Penicillin

|
WikiDoc Resources for Penicillin |
|
Articles |
|---|
|
Most recent articles on Penicillin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Penicillin at Clinical Trials.gov Clinical Trials on Penicillin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Penicillin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Penicillin Discussion groups on Penicillin Patient Handouts on Penicillin Directions to Hospitals Treating Penicillin Risk calculators and risk factors for Penicillin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Penicillin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Overview
Penicillin (sometimes abbreviated PCN) refers to a group of beta-lactam antibiotics used in the treatment of bacterial infections caused by susceptible, usually Gram-positive, organisms. The name “penicillin” can also be used in reference to a specific member of the penicillin group Penam Skeleton, which has the molecular formula R-C9H11N2O4S, where R is a variable side chain.
History
The discovery of penicillin is usually attributed to Scottish scientist Sir Alexander Fleming in 1928, though others had earlier noted the antibacterial effects of Penicillium. The development of penicillin for use as a medicine is attributed to the Australian Nobel Laureate Howard Walter Florey. In March 2000, doctors of the San Juan de Dios Hospital in San Jose (Costa Rica) published manuscripts belonging to the Costa Rican scientist and medical doctor Clodomiro (Clorito) Picado Twight (1887-1944). The manuscripts explained Picado's experiences between 1915 and 1927 about the inhibitory actions of the fungi of genera Penic. Apparently Clorito Picado had reported his discovery to the Paris Academy of Sciences in Paris, yet did not patent it, even though his investigation had started years before Fleming's. Fleming, at his laboratory in St. Mary's Hospital (now one of Imperial College's teaching hospitals) in London, noticed a halo of inhibition of bacterial growth around a contaminant blue-green mold Staphylococcus plate culture. Fleming concluded that the mold was releasing a substance that was inhibiting bacterial growth and lysing the bacteria. He grew a pure culture of the mold and discovered that it was a Penicillium mold, now known to be Penicillium notatum. Fleming coined the term "penicillin" to describe the filtrate of a broth culture of the Penicillium mold. Even in these early stages, penicillin was found to be most effective against Gram-positive bacteria, and ineffective against Gram-negative organisms and fungi. He expressed initial optimism that penicillin would be a useful disinfectant, being highly potent with minimal toxicity compared to antiseptics of the day, but particularly noted its laboratory value in the isolation of "Bacillus influenzae" (now Haemophilus influenzae).[1] After further experiments, Fleming was convinced that penicillin could not last long enough in the human body to kill pathogenic bacteria and stopped studying penicillin after 1931, but restarted some clinical trials in 1934 and continued to try to get someone to purify it until 1940. .[2]
In 1939, Australian scientist Howard Walter Florey and a team of researchers (Ernst Boris Chain, A. D. Gardner, Norman Heatley, M. Jennings, J. Orr-Ewing and G. Sanders) at the Sir William Dunn School of Pathology, University of Oxford made significant progress in showing the in vivo bactericidal action of penicillin. Their attempts to treat humans failed due to insufficient volumes of penicillin (the first patient treated was Reserve Constable Albert Alexander), but they proved its harmlessness and effect on mice.
A mouldy cantaloupe in a Peoria market in 1941 was found to contain the best and highest quality penicillin after a world-wide search.[3]
Some of the pioneering trials of penicillin took place at the Radcliffe Infirmary in Oxford. On March 3, 1942 John Bumstead and Orvan Hess became the first in the world to successfully treat a patient using penicillin.[4][5]

During World War II, penicillin made a major difference in the number of deaths and amputations caused by infected wounds amongst Allied forces; saving an estimated 12-15% of lives. Availability was severely limited, however, by the difficulty of manufacturing large quantities of penicillin and by the rapid renal clearance of the drug necessitating frequent dosing. Penicillins are actively secreted and about 80% of a penicillin dose is cleared within three to four hours of administration. During those times it became common procedure to collect the urine from patients being treated so that the penicillin could be isolated and reused.[6]
This was not a satisfactory solution, however, so researchers looked for a way to slow penicillin secretion. They hoped to find a molecule that could compete with penicillin for the organic acid transporter responsible for secretion such that the transporter would preferentially secrete the competitive inhibitor. The uricosuric agent probenecid proved to be suitable. When probenecid and penicillin are concomitantly administered, probenecid competitively inhibits the secretion of penicillin, increasing its concentration and prolonging its activity. The advent of mass-production techniques and semi-synthetic penicillins solved supply issues, and this use of probenecid declined.[6]Probenecid is still clinically useful, however, for certain infections requiring particularly high concentrations of penicillins.[7]
The chemical structure of penicillin was determined by Dorothy Crowfoot Hodgkin in the early 1940s. A team of Oxford research scientists led by Australian Howard Walter Florey and including Ernst Boris Chain and Norman Heatley discovered a method of mass producing the drug. Chemist John Sheehan at MIT completed the first total synthesis of penicillin and some of its analogs in the early 1950s, but his methods were not efficient for mass production. Florey and Chain shared the 1945 Nobel prize in medicine with Fleming for this work. Penicillin has since become the most widely used antibiotic to date and is still used for many Gram-positive bacterial infections.
