Glioma pathophysiology
|
Glioma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Glioma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glioma pathophysiology |
|
Risk calculators and risk factors for Glioma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2], Sujit Routray, M.D. [3]
Overview
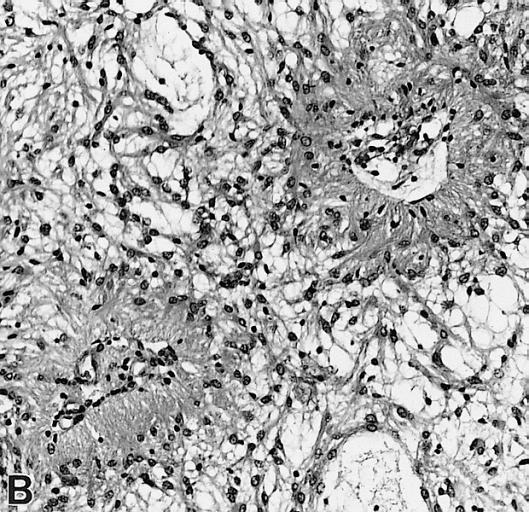
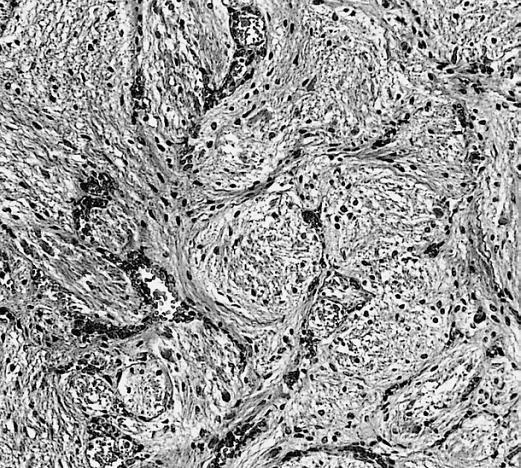
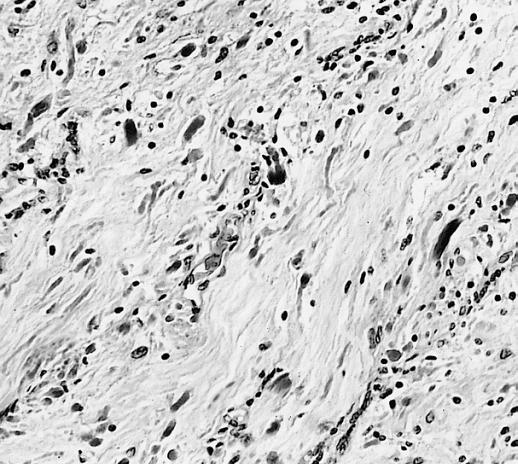
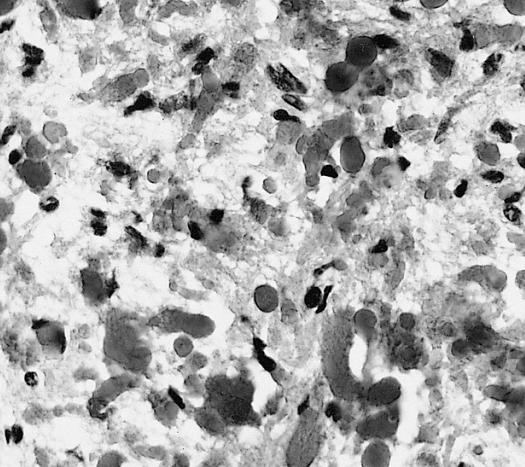
The pathogenesis of cerebral glioma involves invasion of the tumor cells into the adjacent normal brain tissue. Although in certain areas the margin of the tumor may seem to be macroscopically well defined from the brain, there are always microscopic nests of tumor cells extending well out into the brain.[1] Genes involved in the pathogenesis of glioma include ERCC1, ERCC2, XRCC1, MGMT, IDH1, IDH2, p53, EGFR, TSC1, TSC2, RB1, APC, hMLH1, hMSH2, PMS2, PTEN, NF1, and NF2.[2][3] The gross and histopathological appearance of glioma varies with the tumor grade and type.[1][4][5][6][7]
Pathophysiology
Pathogenesis
- The pathogenesis of cerebral glioma involves invasion of the tumor cells into the adjacent normal brain tissue. Although in certain areas the margin of the tumor may seem to be macroscopically well defined from the brain, there are always microscopic nests of tumor cells extending well out into the brain.[1]
- Astrocytic projections interact with vessels and act as additional elements of the blood brain barrier (BBB). The tumors take advantage of the blood brain barrier to ensure survival and continuous growth.
- Glioma cells migrate to different regions of the brain guided by the extension of blood vessels, colonizing the healthy adjacent tissue.
