposed - not covered by bone or skin.
Historical Perspective
- Anencephaly was first recognized in the 16th century. In 1989, Aubrey Milunsky and his colleagues observed a reduction in the cases of neural tube defects in the mothers who took folic acid supplements during pregnancy.[1]
- In 1992, Thresa was born with anencephaly. Her parents knew their daughter is going to die. So, they requested if her organs could be used for transplantation. This initiated a debate on anencephalic infant organ donation.[2]
Classification
| Type of Anencephaly | Clinical features |
|---|---|
| Meroanencephaly | Meroanencephaly is a rare form of anencephaly. Clinical features include malformed cranial bones with a median cranial defect. The fetus also has a cranial protrusion called cerebrovasculosa. It is a spongy vascular tissue mixed with the glial tissue. [3] |
| Holoanencephaly | It is the most common type of anencephaly. In this condition, the entire fetus brain fails to develop except for the brain stem. Usually, infant survives for only one day after birth.[3] |
| Craniorachischisis | It is the most severe type of anencephaly. Along with the cranial defect, fetus also has a defect in the vertebral column exposing the underlying neural tissue. Area cerebrovasculosa and area medullovasculosa fill the areas with cranial and spinal defects |
Embryology
By the 3rd week of gestation, the embryo has 3 layers, from outer to inner these layers are ectoderm, mesoderm and endoderm. Notochord in the mesoderm sends signal molecule called Sonic Hedgehog protein to the overlying ectoderm forming it a neuroectoderm. Neuroectoderm becomes a neural plate. The neural plate folds and closes to form a tube-like structure called neural tube. It also forms neural crest cells. The neural tube then goes on to form structures of the adult brain.
Pathogenesis
A neural tube defect occurs if the neuropore, opening of the neural tube, fails to close in week 4 of gestation. There are two neuropores, rostral and caudial. Anencephaly results from failure of the rostral neuropore to close anteriorly around day 25.(book reference). The craniofacial abnormalities in anencephaly are caused by abnormal induction by the neural crest cells.[4]
Etiology and Risk factors
Folate deficiency
- Studies show that most of the neural tube defects are caused by folic acid deficiency[5].
- This inadequate folate could be due to less oral intake, decreased intestinal absorption, or due to abnormal folate metabolism due to gene mutation. Some drugs antagonize the effect of folic acid resulting in folic acid deficiency and hence NTD. Most important ones are anti-epileptic drugs such as valproic acid and carbamazepine. Also, methotrexate, which is an antineoplastic drug also used for the treatment of ectopic pregnancy, has been linked with increased risk of NTD.[6]
Genetics
Neural tube defects do not follow direct patterns of heredity, though there is some indirect evidence of inheritance[7]
, and recent animal models indicating a possible association with deficiencies of the transcription factor TEAD2.[8]
The motivation behind studying the genetic patterns is following:
- NTDs are consistently prevalent among monozygotic twins as compared to dizygotic twins.[9]
- There is a high recurrence rate within families. Statistics show the recurrence risk of 1/20 if one previous pregnancy is affected and 1/10 if two pregnancies are affected in a family.[10]
- Due to predilection towards female fetuses as compared to male.[11]
Syndromes
- Anencephaly is associated with:
- Trisomy 13 or Trisomy 18
- Meckel-Gruber syndrome
- Roberts syndrome
- Jarcho-Levin syndrome
- HARD (hydrocephalus, agyria and retinal dysplasia)
- OEIS complex (omphalocele, exstrophy of the cloaca, imperforate anus and spinal defects)
- Limb-body wall complex (LBWC)
Fever/hyperthermia
- If pregnant mother’s core body temperature elevates from baseline it could lead to congenital anomalies such neural tube defects, including anencephaly.[12][13]
- The risk is more profound if this happens during the first trimester as this is the time during which organogenesis takes place. The National Birth Defects Prevention Study (NBDPS) infers that the risk of birth defects due to maternal infection-related fever can be reduced by the usage of acetaminophen.[14]
Amniotic bands
- If a pregnant mother develops amniotic bands, it could effect the normal development and growth of the central nervous system. It could result in neural tube defects including anencephaly
Pregestational diabetes
- If a woman has uncontrolled diabetes mellitus before conception, it could result in neural tube defects including anencephaly.[11]
- Therefore, close monitoring of periconceptional glycemic level is essential to prevent neural tube defects and other congenital anomalies.