Developments from penicillin
The narrow spectrum of activity of the penicillins, along with the poor activity of the orally-active phenoxymethylpenicillin, led to the search for derivatives of penicillin which could treat a wider range of infections.
The first major development was ampicillin, which offered a broader spectrum of activity than either of the original penicillins. Further development yielded beta-lactamase-resistant penicillins including flucloxacillin, dicloxacillin and methicillin. These were significant for their activity against beta-lactamase-producing bacteria species, but are ineffective against the methicillin-resistant Staphylococcus aureus strains that subsequently emerged.
The line of true penicillins were the antipseudomonal penicillins, such as ticarcillin and piperacillin, useful for their activity against Gram-negative bacteria. However, the usefulness of the beta-lactam ring was such that related antibiotics, including the mecillinams, the carbapenems and, most importantly, the cephalosporins, have this at the centre of their structures.
Mechanism of action
β-lactam antibiotics work by inhibiting the formation of peptidoglycan cross-links in the bacterial cell wall. The β-lactam moiety (functional group) of penicillin binds to the enzyme (DD-transpeptidase) that links the peptidoglycan molecules in bacteria, and this weakens the cell wall of the bacterium (in other words, the antibiotic causes cytolysis or death). In addition, the build-up of peptidoglycan precursors triggers the activation of bacterial cell wall hydrolases and auto lysins which further digest the bacteria's existing peptidoglycan.
When the bacteria lose their cell walls they are then called spheroplasts.
Penicillin shows a synergistic effect with aminoglycosides since the inhibition of peptidoglycan synthesis allows aminoglycosides to penetrate the bacterial cell wall more easily, allowing its disruption of bacterial protein synthesis within the cell. This results in a lowered MBC for susceptible organisms.
Variants in clinical use
The term “penicillin” is often used generically to refer to one of the narrow-spectrum penicillins, particularly benzylpenicillin.
Benzathine benzylpenicillin
Benzathine benzylpenicillin (rINN), also known as benzathine penicillin, is slowly absorbed into the circulation, after intramuscular injection, and hydrolysed to benzylpenicillin in vivo. It is the drug-of-choice when prolonged low concentrations of benzylpenicillin are required and appropriate, allowing prolonged antibiotic action over 2–4 weeks after a single IM dose. It is marketed by Wyeth under the trade name Bicillin L-A.
Specific indications for benzathine pencillin include:[7]
- Prophylaxis of rheumatic fever
- Early or latent syphilis
Benzylpenicillin (penicillin G)
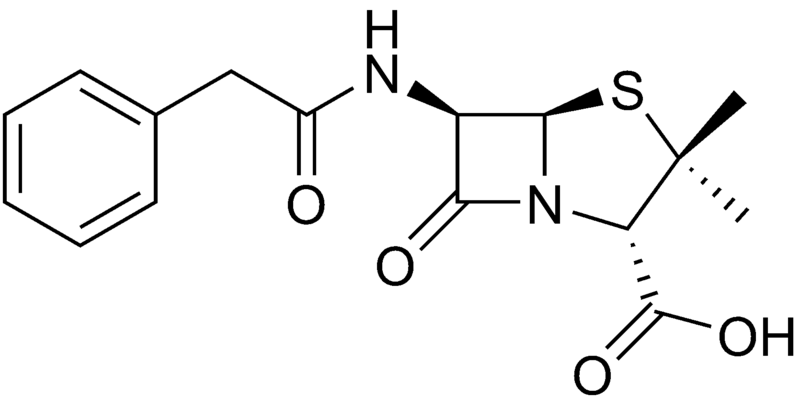 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | parenteral |
| Identifiers | |
| |
| CAS Number | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H18N2O4S |
| Molar mass | 334.4 g/mol |
Benzylpenicillin, commonly known as penicillin G, is the gold standard penicillin. Penicillin G is typically given by a parenteral route of administration (not orally) because it is unstable in the hydrochloric acid of the stomach. Because the drug is given parenterally, higher tissue concentrations of penicillin G can be achieved than is possible with phenoxymethylpenicillin. These higher concentrations translate to increased antibacterial activity.