- Uncontrolled and fast growth also leads to the disruption of the chimeric and fragile vessels in the tumor mass resulting in peritumoral edema.[8]
Genetics
Genes involved in the pathogenesis of glioma include:[2][3]
Associated Conditions
Gliomas may be associated with:[3][1]
- Neurofibromatosis type 1
- Neurofibromatosis type 2
- Tuberous sclerosis
- Li-Fraumeni syndrome
- Turcot syndrome
- Maffucci syndrome
- Von Hippel-Lindau disease
- Retinoblastoma
Gross Pathology
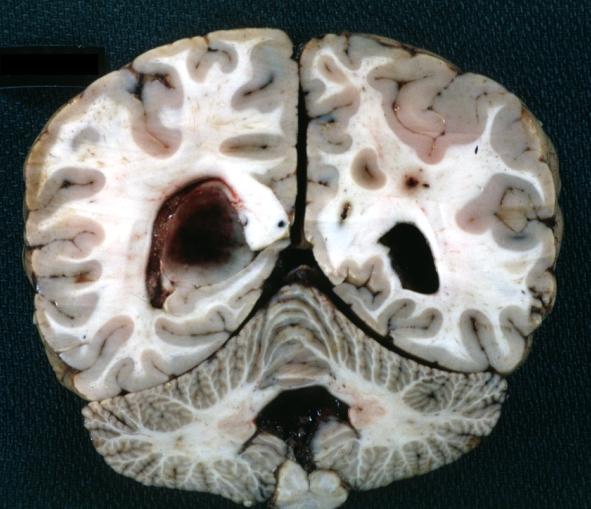
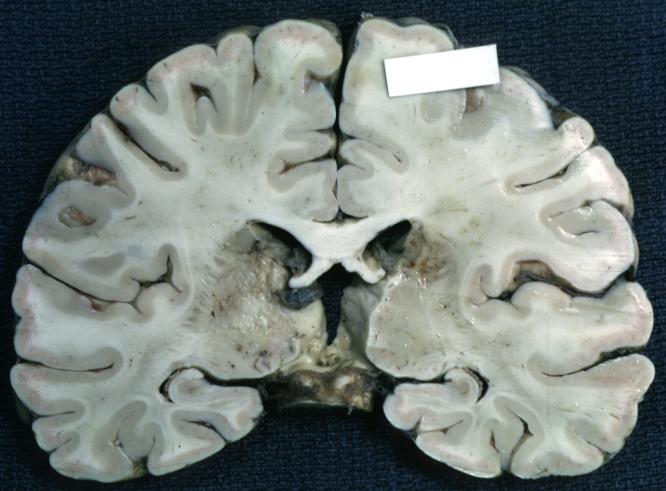
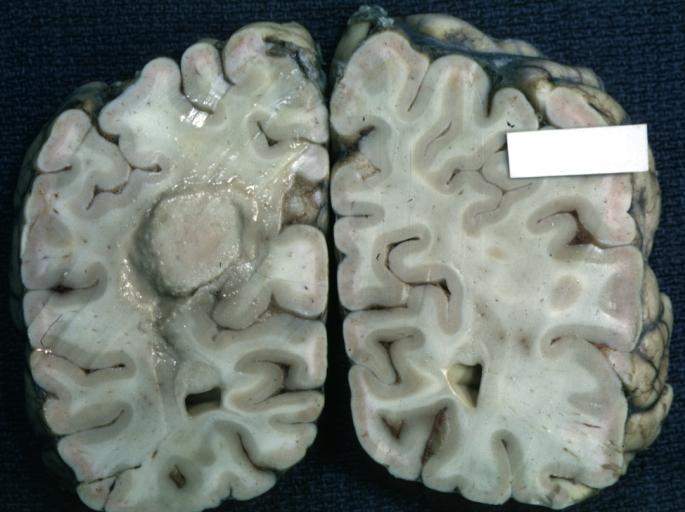
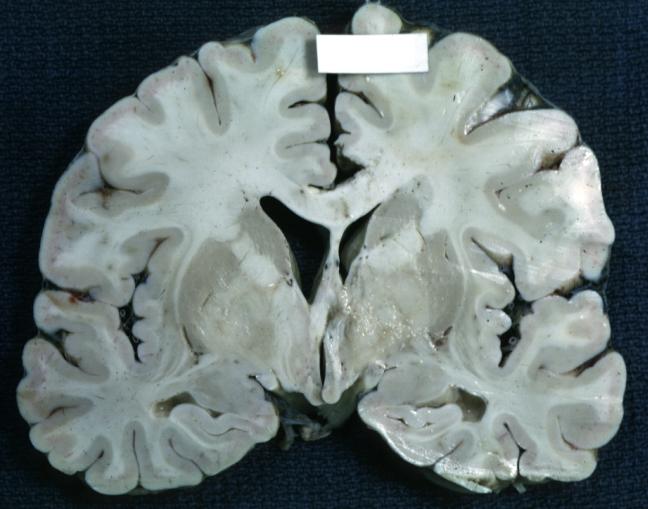
The gross pathological appearance of glioma varies with the tumor grade and type. Common findings are listed below:[1][4][5][6][7]
| Type of glioma | Gross pathological features |
|---|---|
| |
| |
| |
| |
| |
|
Gallery
-
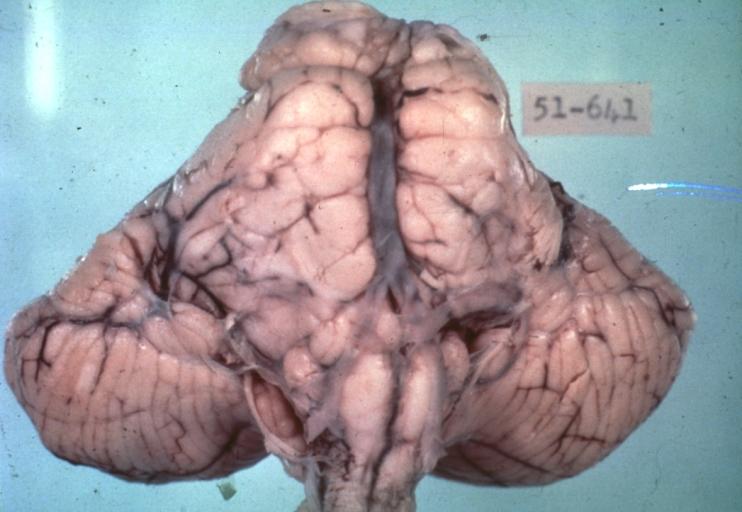
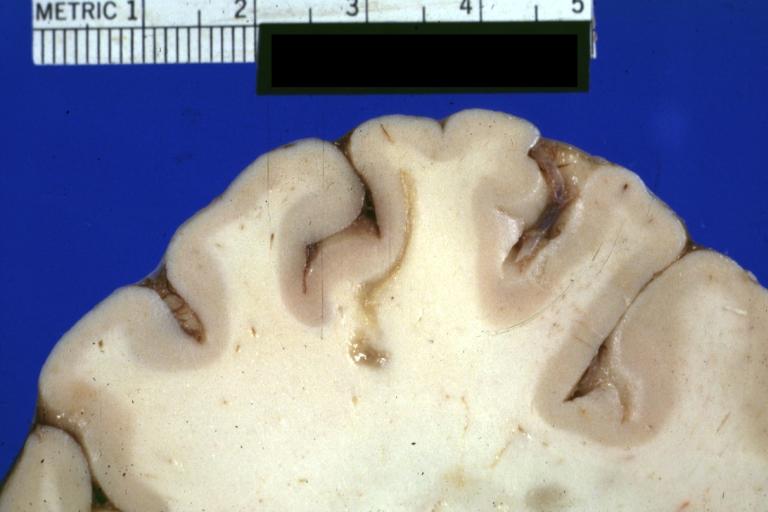
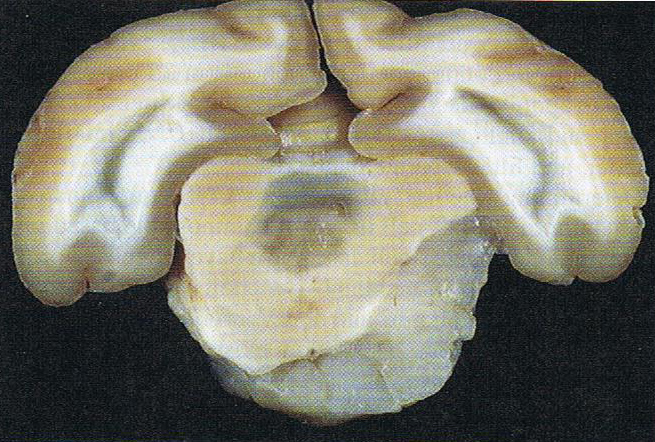
Brain: Pontine Glioma: Gross; fixed tissue, anterior view of brain stem and cerebellum with bosselated tumor adjacent to basilar artery
-
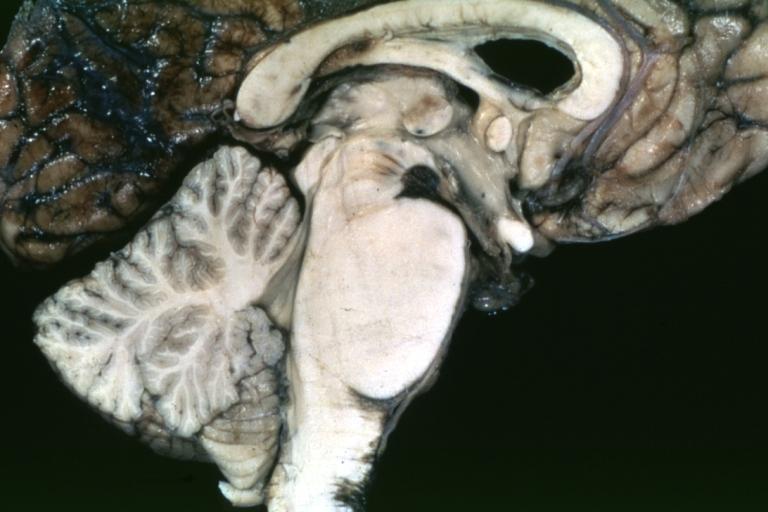
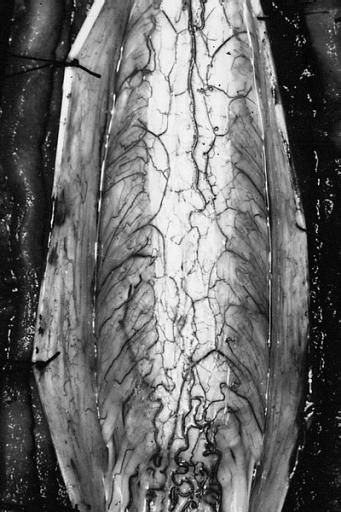
Brain: Pontine Glioma: Gross; fixed tissue, sagittal section brain stem and cerebellum
-
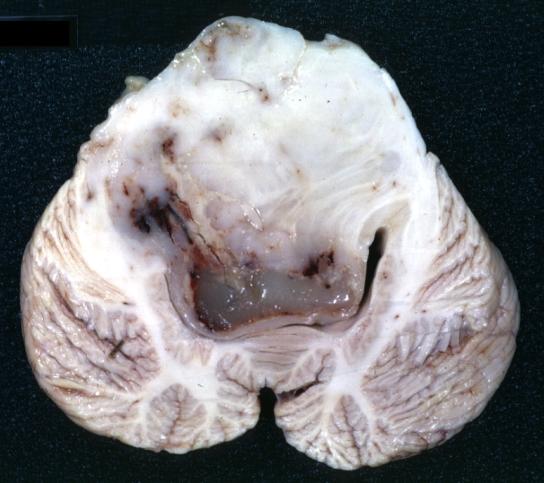
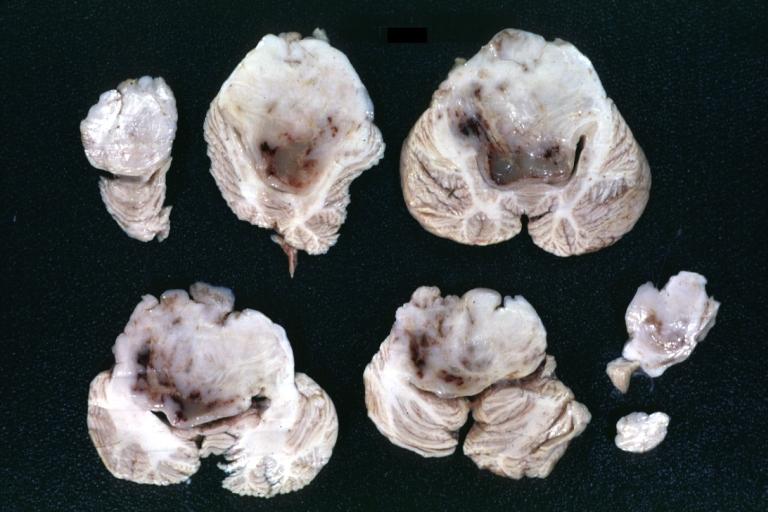
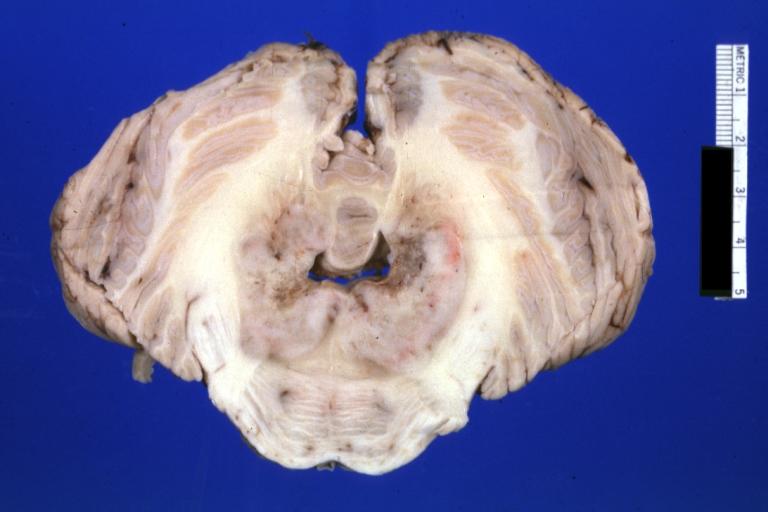
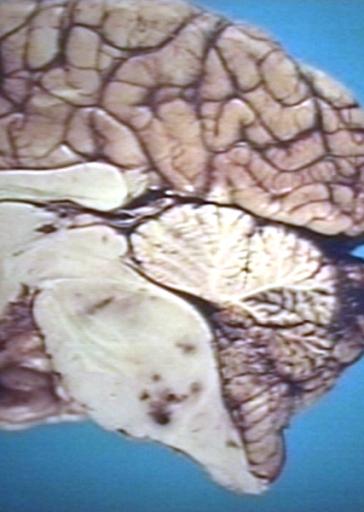
Brain: Glioma: Gross; fixed tissue, horizontal section brain stem and cerebellum with obvious gelatinous appearing neoplasm a pontine glioma
-
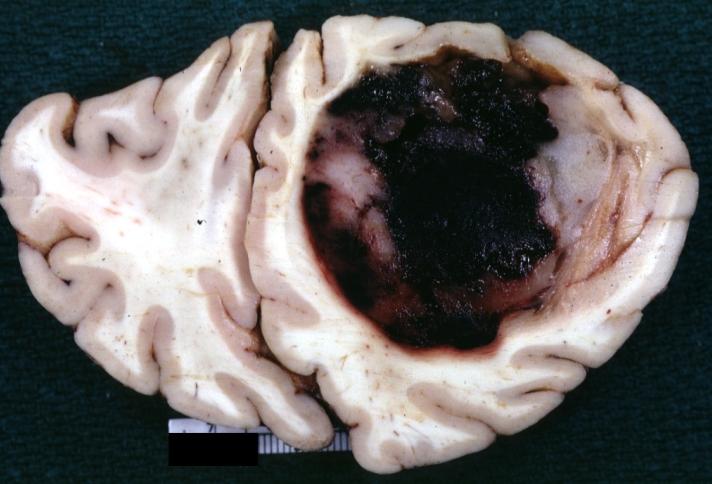
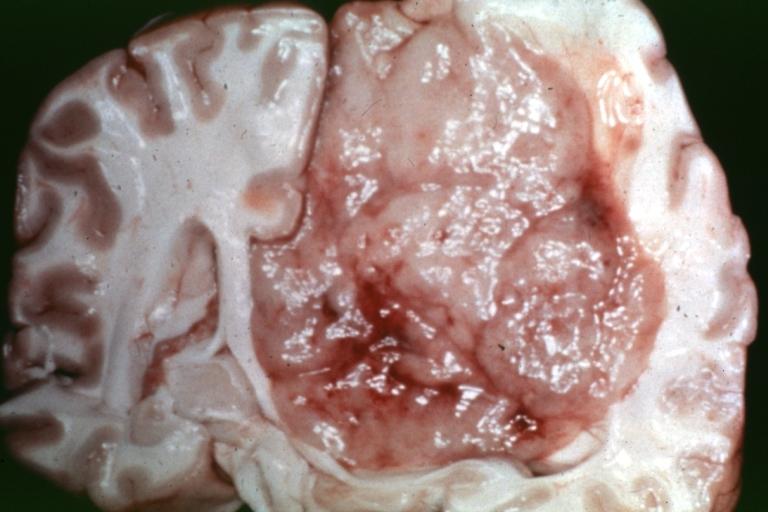
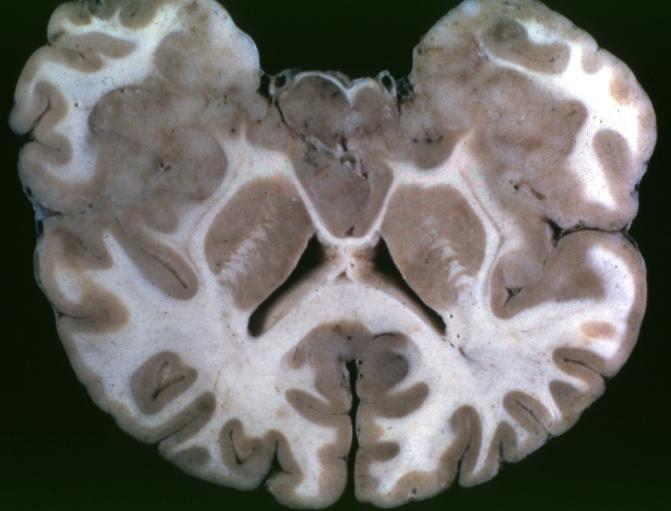
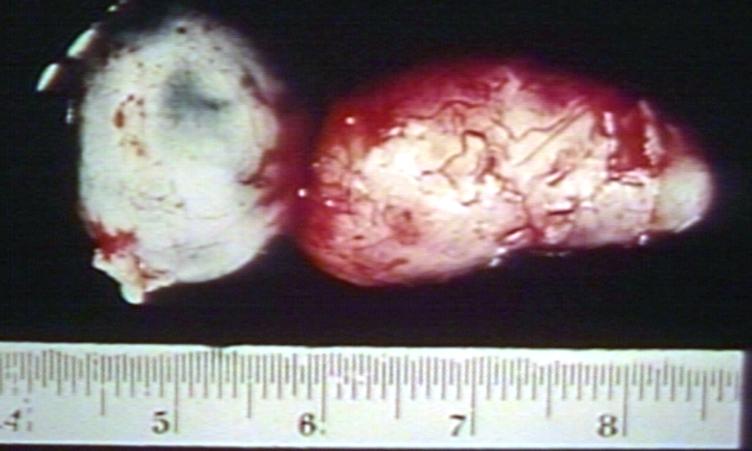