Maternal Obesity
- Obesity doubles the risk of neural tube defects. Also, it provides a limitation to fetal imaging.[15]
- Folic acid supplementation does not decrease this risk of NTD, therefore it is mandatory to control weight before conceiving.[16]
Toxins
- Anencephaly and other physical and mental deformities have also been blamed on high exposure to such toxins as lead, chromium, mercury, and nickel. [17]
Differentiating Anencephalopathy from other Disorders
There are many false diagnoses for anencephaly, as it is not a common diagnosis, often confused with exencephaly or microcephaly.
Epidemiology and Demographics
Incidence
In the United States, approximately 1,000 to 2,000 babies are born with anencephaly each year. In 2001, the National Center for Health Statistics reported 9.4 cases among 100,000 live births.[18]
Demographics
The highest prevalence has been seen among the Hispanic population. Female babies, whites and children born to mothers who are at extreme of ages are more likely to be affected by the disorder. Worldwide, Ireland and British Islands has higher prevalence as compared to Asia and Africa which has a lower prevalence rate.
Recurrence rate
Like any other neural tube defect, the recurrence rate of anencephaly is 2-4% if one sibling is affected[19][20] and 10 percent if two siblings are affected. This familial tendency is due to genetics, environmental factors, or both.[21]
Natural History, Complications, Prognosis
There is no cure or standard treatment for anencephaly and the prognosis for affected individuals is poor. Most anencephalic babies do not survive birth, accounting for 55% of non-aborted cases. If the infant is not stillborn, then he or she will usually die within a few hours or days after birth from cardiorespiratory arrest.
In almost all cases anencephalic infants are not aggressively resuscitated since there is no chance of the infant ever achieving a conscious existence. Instead, the usual clinical practice is to offer hydration, nutrition and comfort measures and to "let nature take its course". Artificial ventilation, surgery (to fix any co-existing congenital defects), and drug therapy (such as antibiotics) are usually regarded as futile efforts. Clinicians and medical ethicists may view the provision of nutrition and hydration as medically futile. Occasionally some may even go one step further to argue that euthanasia is morally and clinically appropriate in such cases.
Prenatal screening
- American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) advocates universal screening of all the pregnant women for the NTD. [22][23]
- This results in early diagnosis which helps the couple to plan either pregnancy termination or otherwise prepare them for the birth. The maternal serum alpha-fetoprotein (AFP screening) and detailed fetal ultrasound[24] can be useful for screening for neural tube defects such as spina bifida or anencephaly.[25]
Ultrasound
Ultrasound provides a definitive diagnosis of anencephaly before birth. It can often be diagnosed through a transvaginal ultrasound performed at 12th week postmenstrual.[26] The ultrasound findings include open calvaria and characteristic frog-like appearance of the fetus. Also, a large amount of amniotic fluid called polyhydromnios is also seen.
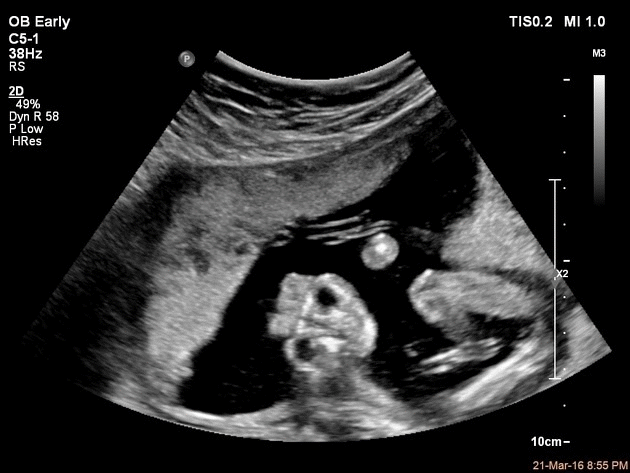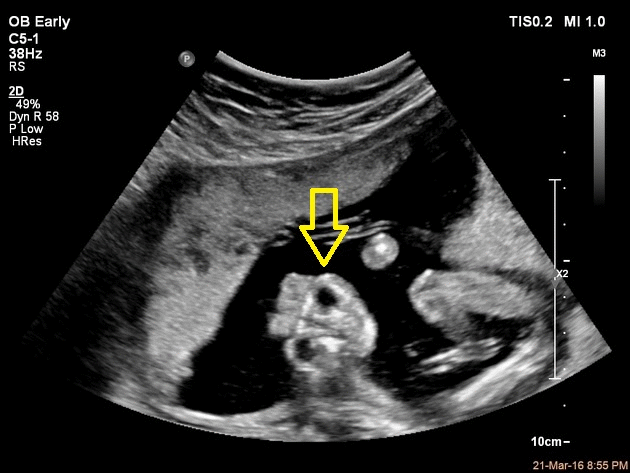
Alpha-Fetoprotein
- Alpha-fetoprotein (AFP) levels can be measured in maternal serum (MSAFP), amniotic fluid, and fetal plasma.[27]
- Normally, the level rises in early pregnancy, peaks between 10 and 13th week, and then the level declines becoming extremely low near the term.[28]
- For the purpose of prenatal screening, alpha-fetoprotein levels are checked and are found to elevated. This test is non-specific for NTD, as AFP can also be elevated due to other maternal and fetal factors. Therefore, results should always be combined with ultrasound findings and the levels of Acetylcholinesterase (AChE).
