Chromium
Overview
Chromium (Template:PronEng) is a chemical element which has the symbol Cr and atomic number 24. It is a steel-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odourless, tasteless, and malleable.
History
On 26 July 1761, Johann Gottlob Lehmann found an orange-red mineral in the Ural Mountains which he named Siberian red lead. Though misidentified as a lead compound with selenium and iron components, the material was in fact lead chromate with a formula of PbCrO4, now known as the mineral crocoite.
In 1770, Peter Simon Pallas visited the same site as Lehmann and found a red "lead" mineral that had very useful properties as a pigment in paints. The use of Siberian red lead as a paint pigment developed rapidly. A bright yellow made from crocoite became a color in fashion.
In 1797, Louis Nicolas Vauquelin received samples of crocoite ore. He was able to produce chromium oxide with a chemical formula of CrO3, by mixing crocoite with hydrochloric acid. In 1798, Vauquelin discovered that he could isolate metallic chromium by heating the oxide in a charcoal oven. He was also able to detect traces of chromium in precious gemstones, such as ruby, or emerald. Later that year he successfully isolated chromium atoms.
During the 1800s chromium was primarily used as a component of paints and in tanning salts but now metal alloys account for 85% of the use of chromium. The remainder is used in the chemical industry and refractory and foundry industries.
Chromium was named after the Greek word "Chrôma" meaning color, because of the many colorful compounds made from it.
Occurrence and production
Chromium is mined as chromite (FeCr2O4) ore. About two-fifths of the chromite ores and concentrates in the world are produced in South Africa. Kazakhstan, India, Russia and Turkey are also substantial producers. Untapped chromite deposits are plentiful, but geographically concentrated in Kazakhstan and southern Africa.
Approximately 15 million tons of marketable chromite ore were produced in 2000, and converted into approximately 4 million tons of ferro-chrome with an approximate market value of 2.5 billion United States dollars.
Though native chromium deposits are rare, some native chromium metal has been discovered. The Udachnaya Mine in Russia produces samples of the native metal. This mine is a kimberlite pipe rich in diamonds, and the reducing environment so provided helped produce both elemental chromium and diamond. (See also chromium minerals)
Chromium is obtained commercially by heating the ore in the presence of aluminium or silicon.
Chemical properties

Chromium is a member of the transition metals, in group 6. Chromium(0) has an electronic configuration of 4s13d5, due to the lower energy of the high spin configuration. Chromium exhibits a wide range of possible oxidation states. The most common oxidation states of chromium are +2, +3, and +6, with +3 being the most stable. +1, +4 and +5 are rare. Chromium compounds of oxidation state +6 are powerful oxidants.
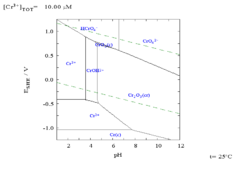
Chromium is passivated by oxygen, forming a thin (usually a few atoms thick being transparent because of thickness) protective oxide surface layer with another element such as nickel, and/or iron. It forms a compound called a spinal structure which, being very dense, prevents diffusion of oxygen into the underlying layer. (In iron or plain carbon steels the oxygen actually migrates into the underlying material.) Chromium is usually plated on top of a nickel layer which may or may not have been copper plated first. Chromium as opposite to most other metals such as iron and nickel does not suffer from hydrogen embrittlement. It does though suffer from nitrogen embrittlement and hence no straight chromium alloy has ever been developed. Below the pourbaix diagram can be seen, it is important to understand that the diagram only displays the thermodynamic data and it does not display any details of the rates of reaction.
Compounds
Potassium dichromate is a powerful oxidizing agent and is the preferred compound for cleaning laboratory glassware of any trace organics. It is used as a saturated solution in concentrated sulfuric acid for washing the apparatus. For this purpose, however, sodium dichromate is sometimes used because of its higher solubility (5 g/100 ml vs. 20 g/100 ml respectively). Chrome green is the green oxide of chromium, Cr2O3, used in enamel painting, and glass staining. Chrome yellow is a brilliant yellow pigment, PbCrO4, used by painters.
