Early-onset Alzheimer's disease
|
Alzheimer's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Early-onset Alzheimer's disease On the Web |
|
American Roentgen Ray Society Images of Early-onset Alzheimer's disease |
|
Risk calculators and risk factors for Early-onset Alzheimer's disease |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Early-onset Alzheimer's; early-onset AD; early onset AD; Early onset Alzheimer's; Early onset Alzheimer's disease; Early-onset Alzheimer's disease
Overview
Early-onset Alzheimer's disease is the term used for cases of Alzheimer's disease diagnosed in patients under the age of 65. It is an uncommon form of Alzheimer's, accounting for only 5-10% of all Alzheimer's cases. Approximately half the cases of early-onset Alzheimer's are Familial Alzheimer's disease, in which a genetic predisposition leads to the disease. The other incidences of early onset Alzheimer's, however, share the same traits as the late onset form, commonly referred to simply as Alzheimer's disease, and little is understood about how it develops.
Non-Familial early-onset Alzheimer's can develop in people who are in their 30s or 40s, although that is extremely rare. The majority of sufferers are in their 50s or early 60s. Familial Alzheimer's can appear as early as 16 years of age.
Sometimes this disease is referred to as "early-onset dementia," although Alzheimer's disease is just one type of dementia.
Clinical features of Alzheimer's disease
Alzheimer disease (AD) is the most common form of dementia. It usually occurs in old age. It is invariably fatal, generally within ten years of the first signs. Normal aging involves forgetfulness, but the early signs of AD include unusual memory loss, and particularly defects in remembering recent events and the names of people and things. As the disease progresses, the patient exhibits more serious problems, becoming subject to mood swings and unable to perform complex activities such as driving. In the latter stages, patients forget how to do simple things such as brushing their hair, and then require full-time care.
History of Alzheimer's disease
The symptoms of the disease as a distinct nosologic entity were first identified by Emil Kraepelin, and the characteristic neuropathology was first observed by Alois Alzheimer in 1906. In this sense, the disease was co-discovered by Kraepelin and Alzheimer, who worked in Kraepelin's laboratory. Because of the overwhelming importance Kraepelin attached to finding the neuropathological basis of psychiatric disorders, Kraepelin made the generous decision that the disease would bear Alzheimer's name (J. Psychiat. Res., 1997, Vol 31, No. 6, pp. 635-643).
Familial Alzheimer's disease
Familial Alzheimer's disease (FAD) is an uncommon form of Alzheimer's disease that usually strikes earlier in life, defined as before the age of 65 (usually between 16 and 65 years of age) and is inherited in an autosomal dominant fashion. It accounts for approximately half the cases of early-onset Alzheimer's disease. Familial AD requires the patient to have at least two first-degree relatives with a history of AD. Non-familial cases of AD are referred to as "sporadic" AD, but still involve genetic risk factors with unclear modes of inheritance. While early-onset familial AD is estimated to account for only 4-5% of total cases of Alzheimer's disease, it has proven a useful model for studying various aspects of the disorder. Moreover, the early-onset familial AD gene mutations guide the vast majority of therapeutic discovery and development for AD. The genetic causes of AD are summarized in "Decoding Darkness: The Search for the Genetics Causes of Alzheimer's Disease" by Rudolph Tanzi and Ann Parson (Perseus Press, 2000).
Clinical features
Alzheimer disease (AD) is the most common form of dementia and usually occurs in old age. It is invariably fatal, generally within ten years of the first signs. Normal aging involves forgetfulness, but the early signs of AD include unusual memory loss, particularly in remembering recent events and the names of people and things. As the disease progresses the patient exhibits more serious problems, becoming subject to mood swings and unable to perform complex activities such as driving. In the latter stages, patients forget how to do simple things such as brushing their hair, and then require full-time care.
Familial Alzheimer disease is an uncommon form of Alzheimer's that comes on earlier in life, before the age of 65 (incidents occurring before 50 years of age are rarer) and is inherited in an autosomal dominant fashion. There are a number of types of familial (or early-onset) AD, which are identified by their genetics and other characteristics such as the age of onset. As a whole, this form of the disease accounts for only about 5% of all cases of AD.