Acetylcholinesterase
AChE is an enzyme contained in blood cells, muscle, and nerve tissue. Its levels can be checked from the amniotic fluid via amniocentesis. An elevation of both AFP and AChE values is highly suggestive and accurate for fetal NTD.
Triple screen
Triple screen is a prenatal screening test which includes 3 biomarkers; AFP, hCG and estradiol. It helps to detect certain congenital anomalies, including neural tube defect. The following table shows the triple screening of neural tube defect and how it is differentiated from other anomalies."Triple Screening in Pregnancy--What It Is and What to Expect - American Family Physician".
| Anomaly | AFP | hCG | Estradiol |
|---|---|---|---|
| NTD | Increased | Normal | Normal |
| Trisomy 21 | Decreased | Increased | Decreased |
| Trisomy 18 | Decreased | Decreased | Decreased |
Diagnosis
Clinical features
- Infants born with anencephaly have the following clinical features[29]:
- Absence of bony covering over the back of the head.
- Facial features: Cleft lip, cleft palate.
- CNS: Absence of bony covering over the back of the head, spina bifida, blindness, deafness.
- GIT: Absence or underdeveloped organs, gastroschisis, omphalocele [29]
- Genito-urinary system: Hypospadias, penile hypoplasia, renal agenesis.
- Skeletal system: Clubbed foot, clubbed hands.
- Lungs: Diapharagmatic hernia.
Physical Examination
Neurologic
- Reflex actions such as breathing and responses to sound or touch may occur. Although some individuals with anencephaly may be born with a rudimentary brainstem, which controls autonomic and regulatory function, the lack of a functioning cerebrum is usually thought of as ruling out the possibility of ever gaining consciousness, even though it has been disputed specifically. [30]
Cardio-vascular system
- A murmur could be heard due to associated congenital heart disease.
Respiratory system
- Decreased breath sounds if associated diaphragmatic hernia is present.[29]
Gastrointestinal system
- On inspection, abdominal wall is not intact. There could be an abdominal wall defect with organs protruding out in an abdominal swelling, called omphalocele, or without a swelling called gastroschisis can be seen.[29]
Management
So far, prevention is the best management of anencephaly.
Medical Therapy
- There is no known medical therapy available for anencephaly."Anencephaly: Causes, Symptoms, Diagnosis and Treatment for Newborns | St. Louis Childrens Hospital".
Surgery
- At this point in time there are no surgical options available.
Prevention
- Recent studies have shown that the addition of folic acid to the diet of women of child-bearing age may significantly reduce, although not eliminate, the incidence of neural tube defects. Therefore, it is recommended that all women of child-bearing age consume 0.4 mg of folic acid daily, especially those attempting to conceive or who may possibly conceive, as this can reduce the risk to 0.03%.[31]
- It is not advisable to wait until pregnancy has begun, since by the time a woman knows she is pregnant, the critical time for the formation of a neural tube defect has usually already passed. A physician may prescribe even higher dosages of folic acid (4 mg/day) for women who have had a previous pregnancy with a neural tube defect.