Brain: Oligodendroglioma: Gross; natural color, large, well circumscribed lesion in left frontal lobe
-
Brain: Glioma: Gross; fixed tissue, horizontal sections brain stem and cerebellum showing large pontine glioma
-
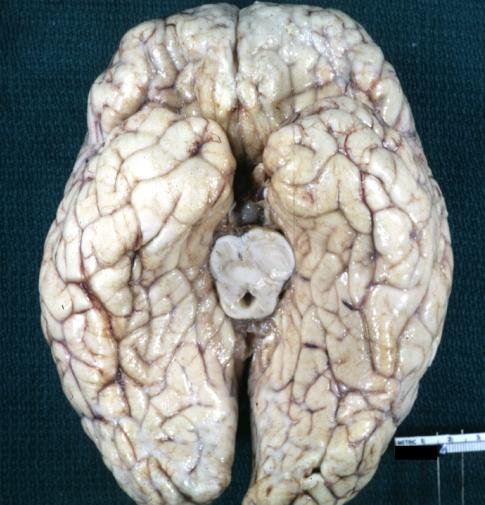
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis: Gross; fixed tissue, view of cerebral hemispheres from inferior with brain stem and cerebellum removed. Pontine asymmetry is easily seen due to low grade astrocytoma and meningeal gliomatosis is easily seen over frontal lobes
-
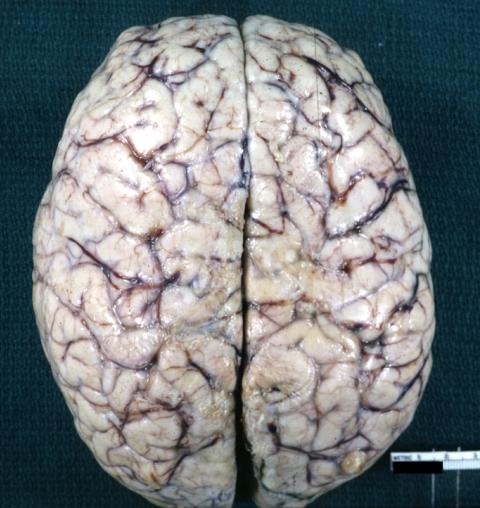
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis in 7 yo boy: Gross; fixed tissue, view of cerebral hemispheres from vertex meningeal gliomatosis.
-
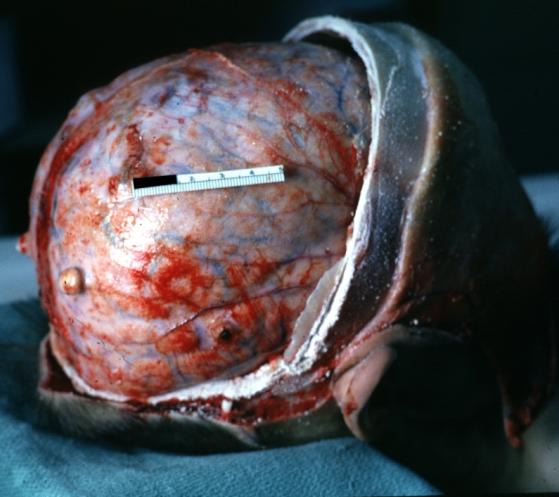
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis: Gross; in situ dural nodule
-
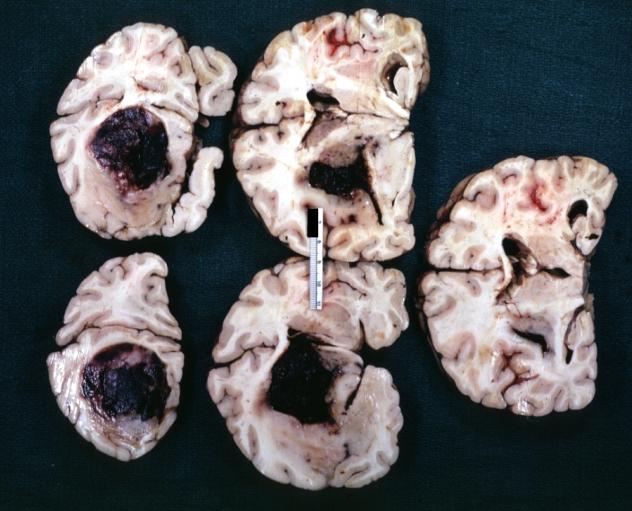
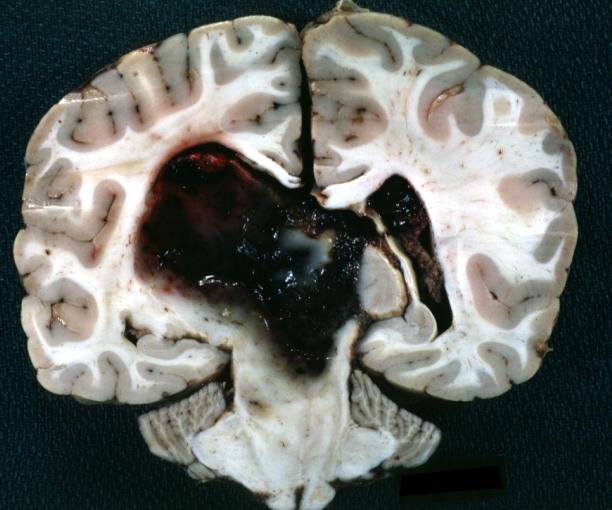
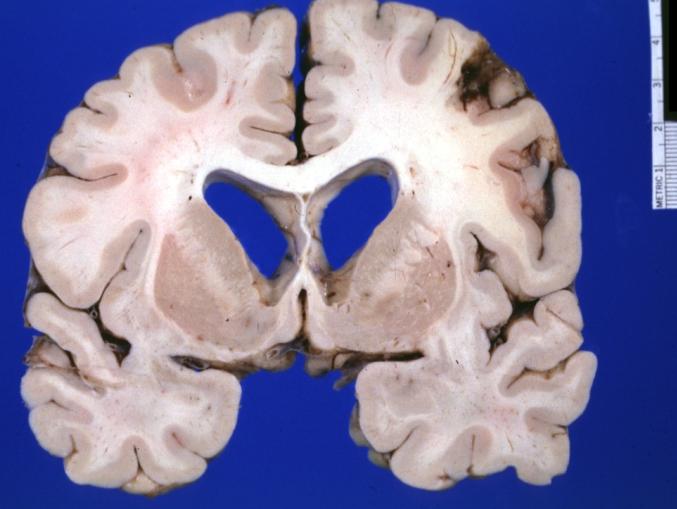
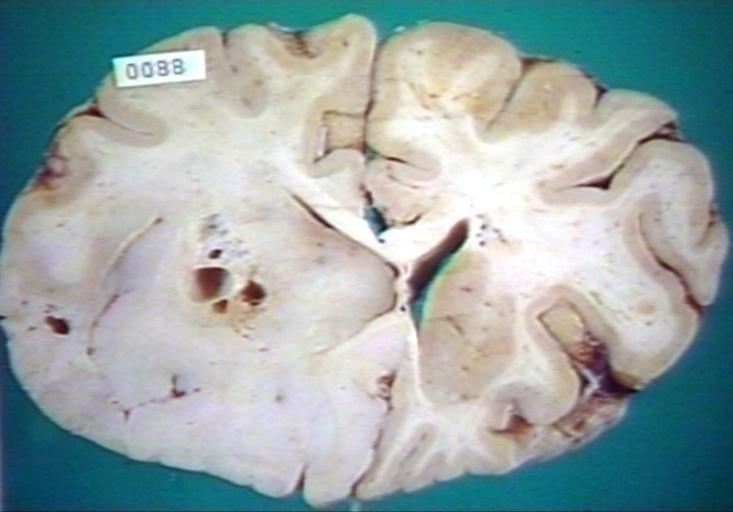
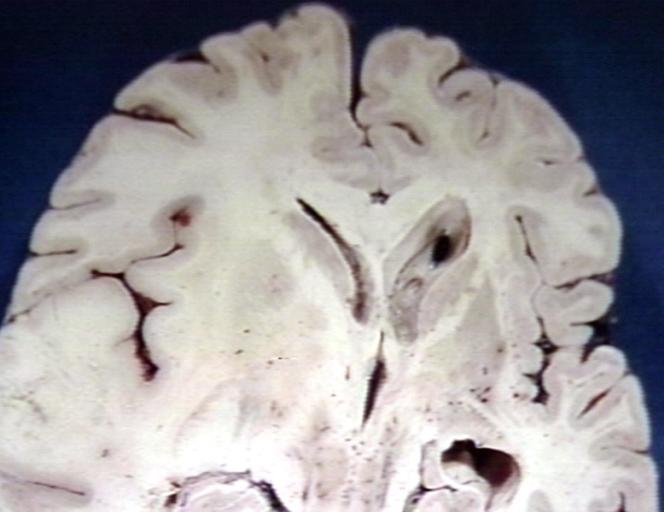
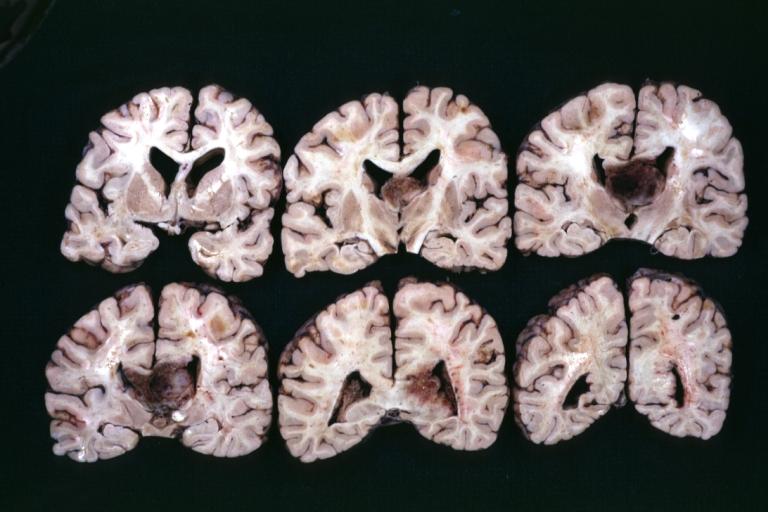
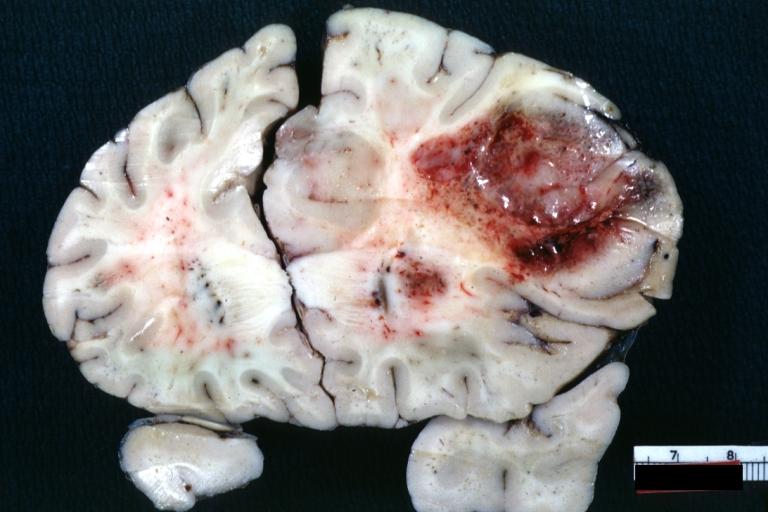
Brain: Oligodendroglioma: Gross; fixed tissue, multiple coronal sections, cerebral hemispheres with large tumor and hemorrhage into tumor
-
Brain: Oligodendroglioma: Gross; fixed tissue, coronal section, cerebral hemispheres, large hemorrhagic lesion in one hemisphere
-
Brain: Oligodendroglioma: Gross; fixed tissue, ischemic tissue, anterior to tumor mass
-
Brain: Oligodendroglioma: Gross; natural color, coronal section, cerebral hemispheres, large lesion, left parieto occipital white matter
-
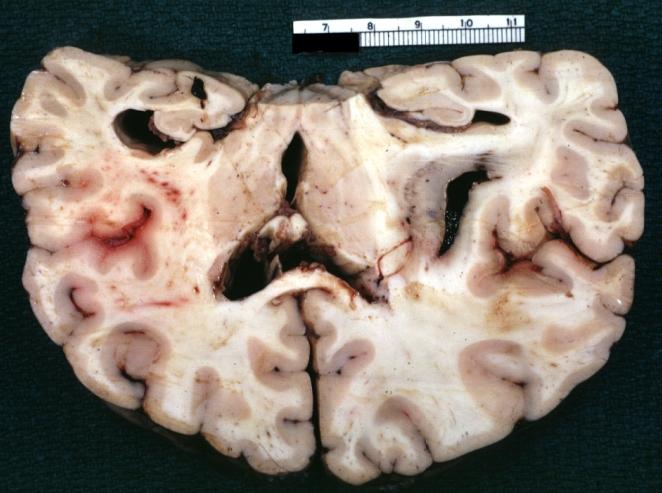
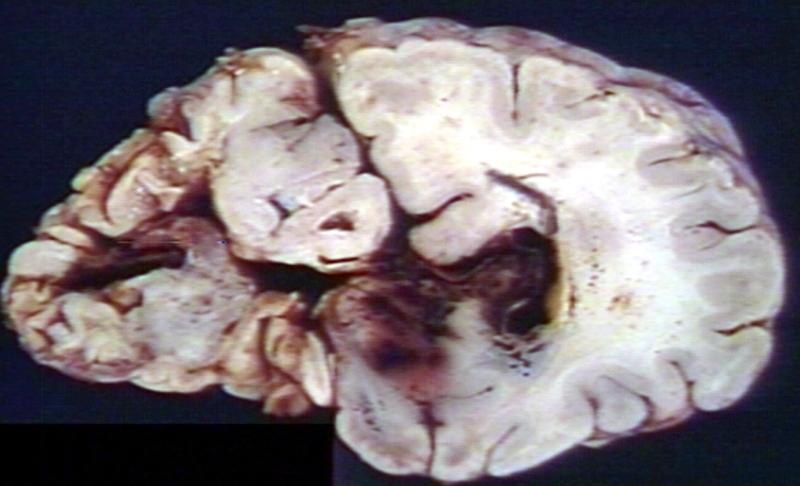