Specific indications for benzylpenicillin include:[7]
- Cellulitis
- Bacterial endocarditis
- Gonorrhea
- Meningitis
- Aspiration pneumonia, lung abscess
- Community-acquired pneumonia
- Syphilis
- Septicaemia in children
Phenoxymethylpenicillin (penicillin V)
Phenoxymethylpenicillin, commonly known as penicillin V, is the orally-active form of penicillin. It is less active than benzylpenicillin, however, and is only appropriate in conditions where high tissue concentrations are not required.
Specific indications for phenoxymethylpenicillin include:[7]
- Infections caused by Streptococcus pyogenes
- Tonsillitis
- Pharyngitis
- Skin infections
- Prophylaxis of rheumatic fever
- Moderate-to-severe gingivitis (with metronidazole)
Penicillin V is the first choice in the treatment of odontogenic infections.
Procaine benzylpenicillin
Procaine benzylpenicillin (rINN), also known as procaine penicillin, is a combination of benzylpenicillin with the local anaesthetic agent procaine. Following deep intramuscular injection, it is slowly absorbed into the circulation and hydrolysed to benzylpenicillin — thus it is used where prolonged low concentrations of benzylpenicillin are required.
This combination is aimed at reducing the pain and discomfort associated with a large intramuscular injection of penicillin. It is widely used in veterinary settings.
Specific indications for procaine penicillin include:[7]
- Syphilis
- It should be noted that in the United States, Bicillin C-R (a injectable suspension which 1.2 million units of benzathine penicillin & 1.2 million units of procaine penicillin per 4 mL) is not recommended for treating syphilis, since it contains only half the recommended dose of benzathine penicillin. Medication errors have been made due to the confusion between Bicillin L-A & Bicillin C-R.[8] As a result, changes in product packaging have been made; specifically, the statement "Not for the Treatment of Syphilis" has been added in red text to both the Bicillin CR and Billin CR 900/300 syringe labels.[9]
- Respiratory tract infections where compliance with oral treatment is unlikely
- Cellulitis, erysipelas
Procaine penicillin is also used as an adjunct in the treatment of anthrax.
Semi-synthetic penicillins
Structural modifications were made to the side chain of the penicillin nucleus in an effort to improve oral bioavailability, improve stability to beta-lactamase activity, and increase the spectrum of action.
Narrow spectrum penicillinase-resistant penicillins
This group was developed to be effective against beta-lactamases produced by Staphylococcus aureus, and are occasionally known as anti-staphylococcal penicillin. Penicillin is rampantly used for curing infections and to prevent growth of harmful mold.
- Methicillin discontinued (not used clinically)
- Dicloxacillin
- Flucloxacillin
- Oxacillin
- Nafcillin
- Cloxacillin
Narrow spectrum β-lactamase-resistant penicillins
This molecule has a spectrum directed towards Gram negative bacteria without activity on Pseudomonas aeruginosa or Acinetobacter spp. with remarkable resistance to any type of β-lactamase.
Moderate spectrum penicillins
This group was developed to increase the spectrum of action and, in the case of amoxicillin, improve oral bioavailability.
And the prodrugs of ampicillin that are converted in the body to ampicillin:
- Hetacillin, not used now.
- Bacampicillin
- Pivampicillin
Extended Spectrum Penicillins
This group was developed to increase efficacy against Gram-negative organisms. Some members of this group also display activity against Pseudomonas aeruginosa.
Penicillins with beta-lactamase inhibitors
Penicillins may be combined with beta-lactamase inhibitors to increase efficacy against β-lactamase-producing organisms. The addition of the beta-lactamase inhibitor does not generally, in itself, increase the spectrum of the partner penicillin.
- Amoxicillin/clavulanic acid
- Ampicillin/sulbactam
- Ticarcillin/clavulanic acid
- Piperacillin/tazobactam
Other Penicillins
- Metampicillin
- Broadcillin
- Epicillin
- Ampicillin benzathine
- Talampicillin
- Combipenix
- Ampicillinoic acid
- N-(N'-Methylasparaginyl)amoxicillin
- Aspoxicillin
- N-Propionylampicillin
- Lenampicillin
- Sulacillin
Adverse effects
Adverse drug reactions
Common adverse drug reactions (≥1% of patients) associated with use of the penicillins include: diarrhea, nausea, rash, urticaria, and/or superinfection (including candidiasis). Infrequent adverse effects (0.1–1% of patients) include: fever, vomiting, erythema, dermatitis, angioedema, seizures (especially in epileptics) and/or pseudomembranous colitis.[7]
Pain and inflammation at the injection site is also common for parenterally-administered benzathine benzylpenicillin, benzylpenicillin, and to a lesser extent procaine benzylpenicillin.
