Topiramate adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
For patient information about topiramate, click here.
Adverse Reactions
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions are discussed in more detail in other sections of the labeling:
Acute Myopia and Secondary Angle Closure [see Warnings and Precautions (5.1)]
Oligohidrosis and Hyperthermia [see Warnings and Precautions (5.2)]
Metabolic Acidosis [see Warnings and Precautions (5.3)]
Suicidal Behavior and Ideation [see Warnings and Precautions (5.4)]
Cognitive/Neuropsychiatric Adverse Reactions [see Warnings and Precautions (5.5)]
Fetal Toxicity [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)]
Withdrawal of Antiepileptic Drugs (AEDs) [see Warnings and Precautions (5.7)]
Sudden Unexplained Death in Epilepsy (SUDEP) [see Warnings and Precautions (5.8)]
Hyperammonemia and Encephalopathy (Without and With Concomitant Valproic Acid [VPA] Use) [see Warnings and Precautions (5.9)]
Kidney Stones [see Warnings and Precautions (5.10)]
Hypothermia with Concomitant Valproic Acid (VPA) Use [see Warnings and Precautions (5.11)]
Paresthesia [see Warnings and Precautions (5.12)]
The data described in the following sections were obtained using topiramate tablets.
Monotherapy Epilepsy
Adults ≥16 Years
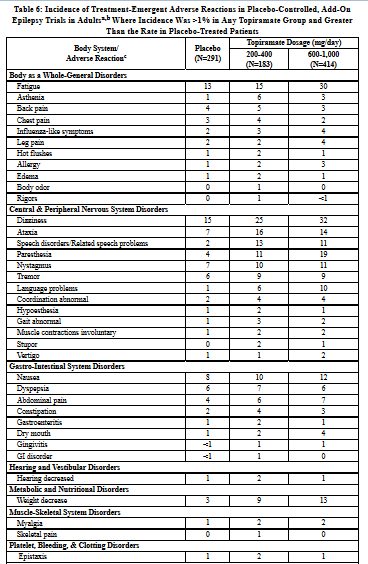
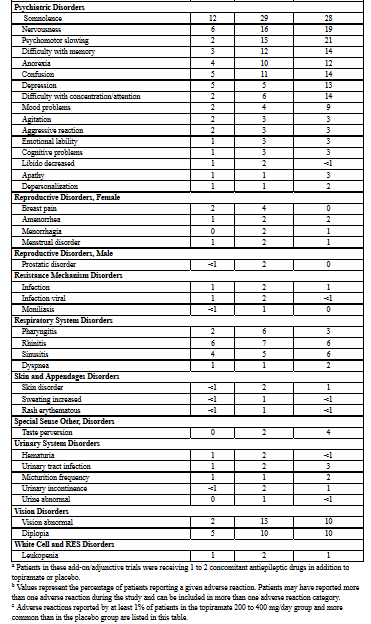
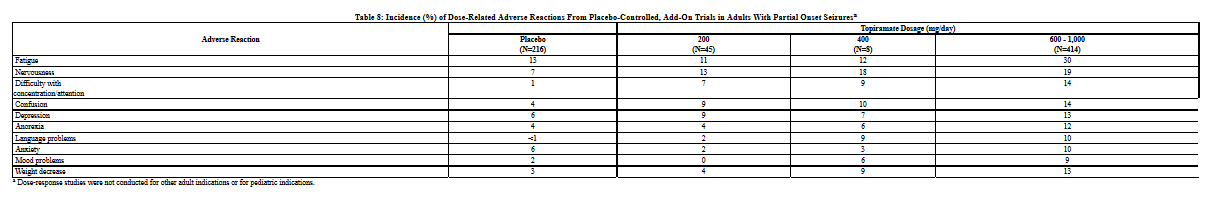
The adverse reactions in the controlled trial that occurred most commonly in adults in the 400 mg/day topiramate group and at a rate higher (≥ 5%) than in the 50 mg/day group were: paresthesia, weight decrease, anorexia, somnolence, and difficulty with memory (see Table 5).
Approximately 21% of the 159 adult patients in the 400 mg/day group who received topiramate as monotherapy in the controlled clinical trial discontinued therapy due to adverse reactions. The most common (≥ 2% more frequent than low-dose 50 mg/day topiramate) adverse reactions causing discontinuation in this trial were difficulty with memory, fatigue, asthenia, insomnia, somnolence, and paresthesia.
Pediatric Patients 6 to <16 Years of Age
The adverse reactions in the controlled trial that occurred most commonly in pediatric patients in the 400 mg/day topiramate group and at a rate higher (≥ 5%) than in the 50 mg/day group were fever, weight decrease, mood problems, cognitive problems, infection, flushing, and paresthesia (see Table 5).