Chromic acid has the hypothetical structure H2CrO4. Neither chromic nor dichromic acid is found in nature, but their anions are found in a variety of compounds. Chromium trioxide, CrO3, the acid anhydride of chromic acid, is sold industrially as "chromic acid".
Chromium and the quintuple bond
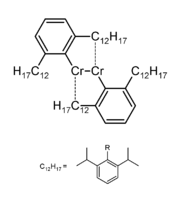
Chromium is notable for its ability to form quintuple covalent bonds. The synthesis of a compound of chromium(I) and a hydrocarbon radical was shown via X-ray diffraction to contain a quintuple bond of length 183.51(4) pm (1.835 angstroms) joining the two central chromium atoms.[2] This was accomplished through the use of an extremely bulky monodentate ligand which through its sheer size prevents further coordination. Chromium currently remains the only element for which quintuple bonds have been observed.
Applications
Uses of chromium:
- In metallurgy, to impart corrosion resistance and a shiny finish:
- as an alloy constituent, such as in stainless steel in cutlery
- in chrome plating,
- in anodized aluminium, literally turning the surface of aluminium into ruby.
- As dyes and paints :
- Chromium(III) oxide is a metal polish known as green rouge.
- Chromium salts color glass an emerald green.
- Chromium is what makes a ruby red, and therefore is used in producing synthetic rubies.
- also makes a brilliant yellow for painting
- As a catalyst.
- Chromite is used to make molds for the firing of bricks.
- Chromium salts are used in the tanning of leather.
- Potassium dichromate is a chemical reagent, used in cleaning laboratory glassware and as a titrating agent. It is also used as a mordant (i.e., a fixing agent) for dyes in fabric.
- Chromium(IV) oxide (CrO2) is used to manufacture magnetic tape, where its higher coercivity than iron oxide tapes gives better performance.
- In well drilling muds as an anti-corrosive.
- In medicine, as a dietary supplement or slimming aid, usually as chromium (III) chloride or chromium(III) picolinate.
- Chromium hexacarbonyl (Cr(CO)6) is used as a gasoline additive.
- Chromium boride (CrB) is used as a high-temperature electrical conductor.
- Chromium (III) sulfate (Cr2(SO4)3) is used as a green pigment in paints, in ceramic, varnishes and inks as well as in chrome plating.
- Chromium (VI) is used in the post Ballard preparation of Gravure (rotogravure) printing Forme Cylinders. By electroplating the metal onto the second coat of copper (after the Ballard skin), the longevity of the printing cylinder is increased.
- Micronutrient, in "health" aware drinks, known to improve the amount of energy you get from food.
Biological role
Trivalent chromium (Cr(III), or Cr3+) is required in trace amounts for sugar metabolism in humans (Glucose Tolerance Factor) and its deficiency may cause a disease called chromium deficiency. In contrast, hexavalent chromium is very toxic and mutagenic when inhaled as publicized by the film Erin Brockovich. Cr(VI) has not been established as a carcinogen when not inhaled but in solution it is well established as a cause of allergic contact dermatitis (ACD).[3]
Recently it was shown that the popular dietary supplement chromium picolinate complex generates chromosome damage in hamster cells. In the United States the dietary guidelines for daily chromium uptake were lowered from 50-200 µg for an adult to 35 µg (adult male) and to 25 µg (adult female).[4]
Isotopes
Naturally occurring chromium is composed of three stable isotopes; 52Cr, 53Cr, and 54Cr with 52Cr being the most abundant (83.789% natural abundance). Nineteen radioisotopes have been characterized with the most stable being 50Cr with a half-life of (more than) 1.8x1017 years, and 51Cr with a half-life of 27.7 days. All of the remaining radioactive isotopes have half-lives that are less than 24 hours and the majority of these have half-lives that are less than 1 minute. This element also has 2 meta states.