Brain: Gliomatosis Cerebri: Gross; fixed tissue, coronal sections, cerebral hemispheres, lesion is in temporal lobes and hypothalamus
-
Brain: Ventriculitis: Gross; fixed tissue, case of glioma with meningitis, a nice view of ventriculitis in one lateral ventricle
-
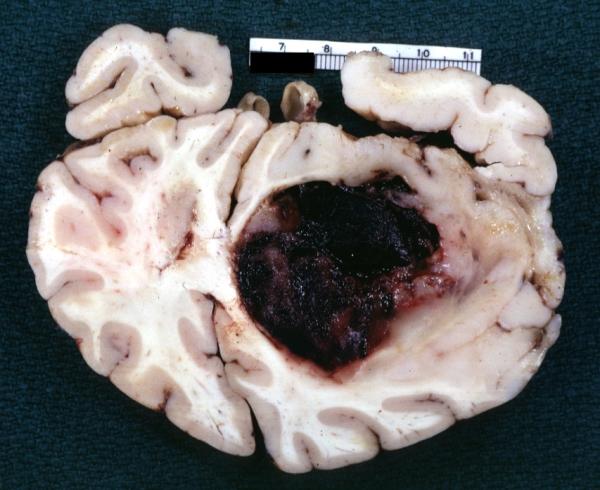
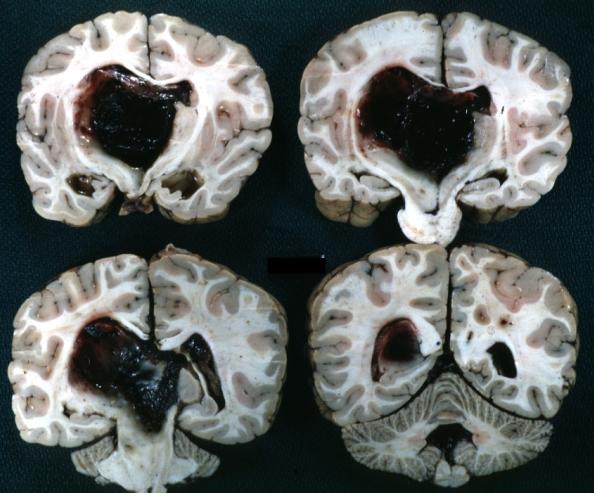
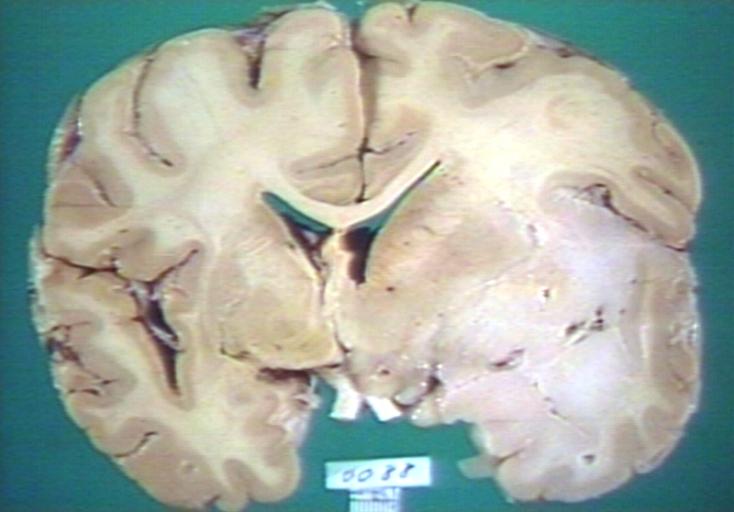
Brain: Glioma Thalamic Grade Ii-Iii: Gross; fixed tissue, four coronal sections, cerebral hemispheres, very large hemorrhagic lesion
-
Brain: Glioma Thalamic Grade Ii-Iii: Gross; fixed tissue, coronal section, cerebral hemispheres with large hemorrhagic lesion
-
Brain: Glioma Thalamic Grade Ii-Iii: Gross fixed tissue coronal section cerebral hemispheres lesions appears to be in choroid plexus of lateral ventricle in this picture. There is blood in fourth ventricle
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres (58 yo man)
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres
-
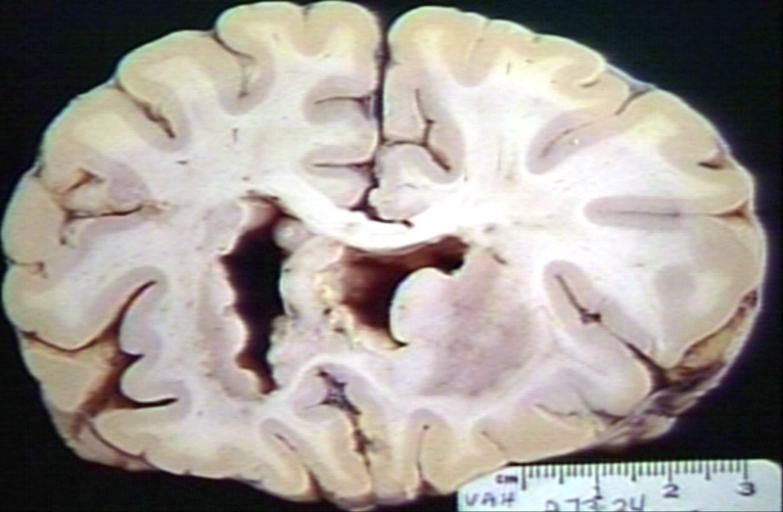
Brain: Infarct Subcortical: Gross; fixed tissue, close-up view of old small subcortical infarct, a case of microglioma
-
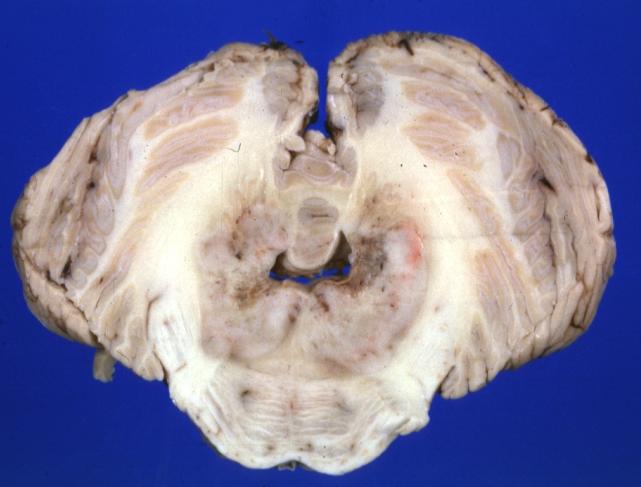
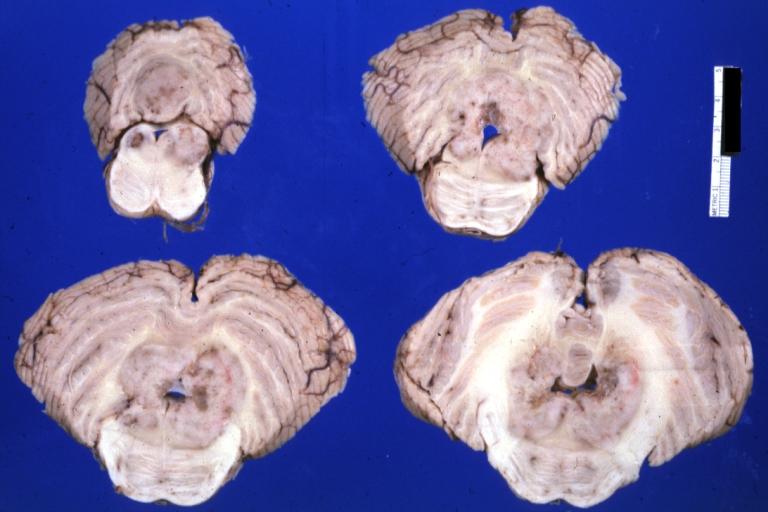
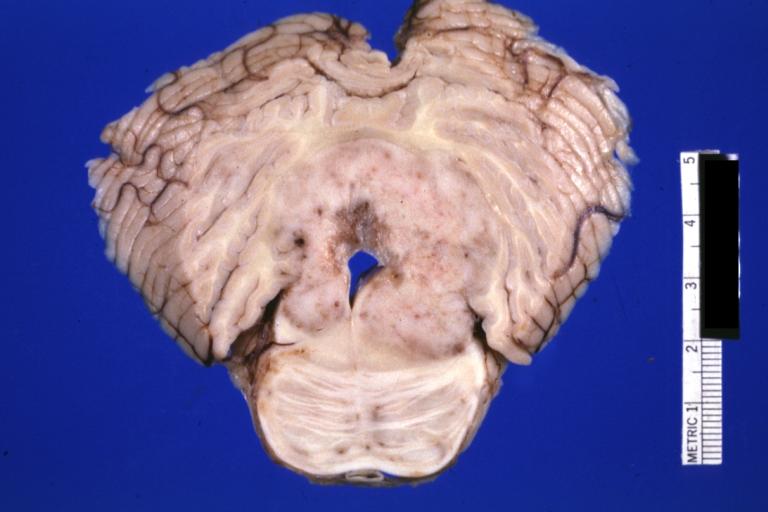
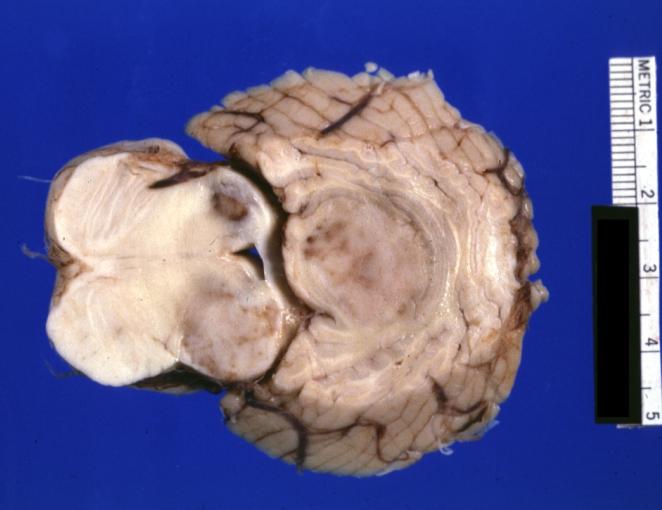
Brain: Microglioma: Gross; fixed tissue; cerebellum and fourth ventricle with periventricular tumor invasion
-
Brain: Microglioma: Gross fixed tissue horizontal sections cerebellum and brain stem with periventricular neoplastic infiltrate
-
Brain: Microglioma: Gross fixed tissue horizontal section midbrain and cerebellum at mid pons level periventricular tumor infiltration
-
Brain: Microglioma: Gross fixed tissue horizontal section rostral pons and cerebellum
-