Histologically, familial AD is practically indistinguishable from other forms of the disease. Deposits of amyloid can be seen in sections brain tissue (visible as an apple-green yellow birefringence under polarised light). This amyloid protein forms plaques and neurofibrillary tangles that progress through the memory centers of the brain. Very rarely, the plaque may be unique, or uncharacteristic of AD; this can happen when there is a mutation in one of the genes that creates a functional, but malformed, protein instead of the ineffective gene products that usually result from mutations.
Genetic causes and mutations
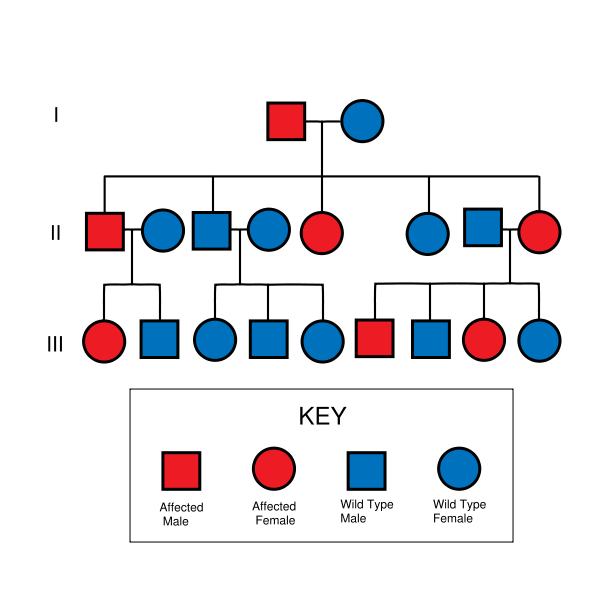
There are multiple genetic causes of familial Alzheimer disease. Two of these are the presenilin mutations in genes on chromosomes 1 and 14, Others include several amyloid precursor protein mutations on chromosome 21 and one of the three common alleles of the apolipoprotein E gene on chromosome 19. Several other gene polymorphisms have also been identified as increasing susceptibility to Alzheimer's disease. A full list of all genes tested for genetic association with AD and the current strength of those associations can be found at AlzGene.org <http://alzgene.org> and in "Decoding Darkness: The Search for the Genetics Causes of Alzheimer's Disease" by Rudolph Tanzi and Ann Parson (Perseus Press, 2000).
PSEN1 - Presenilin 1
The presenilin 1 gene (PSEN1) was identified by Sherrington (1995); multiple relevant mutations have been identified. Mutations in this gene cause familial Alzheimer's type 3 with certainty and usually in patients under 50 years of age. This protein has been identified as part of the enzymatic complex that cleaves amyloid beta peptide from APP (see below).
The gene contains 14 exons, and the coding portion is estimated at 60 kb, as reported by Rogaev (1997) and Del-Favero (1999). The protein the gene codes for (PS1) is an integral membrane protein. As stated by Ikeuchi (2002) it cleaves the protein Notch1, and so is thought by Koizumi (2001) to have a role in somitogenesis in the embryo. It also has an action on an amyloid precursor protein, which explains its probable role in the pathogenesis of FAD. Homologs of PS1 have been found in plants, invertebrates, and other vertebrates.
There are over 150 allelic mutations in PSEN1 that cause AD, including Met146Leu, which has been found in unrelated families in Italy by Sherrington (1995), as well as in Argentina by Morelli (1998). There are other mutations at this same amino acid position including Met146Val, found by the Alzheimer's Disease Collaborative Group (1995), and Met146Ile identified as two different point mutations, one in a Danish family by Jorgensen (1996), and another in a Swedish family by Gustafson (1998).
Some mutations in the gene, of which there are over 90, include: His163Arg, Ala246Glu, Leu286Val, and Cys410Tyr. Most display complete penetrance, though Glu318Gly, a common mutation that predisposes individuals to familial Alzheimer disease demonstrated an incidence of 8.7% in patients with familial AD as determined in a study by Taddei (2002).
PSEN2 - Presenilin 2
The presenilin 2 gene (PSEN2) is very similar in structure and function to PSEN1. It is located on chromosome 1 (1q31-q42), and mutations in this gene cause type 4 FAD. The gene was identified by Rudolph Tanzi and Jerry Schellenberg in 1995 (Levy-Lahad et al. Nature, 1995). A subsequent study by Kovacs (1996) in Nature Medicine showed that PS1 and PS2 proteins are expressed in similar amounts, and in the same organelles as each other, in mammalian neuronal cells. Levy-Lahad (1996) determined that PSEN2 contained 12 exons, 10 of which were coding exons, and that the primary transcript encodes a 448-amino acid polypeptide with 67% homology to PS1. This protein has been identified as part of the enzymatic complex that cleaves amyloid beta peptide from APP (see below).