53Cr is the radiogenic decay product of 53Mn. Chromium isotopic contents are typically combined with manganese isotopic contents and have found application in isotope geology. Mn-Cr isotope ratios reinforce the evidence from 26Al and 107Pd for the early history of the solar system. Variations in 53Cr/52Cr and Mn/Cr ratios from several meteorites indicate an initial 53Mn/55Mn ratio that suggests Mn-Cr isotope systematics must result from in-situ decay of 53Mn in differentiated planetary bodies. Hence 53Cr provides additional evidence for nucleosynthetic processes immediately before coalescence of the solar system.
The isotopes of chromium range in atomic weight from 43 u (43Cr) to 67 u (67Cr). The primary decay mode before the most abundant stable isotope, 52Cr, is electron capture and the primary mode after is beta decay.
Precautions
Chromium metal and chromium(III) compounds are not usually considered health hazards; chromium is an essential trace mineral.[5] However, hexavalent chromium (chromium VI) compounds can be toxic if orally ingested or inhaled. The lethal dose of poisonous chromium (VI) compounds is about one half teaspoon of material. Most chromium (VI) compounds are irritating to eyes, skin and mucous membranes. Chronic exposure to chromium (VI) compounds can cause permanent eye injury, unless properly treated. Chromium(VI) is an established human carcinogen. An investigation into hexavalent chromium release into drinking water formed the plot of the motion picture Erin Brockovich.
World Health Organization recommended maximum allowable concentration in drinking water for chromium (VI) is 0.05 milligrams per liter. Hexavalent chromium is also one of the substances whose use is restricted by the European Restriction of Hazardous Substances Directive.
As chromium compounds were used in dyes and paints and the tanning of leather, these compounds are often found in soil and groundwater at abandoned industrial site, now needing environmental cleanup and remediation per the treatment of brownfield land. Primer paint containing hexavalent chromium is still widely used for aerospace and automobile refinishing applications.
See also
External links
- IARC Monograph "Chromium and Chromium compounds"
- International Chromium Development Association
- It's Elemental – The Element Chromium
- National Pollutant Inventory - Chromium (III) compounds fact sheet
- The Merck Manual – Mineral Deficiency and Toxicity
- National Institute for Occupational Safety and Health - Chromium Page
References
- ↑ Ignasi Puigdomenech, Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology, freely downloadable software at [1]
- ↑ T. Nguyen, A. D. Sutton, M. Brynda, J. C. Fettinger, G. J. Long and P. P. Power (2005). "Synthesis of a Stable Compound with Fivefold Bonding Between Two Chromium(I) Centers". Science. 310 (5749): 844–847. doi:10.1126/science.1116789.
- ↑ "ToxFAQs: Chromium". Agency for Toxic Substances & Disease Registry, Centers for Disease Control and Prevention. February 2001. Retrieved 2007-10-02.
- ↑ Vincent, J.B. (2007). "Recent advances in the nutritional biochemistry of trivalent chromium". Proceedings of the Nutrition Society. 63 (01): 41–47. doi:10.1079/PNS2003315.
|access-date=requires|url=(help) - ↑ "Chromium". Wellness Letter.
af:Chroom ar:كروم ast:Cromu (elementu) az:Xrom bn:ক্রোমিয়াম be:Хром bs:Hrom bg:Хром ca:Crom cs:Chróm co:Cromu da:Krom de:Chrom et:Kroom el:Χρώμιο eo:Kromo fa:کروم fur:Crom gl:Cromo (elemento) ko:크로뮴 hy:Քրոմ hi:क्रोमियम hr:Krom io:Kromio id:Kromium is:Króm it:Cromo he:כרום sw:Chromi ht:Kwòm ku:Krom la:Chromium lv:Hroms lb:Chrom lt:Chromas jbo:rogjinme hu:Króm mk:Хром mi:Konukita ms:Kromium nl:Chroom (metaal) no:Krom nn:Krom oc:Cròme uz:Xrom nds:Chrom qu:Krumu sq:Kromi scn:Cromu simple:Chromium sk:Chróm sl:Krom sr:Хром sh:Hrom fi:Kromi sv:Krom ta:குரோமியம் th:โครเมียม uk:Хром