Allergy/hypersensitivity
Although penicillin is still the most commonly reported allergy, less than 20% of all patients who believe that they have a penicillin allergy are truly allergic to penicillin;[10] nevertheless, penicillin is still the most common cause of severe allergic drug reactions.
Allergic reactions to any β-lactam antibiotic may occur in up to 10% of patients receiving that agent. Anaphylaxis will occur in approximately 0.01% of patients.[7] There is about a 5% cross-sensitivity between penicillin-derivatives, cephalosporins and carbapenems.[11] This risk warrants extreme caution with all β-lactam antibiotics in patients with a history of severe allergic reactions (urticaria, anaphylaxis, interstitial nephritis) to any β-lactam antibiotic.
The PEN-FAST questions can help predict whether a patient has allergies[12]:
- F: Fives years or less since an allergy event (2 points)
- A: Anaphylaxis/angioedema
OR
- S: severe cutaneous adverse reaction (2 points)
- T: Treatment required for reaction (1 point)
Interpretation of the PEN-FAST: 0: Very low risk < 1% 1-2: Low risk - 5%
See also
References
- ↑ Flemming A. (1929). "On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzæ". Br J Exp Pathol. 10 (31): 226–36.
- ↑ Brown, Kevin. (2004). Penicillin Man: Alexander Fleming and the Antibiotic Revolution. Stroud: Sutton. ISBN 0-7509-3152-3.
- ↑ [1]
- ↑ Saxon, W. (1999-06-09). "Anne Miller, 90, first patient who was saved by penicillin". The New York Times. Check date values in:
|date=(help) - ↑ Krauss K, editor (1999). "Yale-New Haven Hospital Annual Report" (PDF). New Haven: Yale-New Haven Hospital.
- ↑ 6.0 6.1 Silverthorn, DU. (2004). Human physiology: an integrated approach. Upper Saddle River (NJ): Pearson Education. ISBN 0-8053-5957-5. Unknown parameter
|edithion=ignored (help) - ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Rossi S, editor, ed. (2006). Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 0-9757919-2-3.
- ↑ "Inadvertent use of Bicillin C-R to treat syphilis infection--Los Angeles, California, 1999-2004". MMWR Morb. Mortal. Wkly. Rep. 54 (9): 217–9. 2005. PMID 15758893.
- ↑ United States Food & Drug Administration. "FDA Strengthens Labels of Two Specific Types of Antibiotics to Ensure Proper Use." Published December 1, 2004. Last accessed June 18, 2007.
- ↑ Salkind AR, Cuddy PG, Foxworth JW (2001). "Is this patient allergic to penicillin? An evidence-based analysis of the likelihood of penicillin allergy". JAMA. 285 (19): 2498&ndash, 2505.
- ↑ Gruchalla RS, Pirmohamed M (2006). "Clinical practice. Antibiotic allergy". N. Engl. J. Med. 354 (6): 601–9. doi:10.1056/NEJMcp043986. PMID 16467547.
- ↑ Su C, Belmont A, Liao J, Kuster JK, Trubiano JA, Kwah JH (2023). "Evaluating the PEN-FAST Clinical Decision-making Tool to Enhance Penicillin Allergy Delabeling". JAMA Intern Med. doi:10.1001/jamainternmed.2023.1572. PMC 10282954 Check
|pmc=value (help). PMID 37338869 Check|pmid=value (help).
ar:بنسلين
bs:Penicilin
bg:Пеницилин
ca:Penicil·lina
cs:Penicilín
da:Penicillin
de:Benzylpenicillin
eo:Penicilino
ko:페니실린
hr:Penicilin
io:Penicilino
id:Penisilin
is:Penisillín
it:Penicillina
kk:Пенициллин
he:פניצילין
hu:Penicillin
nl:Penicilline
ka:პენიცილინი
no:Penicillin
nn:Penicillin
simple:Penicillin
sr:Пеницилин
fi:Penisilliini
sv:Penicillin
th:ฟีนอกซิลเมตทิลเพนิซิลลิน
- Pages with script errors
- CS1 errors: dates
- CS1 maint: Extra text: authors list
- Pages with citations using unsupported parameters
- CS1 maint: Extra text: editors list
- CS1 maint: Multiple names: authors list
- CS1 errors: PMC
- CS1 errors: PMID
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Drugs
- Beta-lactam antibiotics