Mutations have not been studied as much as PSEN1, though distinct allelic variants have been identified. These include Asn141Ile, which was identified first by Rudolph Tanzi and Jerry Schellenberg in Volga German families with familial Alzheimer disease (Levy-Lahad et al. Nature, 1995). One study by Nochlin (1998) found severe amyloid angiopathy in the affected individuals in a family. This phenotype may be explained by a study by Tomita (1997) suggesting that the Asn141Ile mutation alters amyloid precursor protein (APP) metabolism, causing an increased rate of protein deposition into plaques.
Other allelic variants include Met239Val, which was identified in an Italian pedigree by Rogaev (1995), who also suggested early on that the gene may be similar to PSEN1; and a Asp439Ala mutation in exon 12 of the gene, which is suggested by Lleo (2001) to change the endoproteolytic processing of the PS2.
APP – Amyloid beta (A4) precursor protein
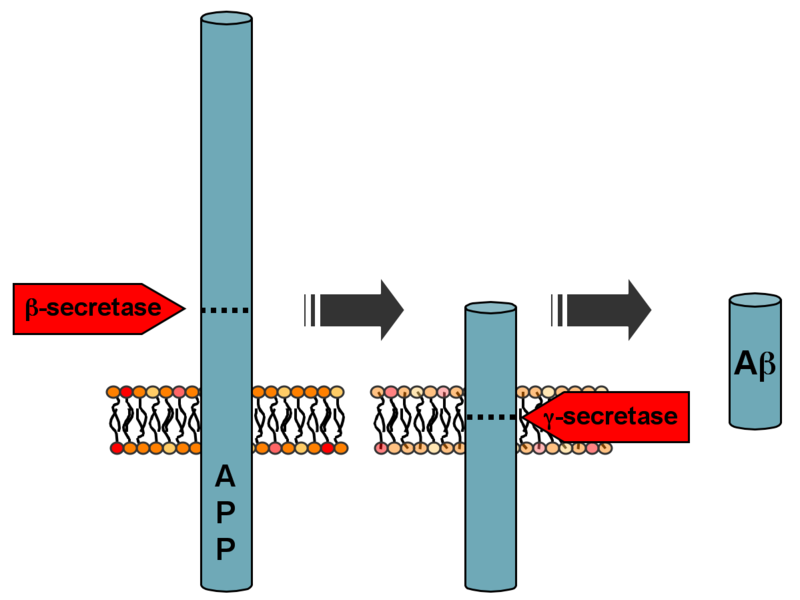
Mutations to the amyloid beta A4 precursor protein (APP) located on the long arm of chromosome 21 (21q21.3) can also cause familial Alzheimer disease (type 1) as well as other problems. The different mutations have different ages of onset. The APP gene was first discovered in 1987 by three different laboratories, including those of Dmitri Goldgaber, Rudolph Tanzi, and Benno Muller-Hill ("Decoding Darkness: The Search for the Genetics Causes of Alzheimer's Disease" by Rudolph Tanzi and Ann Parson, Perseus Press, 2000).
Following cleavage by β-secretase, APP is cleaved by a membrane-bound protein complex called γ-secretase to generate Aβ. Presenilins 1 and 2 are the enzymatic centers of this complex, along with nicastrin, Aph1, and PEN-2. Alpha secretase cleavage of APP, which precludes the production of Aβ, is the most common processing event for APP. 21 allelic mutations have been discovered in the APP gene. These guarantee the manifestation of early-onset familial Alzheimer disease and all occur in the region of the APP gene that encodes the Aβ domain.
APOE - Apolipoprotein E
The APOE gene codes for an apolipoprotein, and is therefore involved with the transport of lipids around the body. Its role in the development of Alzheimer disease is not an autosomal dominant effect as has been observed with the previous three genes; some variants may cause a slight predisposition to AD (Corder, 1993), and Amouyel (1994) reported an increased the risk of other neurological disorders such as Creutzfeldt-Jakob disease.