Brain: Microglioma: Gross fixed tissue horizontal section rostral pons and cerebellum periventricular tumor invasion
-
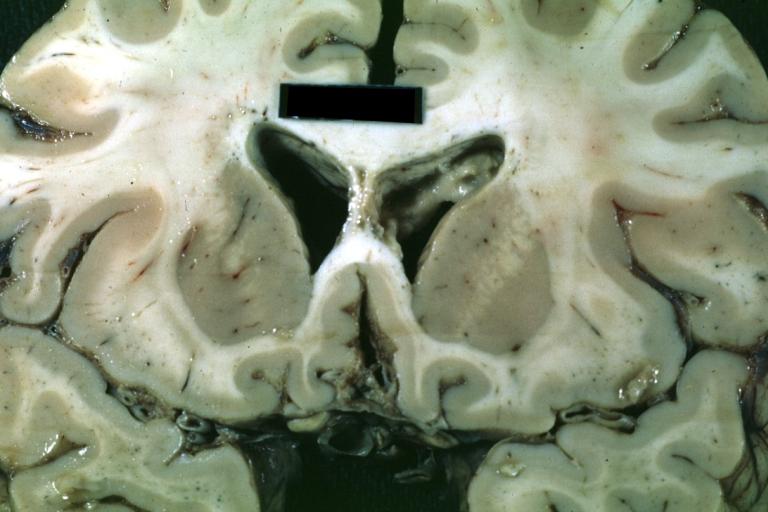
Brain: Microglioma: Gross fixed tissue coronal section cerebral hemispheres with mild ventricular dilation
-
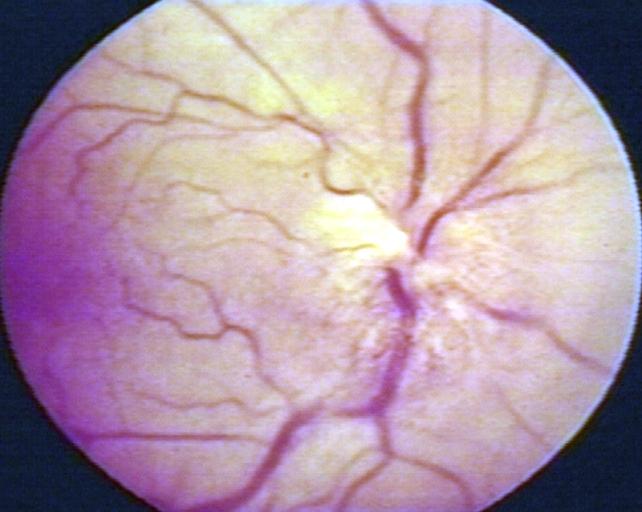
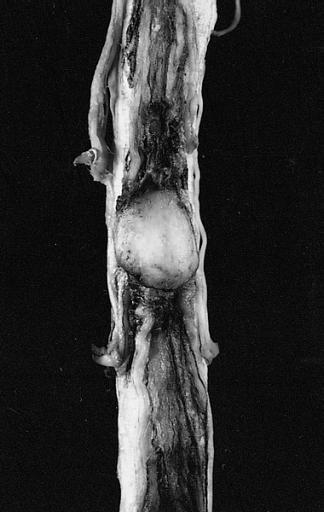
Glioma: Optic Nerve
-
Brain: Oligodendroglioma, Frontal Lobe
-
Brain: Oligodendroglioma, Mixed Astrocytoma & Oligodendroglioma
-
Brain: Oligodendroglioma
-
Brain: Oligodendroglioma
-
Brain: Glioma, Grade II Anaplastic
-
Brain: Glioma, Brain stem, Low Grade
-
Fundoscopy: Eye; Optic Nerve Glioma, Optic Nerve
-
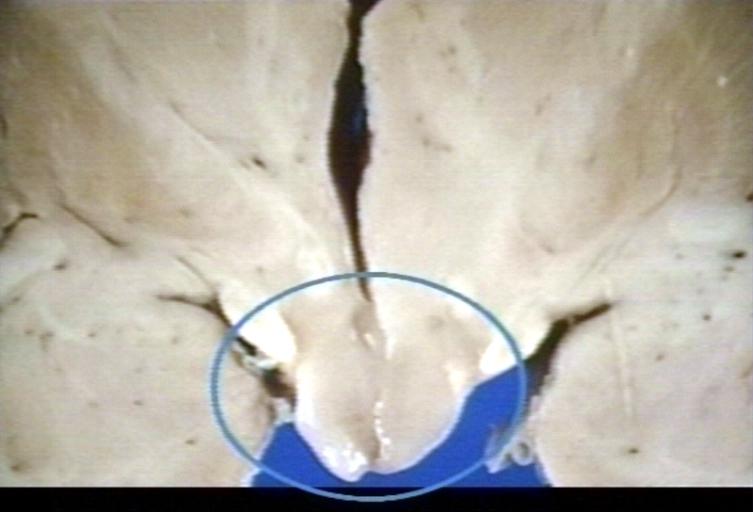
Brain: Glioma, Hypothalamic, Circle Around Region of Tumor
-
CNS: Pilocytic Astrocytoma of the Spinal Cord. The fusiform expansion of the spinal cord produced by this pilocytic astrocytoma is not, on external examination alone, distinguishable from that produced by a nonresectable diffuse glioma.
-
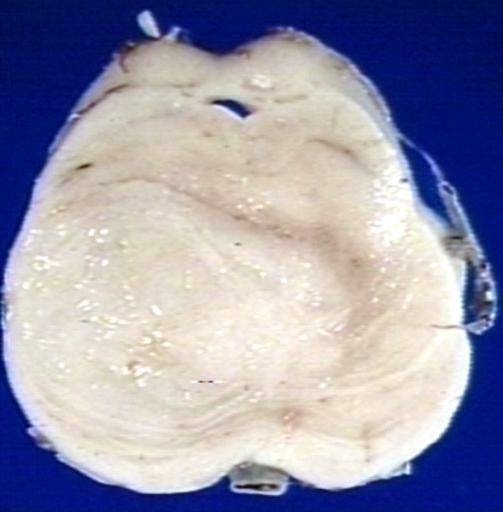
Brain: Glioma, Pontine
-
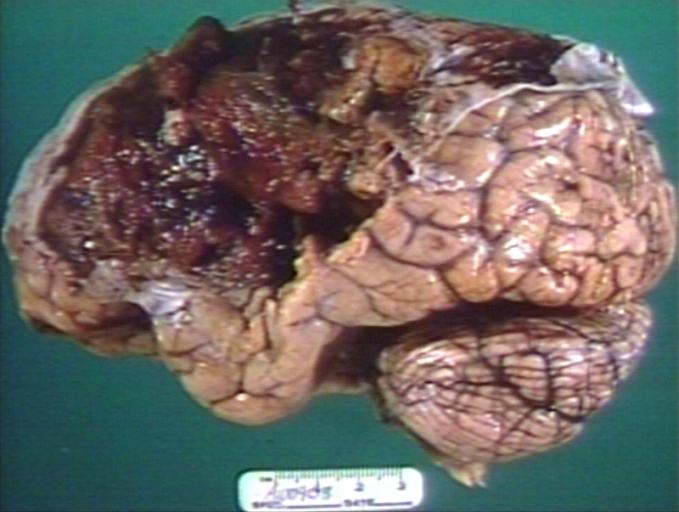
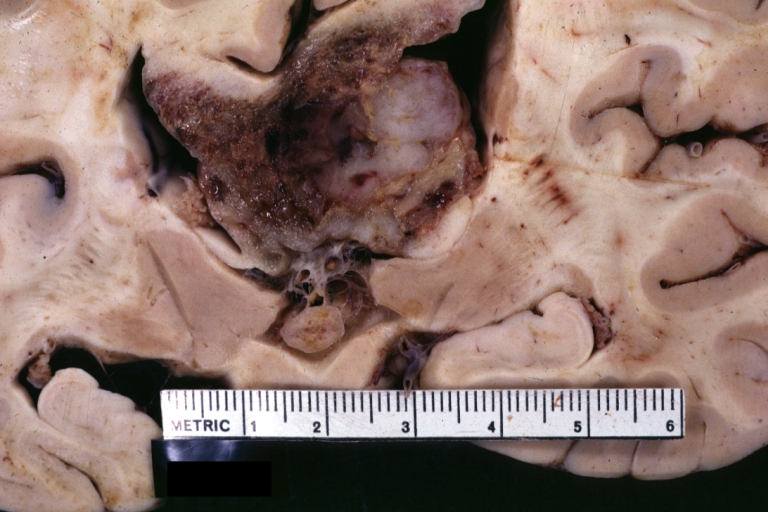
Brain: Glioblastoma Multiforme: Gross fixed tissue close-up large necrotic tumor mass in septum pellucidum
-
Brain: Glioblastoma Multiforme: Gross fixed tissue coronal section of the brain with a large necrotic tumor mass in septum pellucidum diagnosed as astrocytoma grade III
-
Brain: Glioblastoma Multiforme: Gross natural color large hemorrhagic lesion in right centrum semiovale
-
CNS: Malignant pilocytic astrocytoma: A 29-year-old woman died 2 years after a diagnosis of "atypical pilocytic astrocytoma" of the pineal region. At autopsy, multiple tumor implants were present in the craniospinal subarachnoid spaces.