Approximately 14% of the 77 pediatric patients in the 400 mg/day group who received topiramate as monotherapy in the controlled clinical trial discontinued therapy due to adverse reactions. The most common (≥ 2% more frequent than low-dose 50 mg/day topiramate) adverse reactions resulting in discontinuation in this trial were difficulty with concentration/attention, fever, flushing, and confusion.
 |
 |
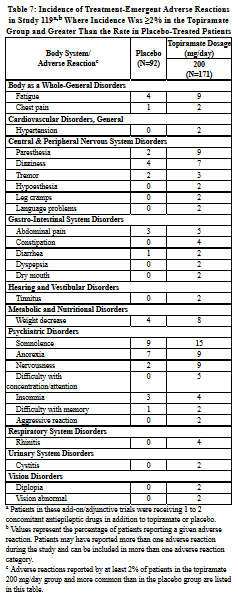
Incidence in Study 119 - Add-On Therapy - Adults with Partial Onset Seizures
Study 119 was a randomized, double-blind, add-on/adjunctive, placebo-controlled, parallel group study with 3 treatment arms: 1) placebo; 2) topiramate 200 mg/day with a 25 mg/day starting dose, increased by 25 mg/day each week for 8 weeks until the 200 mg/day maintenance dose was reached; and 3) topiramate 200 mg/day with a 50 mg/day starting dose, increased by 50 mg/day each week for 4 weeks until the 200 mg/day maintenance dose was reached. All patients were maintained on concomitant carbamazepine with or without another concomitant antiepileptic drug.
The incidence of adverse reactions (Table 7) did not differ significantly between the 2 topiramate regimens. Because the frequencies of adverse reactions reported in this study were markedly lower than those reported in the previous epilepsy studies, they cannot be directly compared with data obtained in other studies.*
 |
 |
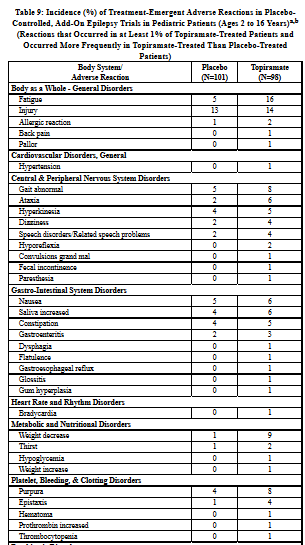 |
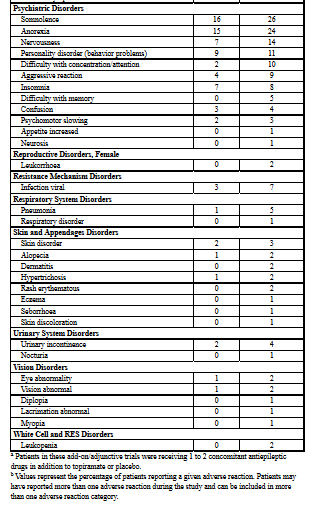 |
Other Adverse Reactions Observed During All Epilepsy Clinical Trials
Topiramate has been administered to 2246 adults and 427 pediatric patients with epilepsy during all clinical studies, only some of which were placebo-controlled. During these studies, all adverse reactions were recorded by the clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals having adverse reactions, similar types of reactions were grouped into a smaller number of standardized categories using modified WHOART dictionary terminology. The frequencies presented represent the proportion of patients who experienced a reaction of the type cited on at least one occasion while receiving topiramate. Reported reactions are included except those already listed in the previous tables or text, those too general to be informative, and those not reasonably associated with the use of the drug.
Reactions are classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent occurring in at least 1/100 patients; infrequent occurring in 1/100 to 1/1000 patients; rare occurring in fewer than 1/1000 patients.
- Autonomic Nervous System Disorders: Infrequent: vasodilation.
- Body as a Whole: Frequent: syncope. Infrequent: abdomen enlarged. Rare: alcohol intolerance.
- Cardiovascular Disorders, General: Infrequent: hypotension, postural hypotension, angina pectoris.
- Central & Peripheral Nervous System Disorders: Infrequent: neuropathy, apraxia, hyperesthesia, dyskinesia, dysphonia, scotoma, ptosis, dystonia, visual field defect, encephalopathy, EEG abnormal. Rare: upper motor neuron lesion, cerebellar syndrome, tongue paralysis.
- Gastrointestinal System Disorders: Infrequent: hemorrhoids, stomatitis, melena, gastritis, esophagitis. Rare: tongue edema.
- Heart Rate and Rhythm Disorders: Infrequent: AV block.
- Metabolic and Nutritional Disorders: Infrequent: dehydration, hypocalcemia, hyperlipemia, hyperglycemia, xerophthalmia, diabetes mellitus. Rare: hypernatremia, hyponatremia, hypocholesterolemia, creatinine increased.
- Musculoskeletal System Disorders: Frequent: arthralgia. Infrequent: arthrosis.
- Neoplasms: Infrequent: thrombocythemia. Rare: polycythemia.
- Platelet, Bleeding, and Clotting Disorders: Infrequent: gingival bleeding, pulmonary embolism.
- Psychiatric Disorders: Frequent: impotence, hallucination, psychosis, suicide attempt. Infrequent: euphoria, paranoid reaction, delusion, paranoia, delirium, abnormal dreaming. Rare: libido increased, manic reaction.