The variant discussed by Corder (1993) was apolipoprotein E type 4 (ApoEε4): the greater the number of copies of this allele a person possessed, the greater their chance of developing Alzheimer's disease. However, recent work with African populations has shown that this rule is not universal (Gureje et al, 2006). This gene is found on chromosome 19 (its locus is 19q13.2), and the subgroup of FAD it causes is type 2.
Other mutations summary
There is considerable genetic heterogeneity in familial Alzheimer's disease, and familial genetic mutations account for a relatively small proportion of cases of AD. With each gene, there are usually many different mutant alleles that are capable of causing the disorder. The types of FAD include type 1, caused by APP mutations; type 2, the susceptibility to which is increased with APOE*E4; and type 3 and type 4, which are caused by PSEN1 and PSEN2 mutations, respectively. The PSEN and the APP mutations show autosomal dominant patterns of inheritance. In addition, there are many other genes that have been implicated in the development of sproadic AD, among the >400 tested for so far for association with both familial and spordic forms of late-onset (>60 years) AD. The list of these genes and summaries of their relative degrees of known genetic association with AD are summarized on AlzGene.org <http://alzgene.org>. The functional properties of these genes, their protein products, and interrelations are summarized at Polygenicpathways.
References
- Alzheimer's Disease Collaborative Group (1995), The structure of the presenilin 1 (S182) gene and identification of six novel mutations in early onset AD families. Nature Genetics, vol. 11, pp. 219-222.
- Amouyel, P., Vidal, O., Launay, J. M., Laplanche, J. L. (1994), The apolipoprotein E alleles as major susceptibility factors for Creutzfeldt-Jakob disease. Lancet, vol. 344, pp. 1315-1318.
- Campion, D., Brice, A., Hannequin, D., Tardieu, S., Dubois, B., Calenda, A., Brun, E., Penet, C., Tayot, J., Martinez, M., et al. (1995), A large pedigree with early-onset Alzheimer's disease: clinical, neuropathologic, and genetic characterization. Neurology, vol. 45, no. 1, pp. 80-85.
- Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., Roses, A. D., Haines, J. L., Pericak-Vance, M. A. (1993), Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science, vol. 261, pp. 921-923.
- Del-Favero, J., Goossens, D., Van den Bossche, D., Van Broeckhoven, C. (1999), YAC fragmentation with repetitive and single-copy sequences: detailed physical mapping of the presenilin 1 gene on chromosome 14. Gene, vol. 229, no. 1-2, pp. 193-201.
- Gureje O., Ogunniyi A., Baiyewu O., Price B., Unverzagt F. W., Evans R. M., Smith-Gamble V., Lane K. A., Gao S, Hall K. S., Hendrie H. C., Murrell J. R. (2006) APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians. Annals of Neurology vol. 59, pp. 182-185.
- Gustafson, L., Brun, A., Englund, E., Hagnell, O., Nilsson, K., Stensmyr, M., Ohlin, A. K., Abrahamson, M. (1998), A 50-year perspective of a family with chromosome-14-linked Alzheimer's disease. Human Genetics, vol. 102, pp. 253-257.
- Levy-Lahad E Wasco W, Poorkaj P, Romano DM, Oshima Jm Pettingell WH, Yu C, Jondro PD, Schmidt SD, Wang K, Crowley AC, Fu Y-H, Guenette SY, Galas D, Nemens E, Wijsman EM, Bird TD, Schellenberg GD, Tanzi RE. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science, 1995; 269: 973-977.
- Levy-Lahad, E., Poorkaj, P., Wang, K., Fu, Y. H., Oshima, J., Mulligan, J., Schellenberg, G. D. (1996),Genomic structure and expression of STM2, the chromosome 1 familial Alzheimer disease gene. Genomics, vol. 34, pp. 198-204.
- Liao, A., Nitsch, R. M., Greenberg, S. M., Finckh, U., Blacker, D., Albert, M., Rebeck, G. W., Gomez-Isla, T., Clatworthy, A., Binetti, G., Hock, C., Mueller-Thomsen, T., Mann, U., Zuchowski, K., Beisiegel, U., Staehelin, H., Growdon, J. H., Tanzi, R. E., Hyman, B. T. (1998), Genetic association of an alpha-2-macroglobulin (val1000ile) polymorphism and Alzheimer's disease. Human Molecular Genetics, vol. 7, pp. 1953-1956.