-
Brain: Oligodendroglioma; Ventricular Cobblestone Effect
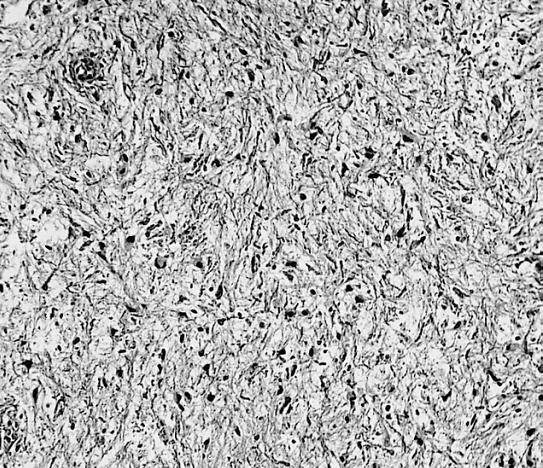
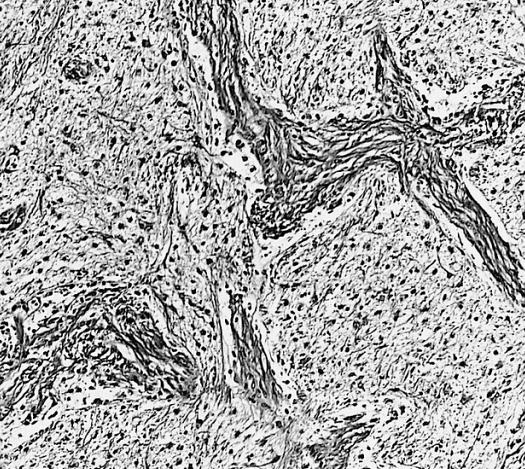
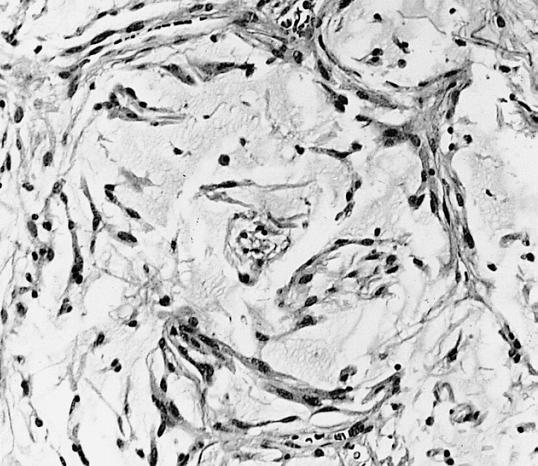
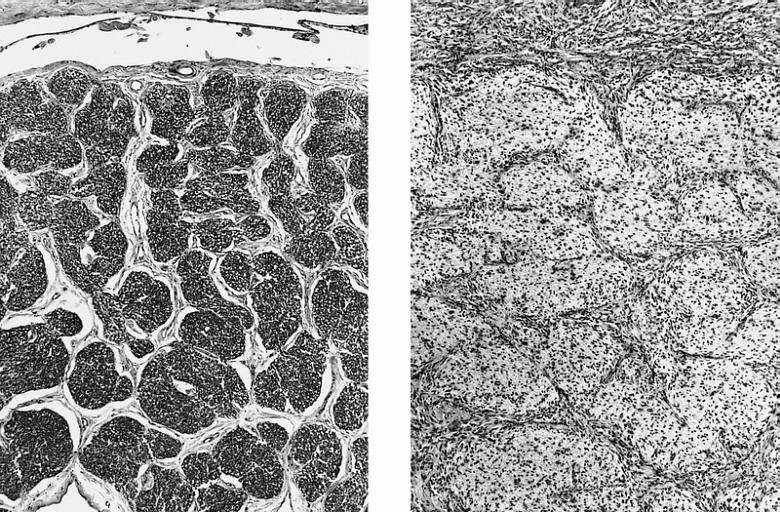
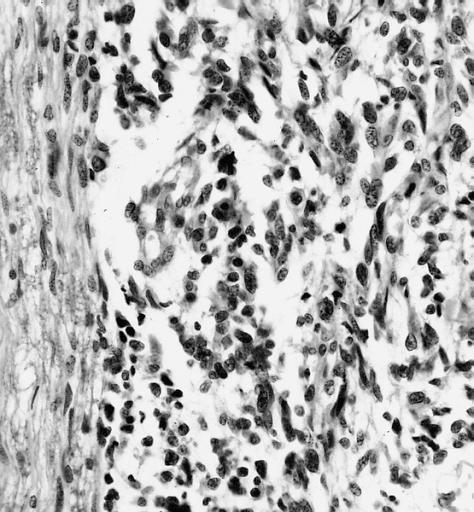
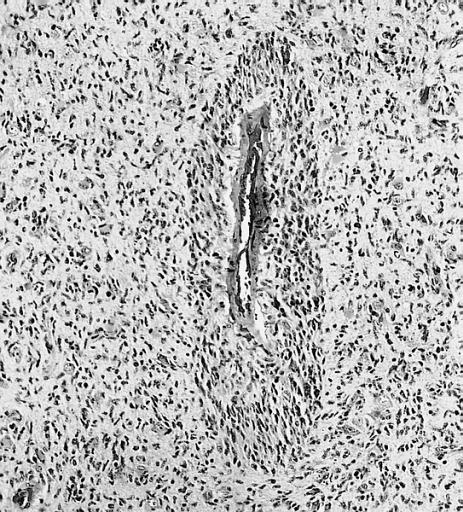
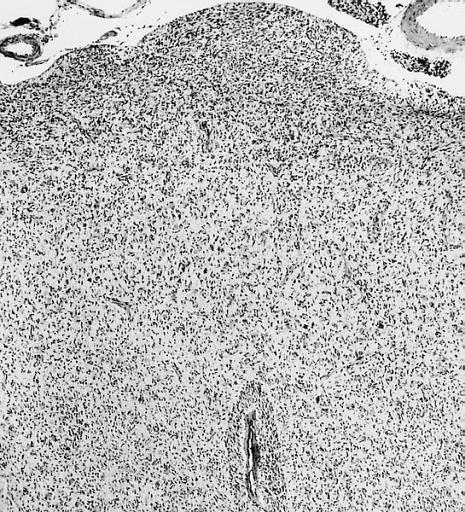
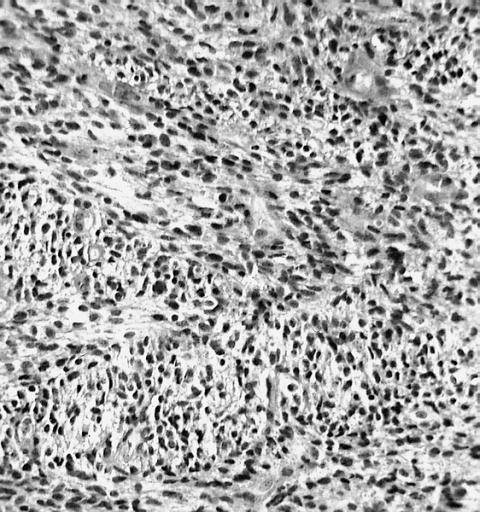
Microscopic Pathology
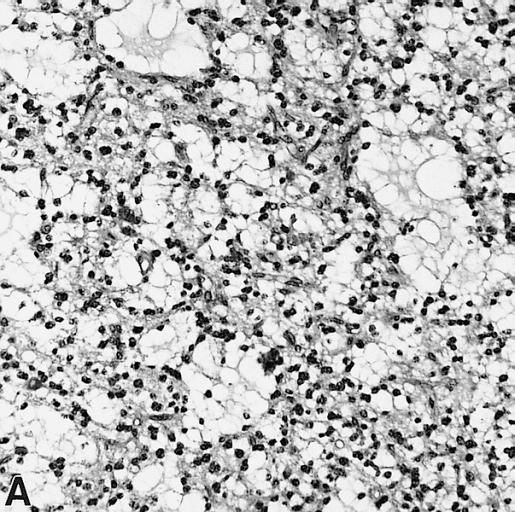
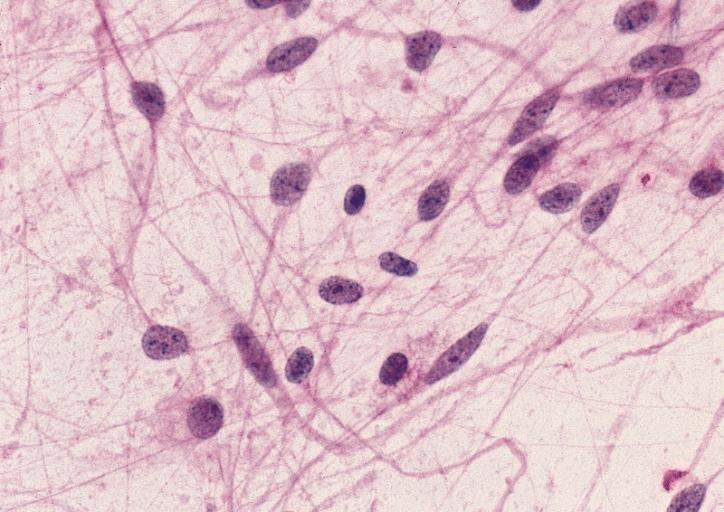
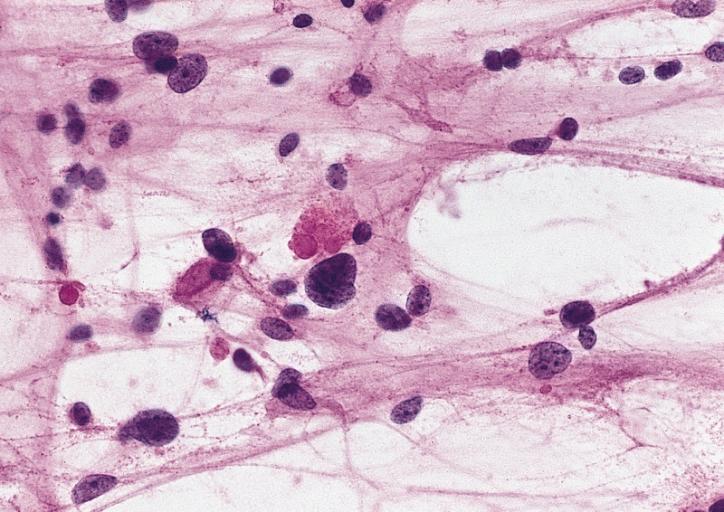
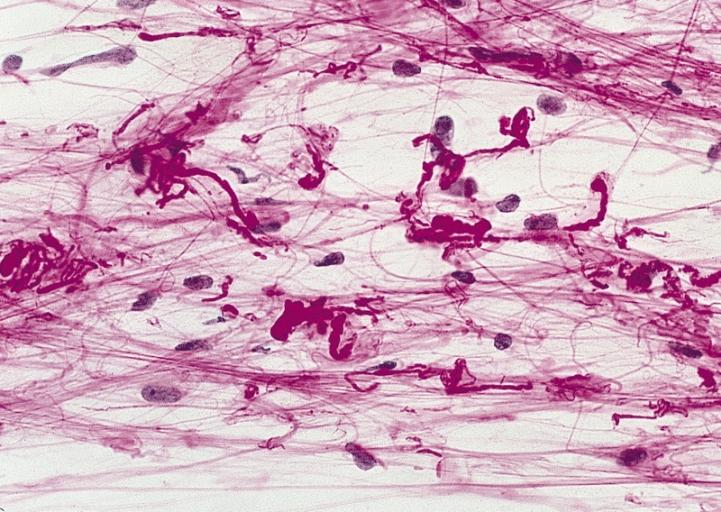
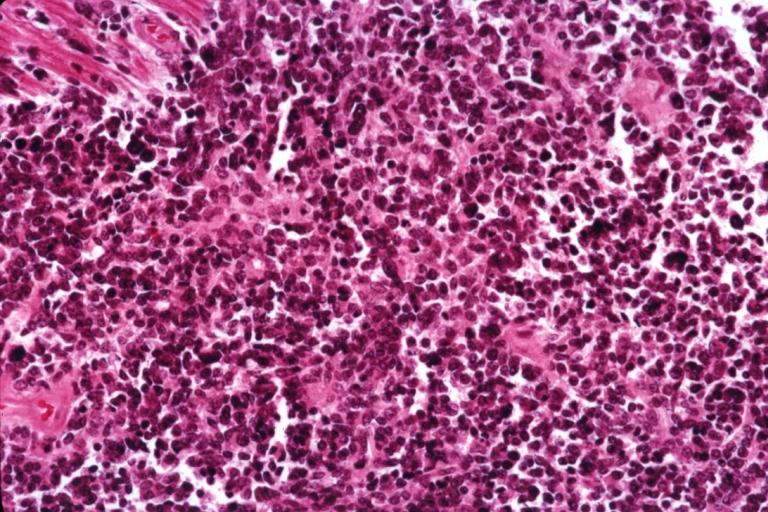
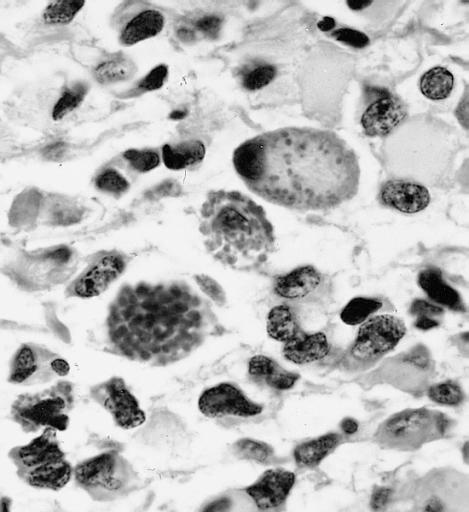
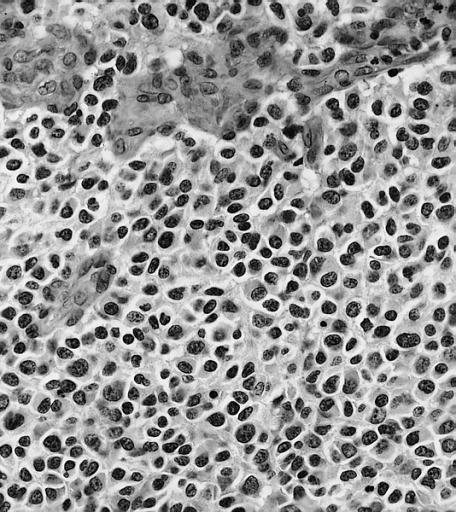
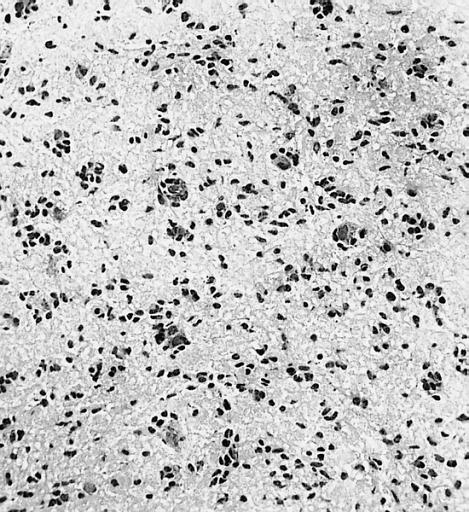
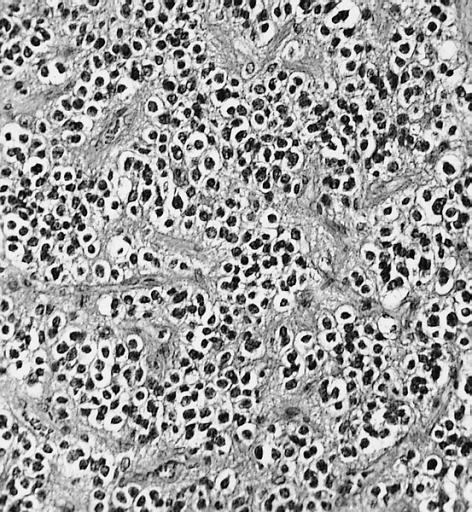
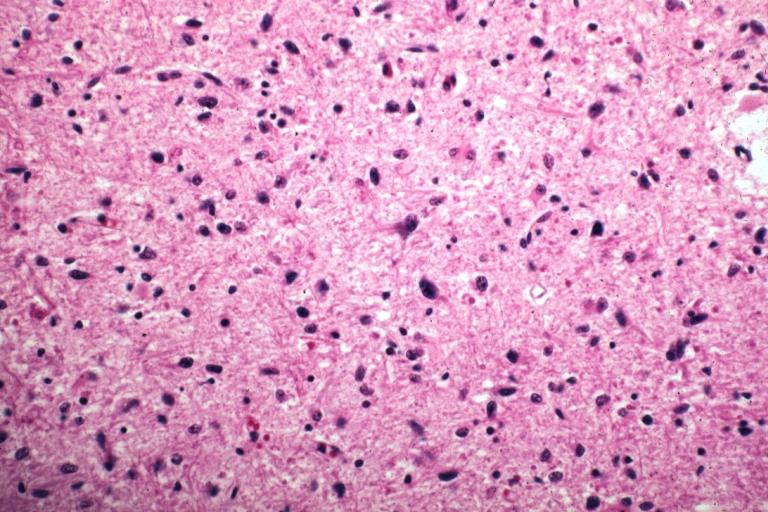
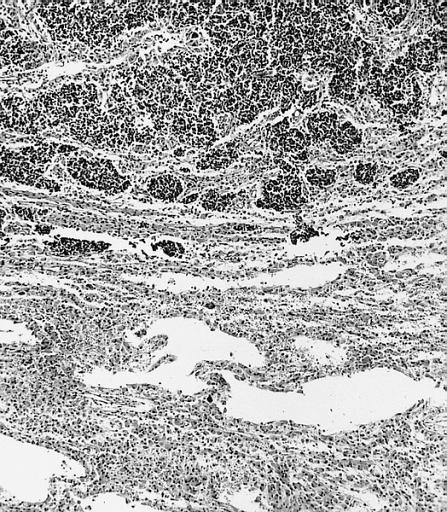
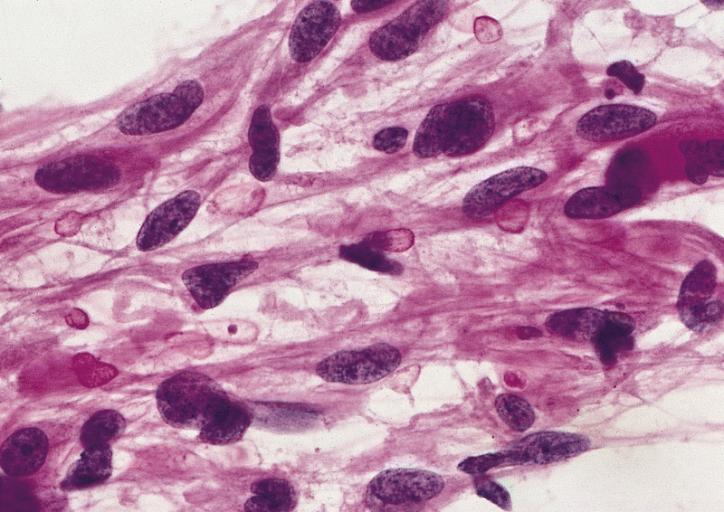
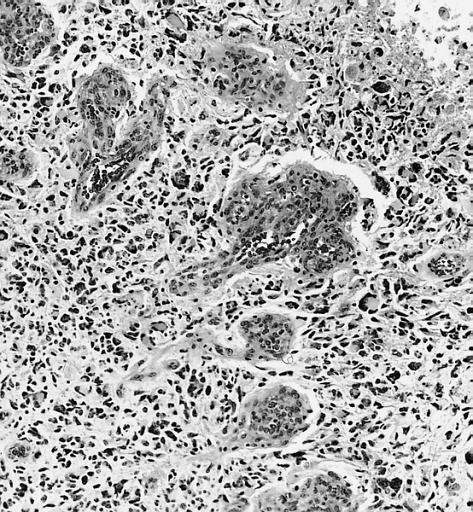