Pregnancy management
- Anencephaly has a poor pregnancy outcome. Majority of the fetuses are either stillborn or die soon after birth, however, few infants do manage to live up to 28 days.[32]
- Therefore, mothers are counselled regarding the termination soon after the prenatal diagnosis. Vaginal delivery is the preferred mode unless there are any maternal indications.[33]
References
- ↑ Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ; et al. (1989). "Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects". JAMA. 262 (20): 2847–52. doi:10.1001/jama.262.20.2847. PMID 2478730 PMID: 2478730 Check
|pmid=value (help). - ↑ Bard JS (1999). "The diagnosis is anencephaly and the parents ask about organ donation: now what? A guide for hospital counsel and ethics committees". West New Engl Law Rev. 21 (1): 49–95. PMID 12774804 PMID 12774804 Check
|pmid=value (help). - ↑ 3.0 3.1 Isada NB, Qureshi F, Jacques SM, Holzgreve W, Tout MJ, Johnson MP; et al. (1993). "Meroanencephaly: pathology and prenatal diagnosis". Fetal Diagn Ther. 8 (6): 423–8. doi:10.1159/000263862. PMID 8286034 PMID 8286034 Check
|pmid=value (help). - ↑ Sarnat HB, Flores-Sarnat L (2005). "Embryology of the neural crest: its inductive role in the neurocutaneous syndromes". J Child Neurol. 20 (8): 637–43. doi:10.1177/08830738050200080101. PMID 16225807 PMID 16225807 Check
|pmid=value (help). - ↑ Copp AJ, Stanier P, Greene ND (2013). "Neural tube defects: recent advances, unsolved questions, and controversies". Lancet Neurol. 12 (8): 799–810. doi:10.1016/S1474-4422(13)70110-8. PMC 4023229. PMID 23790957 PMID: 23790957 Check
|pmid=value (help). - ↑ Wen SW, Walker M (2004). "Risk of fetal exposure to folic acid antagonists". J Obstet Gynaecol Can. 26 (5): 475–80. doi:10.1016/s1701-2163(16)30658-2. PMID 15151734 PMID: 15151734 Check
|pmid=value (help). - ↑ Shaffer LG, Marazita ML, Bodurtha J, Newlin A, Nance WE (1990). "Evidence for a major gene in familial anencephaly". Am. J. Med. Genet. 36 (1): 97–101. doi:10.1002/ajmg.1320360119. PMID 2333913.
- ↑ Kaneko KJ, Kohn MJ, Liu C, Depamphilis ML (2007). "Transcription factor TEAD2 is involved in neural tube closure". Genesis. 45 (9): 577–87. doi:10.1002/dvg.20330. PMID 17868131.
- ↑ Windham GC, Bjerkedal T, Sever LE (1982). "The association of twinning and neural tube defects: studies in Los Angeles, California, and Norway". Acta Genet Med Gemellol (Roma). 31 (3–4): 165–72. doi:10.1017/s0001566000008254. PMID 6763438 PMID: 6763438 Check
|pmid=value (help). - ↑ Ross ME, Mason CE, Finnell RH (2017). "Genomic approaches to the assessment of human spina bifida risk". Birth Defects Res. 109 (2): 120–128. doi:10.1002/bdra.23592. PMC 5388593. PMID 27883265 PMID: 27883265 Check
|pmid=value (help). - ↑ 11.0 11.1 Deak KL, Siegel DG, George TM, Gregory S, Ashley-Koch A, Speer MC; et al. (2008). "Further evidence for a maternal genetic effect and a sex-influenced effect contributing to risk for human neural tube defects". Birth Defects Res A Clin Mol Teratol. 82 (10): 662–9. doi:10.1002/bdra.20511. PMC 2981339. PMID 18937341 PMID: 18937341 Check
|pmid=value (help). - ↑ Li DK, Janevic T, Odouli R, Liu L (2003). "Hot tub use during pregnancy and the risk of miscarriage". Am J Epidemiol. 158 (10): 931–7. doi:10.1093/aje/kwg243. PMID 14607798 PMID: 14607798 Check
|pmid=value (help). - ↑ Edwards MJ (2006). "Review: Hyperthermia and fever during pregnancy". Birth Defects Res A Clin Mol Teratol. 76 (7): 507–16. doi:10.1002/bdra.20277. PMID 16933304 PMID: 16933304 Check
|pmid=value (help). - ↑ Feldkamp ML, Meyer RE, Krikov S, Botto LD (2010). "Acetaminophen use in pregnancy and risk of birth defects: findings from the National Birth Defects Prevention Study". Obstet Gynecol. 115 (1): 109–15. doi:10.1097/AOG.0b013e3181c52616. PMID 20027042 .PMID: 20027042 Check
|pmid=value (help). - ↑ Sukanya S, Bay BH, Tay SS, Dheen ST (2012). "Frontiers in research on maternal diabetes-induced neural tube defects: Past, present and future". World J Diabetes. 3 (12): 196–200. doi:10.4239/wjd.v3.i12.196. PMC 3538985. PMID 23301121 PMID: 23301121 Check
|pmid=value (help). - ↑ Moussa, Hind N; Hosseini Nasab, Susan; Haidar, Ziad A; Blackwell, Sean C; Sibai, Baha M (2016). "Folic acid supplementation: what is new? Fetal, obstetric, long-term benefits and risks". Future Science OA. 2 (2). doi:10.4155/fsoa-2015-0015. ISSN 2056-5623.