- Red Blood Cell Disorders: Frequent: anemia. Rare: marrow depression, pancytopenia.
- Reproductive Disorders, Male: Infrequent: ejaculation disorder, breast discharge.
- Skin and Appendages Disorders: Infrequent: urticaria, photosensitivity reaction, abnormal hair texture. Rare: chloasma.
- Special Senses Other, Disorders: Infrequent: taste loss, parosmia.
- Urinary System Disorders: Infrequent: urinary retention, face edema, renal pain, albuminuria, polyuria, oliguria.
- Vascular (Extracardiac) Disorders: Infrequent: flushing, deep vein thrombosis, phlebitis. Rare: vasospasm.
- Vision Disorders: Frequent: conjunctivitis. Infrequent: abnormal accommodation, photophobia, strabismus. Rare: mydriasis, iritis.
- White Cell and Reticuloendothelial System Disorders: Infrequent: lymphadenopathy, eosinophilia, lymphopenia, granulocytopenia. Rare: lymphocytosis.
Postmarketing and Other Experience
In addition to the adverse experiences reported during clinical testing of topiramate, the following adverse experiences have been reported worldwide in patients receiving topiramate post-approval.
These adverse experiences have not been listed above and data are insufficient to support an estimate of their incidence or to establish causation. The listing is alphabetized: bullous skin reactions (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), hepatic failure (including fatalities), hepatitis, maculopathy, pancreatitis, and pemphigus. The most common (>5% more frequent than placebo or low-dose topiramate in monotherapy) adverse reactions in controlled, epilepsy clinical trials were
- anorexia
- weight decrease
- fatigue
- dizziness
- somnolence
- nervousness
- psychomotor slowing
- difficulty with memory
- difficulty with concentration/attention
- cognitive problem
- confusion, mood problems
- fever
- infection
- flushing
- Rarely, the inhibition of carbonic anhydrase may be strong enough to cause metabolic acidosis of clinical importance.
A GlaxoSmithKline-sponsored Phase IV (post-marketing) study suggested that cognitive side effects may be more common with topiramate than with lamotrigine.[1] In studies of healthy volunteers, comparing the two medications, therapeutic doses of topiramate for bipolar disorder produced greater cognitive deficits than lamotrigine, including short term memory loss and word-finding difficulty. This effect has led to the occasional use of the name "dopamax" by some dissatisfied customers. A flat affect was reported in > 75% patients (n=60).
The most common side effects include a change in taste (carbonated beverages, especially diet sodas and beer, taste particularly bad) and feelings of pins and needles in the head and extremities. Less common side effects include cognitive deficiency (particularly word-finding difficulty); grogginess; lethargy; renal (kidney) stones, impairment of fine motor skills; vision abnormality and transient or permanent vision loss (see below for FDA warning); weight loss; breast pain; abdominal pain; intense sweating; menstrual disorder; taste changes; pharyngitis; sinusitis; diplopia; rash; leukopenia; fatigue; dizziness; insomnia; anxiety; depression; paresthesia; diarrhea; nausea; dyspepsia; constipation; dry-mouth; dysmenorrhea.
The Food and Drug Administration (FDA) has issued a notification alerting physicians who prescribe topiramate, and their patients, to the risk of vision loss (blindness). Acute myopia and secondary angle closure glaucoma, in a small subset of patients who take topiramate regularly, may cause transient (reversible), or permanent, loss of vision. The symptoms, which typically begin in the first month of use, include blurred vision and eye pain. If addressed early in its course, discontinuation of topiramate, along with other measures deemed prudent by the prescribing physician and/or ophthalmologist, may halt the progression of the ocular damage, and may reverse the visual impairment. Patients who take topiramate and who feel pain in or around their eyes, or notice a loss of vision, visual acuity, or blurred vision, are advised to seek consultation with their physician as soon as reasonably possible. According to the FDA: "in more than 825,000 patients...As of August 17, 2001 there have been 23 reported cases: 22 in adults and 1 in pediatric patients. It is generally recognized that postmarketing data are subject to substantial under-reporting."
Another serious side-effect is the development of osteoporosis in adults and children (bones affected break more easily) and rickets (abnormal, deformed growth of bones) in children. Topiramate may also slow the growth of children. All of these conditions should be detected early by performing regular clinical examinations of the patients.
In other postmarketing research, a risk of decreased sweating and hyperthermia was discovered. Pediatric patients (children) are especially prone to this side-effect. It is recommended that children treated with topiramate should be monitored closely for evidence of decreased sweating and increased body temperature, especially in hot weather. All patients, particularly those with other predisposing factors, should be instructed to maintain an adequate fluid intake in order to minimize the risk of kidney stone formation. [2]
References
- ↑ Blum D, Meador K, Biton V; et al. (2006). "Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy". Neurology. 67 (3): 400–6. doi:10.1212/01.wnl.0000232737.72555.06. PMID 16894098.
- ↑ "TOPIRAMATE (TOPIRAMATE ) TABLET, FILM COATED [AUROBINDO PHARMA LIMITED]".
Adapted from the FDA Package Insert.