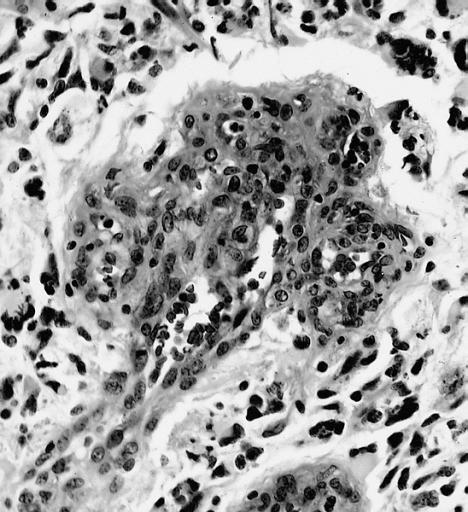
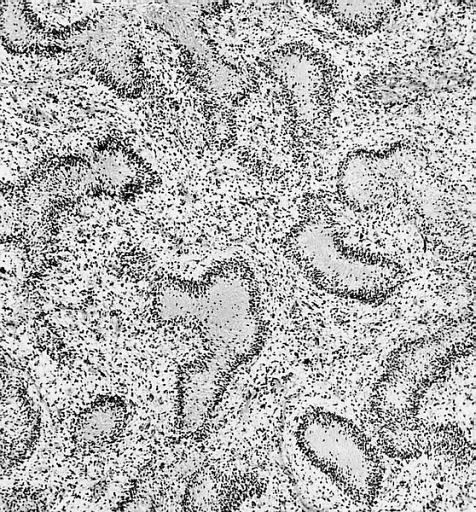
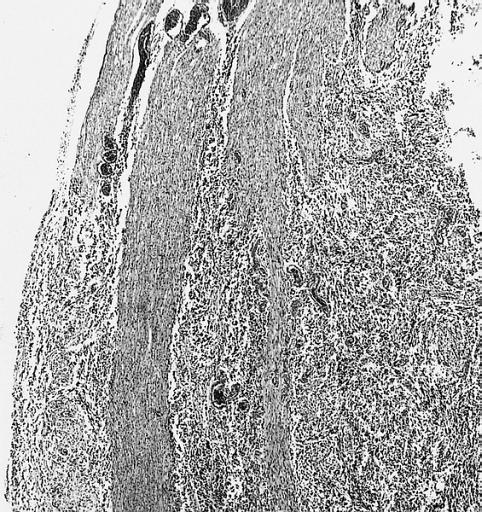
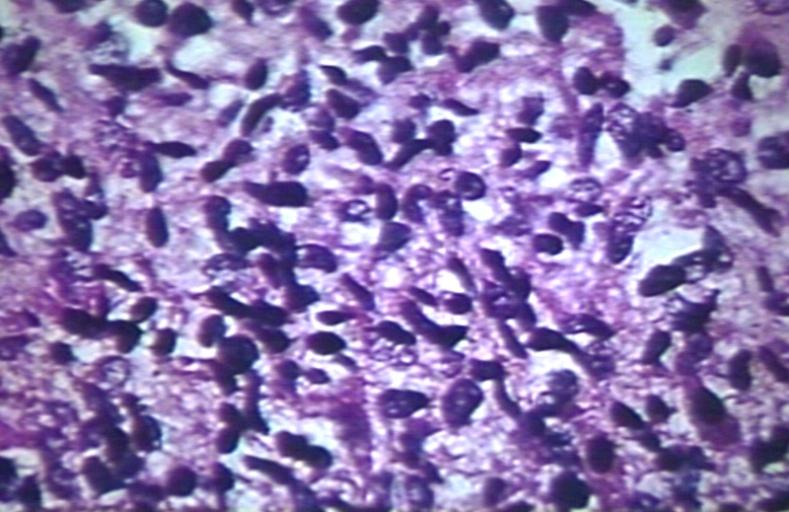
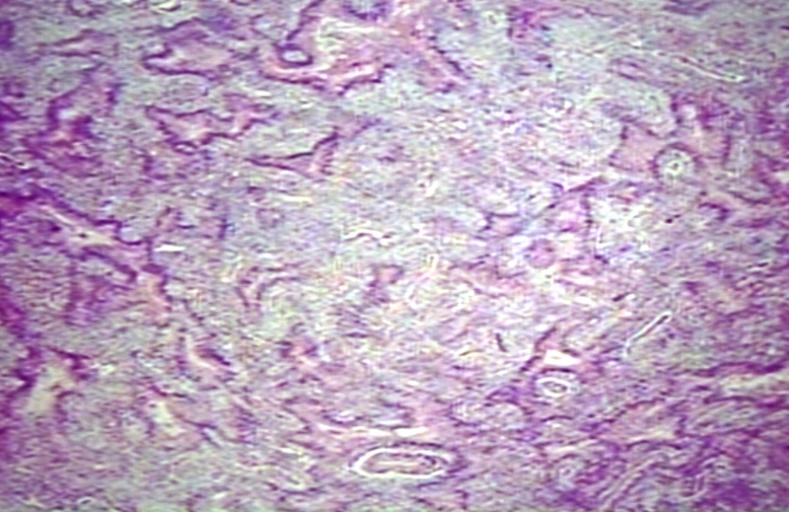
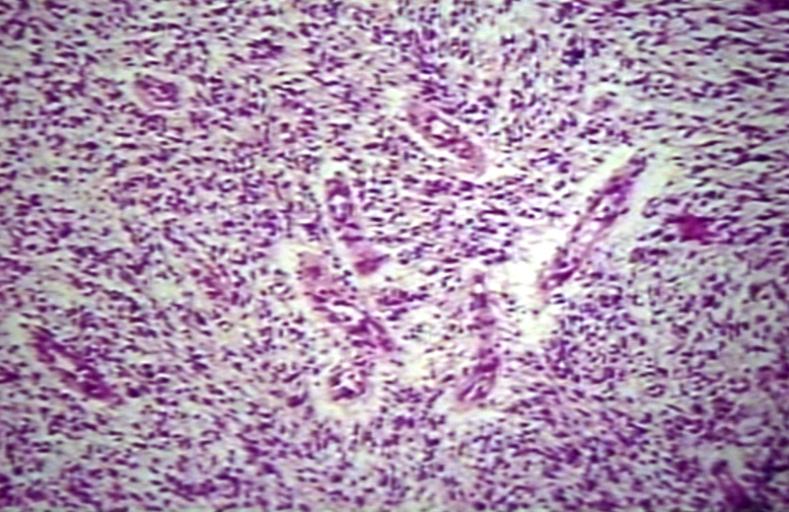
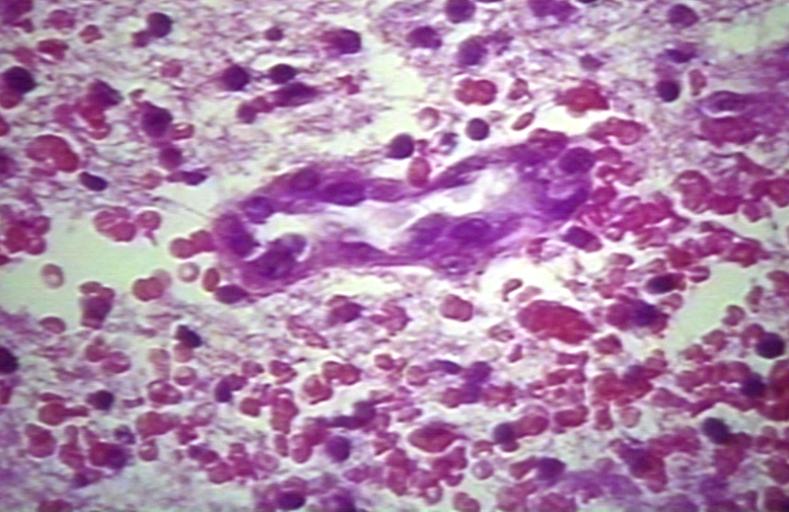
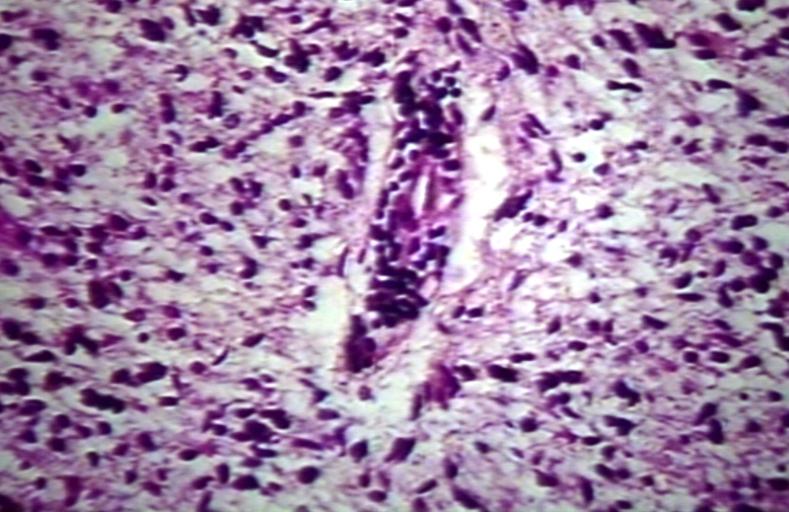
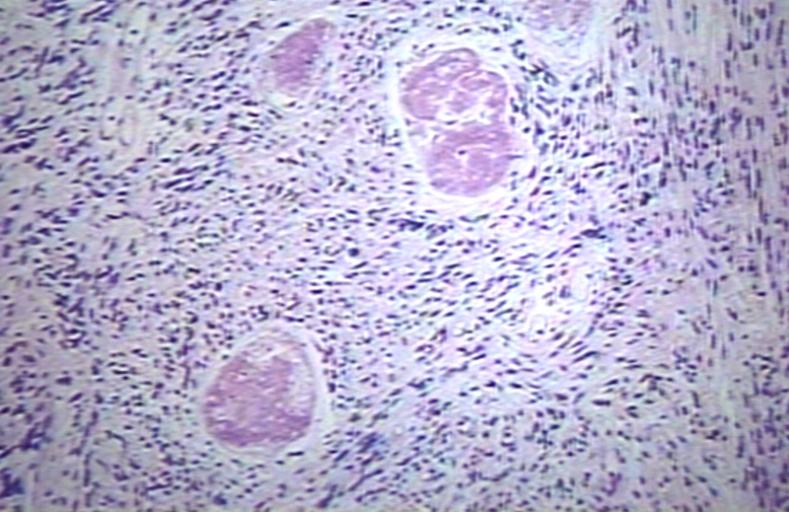
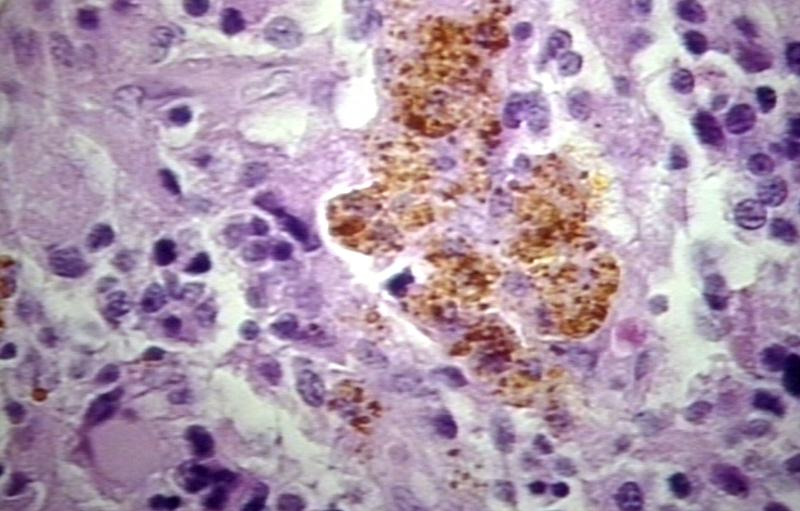
The histopathological appearance of glioma varies with the tumor grade and type, with increasing cellular atypia, mitoses, endothelial cell proliferation, and necrosis. Common findings are listed below:[1][4][5][6][7]
| Type of glioma | Histopathological features |
|---|---|
| |
| |
| |
| |
|
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Pathology of glioma. http://www.surgwiki.com/wiki/Intracranial_tumours,_infection_and_aneurysms#MANAGEMENT
- ↑ 2.0 2.1 Pathology of glioma. Wikipedia. https://en.wikipedia.org/wiki/Glioma