- ↑ Goldsmith, Alexander (1996, quoted by Millen and Holtz, "Dying for Growth")
- ↑ Mathews TJ, Honein MA, Erickson JD (2002). "Spina bifida and anencephaly prevalence--United States, 1991-2001". MMWR Recomm Rep. 51 (RR-13): 9–11. PMID 12353510 PMID: 12353510 Check
|pmid=value (help). - ↑ Cowchock S, Ainbender E, Prescott G, Crandall B, Lau L, Heller R; et al. (1980). "The recurrence risk for neural tube defects in the United States: a collaborative study". Am J Med Genet. 5 (3): 309–14. doi:10.1002/ajmg.1320050314. PMID 7405962 PMID: 7405962 Check
|pmid=value (help). - ↑ Toriello HV, Higgins JV (1983). "Occurrence of neural tube defects among first-, second-, and third-degree relatives of probands: results of a United States study". Am J Med Genet. 15 (4): 601–6. doi:10.1002/ajmg.1320150409. PMID 6614048 PMID: 6614048 Check
|pmid=value (help). - ↑ Copp AJ, Greene ND (2010). "Genetics and development of neural tube defects". J Pathol. 220 (2): 217–30. doi:10.1002/path.2643. PMC 4239538. PMID 19918803 PMID: 19918803 Check
|pmid=value (help). - ↑ Committee on Practice Bulletins-Obstetrics (2017). "Practice Bulletin No. 187: Neural Tube Defects". Obstet Gynecol. 130 (6): e279–e290. doi:10.1097/AOG.0000000000002412. PMID 29189693 PMID: 29189693 Check
|pmid=value (help). - ↑ Driscoll DA, Gross SJ, Professional Practice Guidelines Committee (2009). "Screening for fetal aneuploidy and neural tube defects". Genet Med. 11 (11): 818–21. doi:10.1097/GIM.0b013e3181bb267b. PMC 3111043. PMID 19915395 PMID: 19915395 Check
|pmid=value (help). - ↑ Cedergren M, Selbing A (2006). "Detection of fetal structural abnormalities by an 11-14-week ultrasound dating scan in an unselected Swedish population". Acta obstetricia et gynecologica Scandinavica. 85 (8): 912–5. doi:10.1080/00016340500448438. PMID 16862467.
- ↑ Joó JG, Beke A, Papp C; et al. (2007). "Neural tube defects in the sample of genetic counselling". Prenat. Diagn. 27 (10): 912–21. doi:10.1002/pd.1801. PMID 17602445.
- ↑ Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH (1997). "Ultrasound screening for anencephaly at 10-14 weeks of gestation". Ultrasound Obstet Gynecol. 9 (1): 14–6. doi:10.1046/j.1469-0705.1997.09010014.x. PMID 9060123 PMID: 9060123 Check
|pmid=value (help). - ↑ "StatPearls". 2020. PMID 28613501 PMID 28613501 Check
|pmid=value (help). - ↑ Gitlin D, Perricelli A, Gitlin GM (1972). "Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus". Cancer Res. 32 (5): 979–82. PMID 4111729 PMID 4111729 Check
|pmid=value (help). - ↑ 29.0 29.1 29.2 29.3 Gole RA, Meshram PM, Hattangdi SS (2014). "Anencephaly and its associated malformations". J Clin Diagn Res. 8 (9): AC07–9. doi:10.7860/JCDR/2014/10402.4885. PMC 4225866. PMID 25386414 PMID: 25386414 Check
|pmid=value (help). - ↑ Merker B (2007). "Consciousness without a cerebral cortex: a challenge for neuroscience and medicine". The Behavioral and brain sciences. 30 (1): 63–81, discussion 81–134. doi:10.1017/S0140525X07000891. PMID 17475053.
- ↑ Template:NINDS
- ↑ Jaquier M, Klein A, Boltshauser E (2006). "Spontaneous pregnancy outcome after prenatal diagnosis of anencephaly". BJOG. 113 (8): 951–3. doi:10.1111/j.1471-0528.2006.01014.x. PMID 16827827 PMID: 16827827 Check
|pmid=value (help). - ↑ Osathanondh R, Donnenfeld AE, Frigoletto FD, Driscoll SG, Ryan KJ (1980). "Induction of labor with anencephalic fetus". Obstet Gynecol. 56 (5): 655–7. PMID 7432739 PMID: 7432739 Check
|pmid=value (help).
Template:Congenital malformations and deformations of nervous system
de:Anenzephalie it:Anencefalia he:אננצפלוס nl:Anencefalie fi:Aivottomuus sv:Anencefali