- ↑ 3.0 3.1 3.2 Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006). "Epidemiology and molecular pathology of glioma". Nat Clin Pract Neurol. 2 (9): 494–503, quiz 1 p following 516. doi:10.1038/ncpneuro0289. PMID 16932614.
- ↑ 4.0 4.1 4.2 Pathology of gliomas. Libre Pathology. http://librepathology.org/wiki/index.php/Oligodendroglioma
- ↑ 5.0 5.1 5.2 Pathology of anaplastic astrocytoma. Libre Pathology. http://librepathology.org/wiki/index.php?title=Neuropathology_tumours&redirect=no#Infiltrative_astrocytomas
- ↑ 6.0 6.1 6.2 Pathology of glioblastoma. Libre Pathology. http://librepathology.org/wiki/index.php/Glioblastoma
- ↑ 7.0 7.1 7.2 Pathology of ependymoma. Libre Pathology. http://librepathology.org/wiki/index.php/Ependymoma
- ↑ Dubois LG, Campanati L, Righy C, D'Andrea-Meira I, Spohr TC, Porto-Carreiro I; et al. (2014). "Gliomas and the vascular fragility of the blood brain barrier". Front Cell Neurosci. 8: 418. doi:10.3389/fncel.2014.00418. PMC 4264502. PMID 25565956.