- Lin, F. H., Lin, R., Wisniewski, H. M., Hwang, Y.-W., Grundke-Iqbal, I., Healy-Louie, G., Iqbal, K. (1992), Detection of point mutations in codon 331 of mitochondrial NADH dehydrogenase subunit 2 in Alzheimer's brains. Biochemical & Biophysical Research Communications, vol. 182, pp. 238-246.
- Lleo, A., Blesa, R., Gendre, J., Castellvi, M., Pastor, P., Queralt, R., Oliva, R. (2001), A novel presenilin 2 gene mutation (D439A) in a patient with early-onset Alzheimer's disease. Neurology, vol. 57, pp. 1926-1928.
- Jorgensen, P., Bus, C., Pallisgaard, N., Bryder, M., Jorgensen, A. L. (1996), Familial Alzheimer's disease co-segregates with a met146ile substitution in presenilin-1. Clinical Genetics, vol. 50, pp. 281-286.
- Kovacs, D. M., Fausett, H. J., Page, K. J., Kim, T.-W., Moir, R. D., Merriam, D. E., Hollister, R. D., Hallmark, O. G., Mancini, R., Felsenstein, K. M., Hyman, B. T., Tanzi, R. E., Wasco, W. (1996), Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nature Medicine, vol. 2, pp. 224-229.
- Ikeuchi, T., Sisodia, S. S. (2002), Cell-free generation of the notch1 intracellular domain (NICD) and APP-CTfgamma: evidence for distinct intramembranous "gamma-secretase" activities. Neuromolecular Medicine, vol. 1, no. 1, pp. 43-54.
- Koizumi, K., Nakajima, M., Yuasa, S., Saga, Y., Sakai, T., Kuriyama, T., Shirasawa, T., Koseki, H. (2001), The role of presenilin 1 during somite segmentation. Development, vol. 128, no. 8, pp. 1391-402.
- Morelli, L., Prat, M. I., Levy, E., Mangone, C. A., Castano, E. M. (1998), Presenilin 1 met146leu variant due to an A-T transversion in an early-onset familial Alzheimer's disease pedigree from Argentina. Genetics, vol. 53, pp. 469-473.
- Olson, J. M., Goddard, K. A. B., Dudek, D. M. (2002), A second locus for very-late-onset Alzheimer disease: a genome scan reveals linkage to 20p and epistasis between 20p and the amyloid precursor protein region. American Journal of Human Genetics, vol. 71, pp. 154-161.
- Rogaev, E. I., Sherrington, R., Rogaeva, E. A., Levesque, G., Ikeda, M., Liang, Y., Chi, H., Lin, C., Holman, K., Tsuda, T., Mar, L., Sorbi, S., Nacmias, B., Placentini, S., Amaducci, L., Chumakov, I., Cohen, D., Lannfelt, L., Fraser, P. E., Rommens, J. M., St George-Hyslop, P. H. (1995), Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature, vol. 376, pp. 775-778.
- Rogaev, E. I., Sherrington, R., Wu, C., Levesque, G., Liang, Y., Rogaeva, E. A., Ikeda, M., Holman, K., Lin, C., Lukiw, W. J., de Jong, P. J., Fraser, P. E., Rommens, J. M., St. George-Hyslop, P. (1997), Analysis of the 5-prime sequence, genomic structure, and alternative splicing of the presenilin-1 gene (PSEN1) associated with early onset Alzheimer disease. Genomics, vol. 40, pp. 415-424.
- Sherrington, R., Rogaev, E. I., Liang, Y., Rogaeva, E. A., Levesque, G., Ikeda, M., Chi, H., Lin, C., Li, G., Holman, K., Tsuda, T., Mar, L., Foncin, J. F., Bruni, A. C., Montesi, M. P., Sorbi, S., Rainero, I., Pinessi, L., Nee, L., Chumakov, I., Pollen, D., Brookes, A., Sanseau, P., Polinsky, R. J., Wasco, W., Da Silva, H. A. R., Haines, J. L., Pericak-Vance, M. A., Tanzi, R. E., Roses, A. D., Fraser, P. E., Rommens, J. M., St George P. H. (1995), Cloning of a gene bearing mis-sense mutations in early-onset familial Alzheimer's disease. Nature, vol. 375, pp. 754-760.
- Taddei, K., Fisher, C., Laws, S. M., Martins, G., Paton, A., Clarnette, R. M., Chung, C., Brooks, W. S., Hallmayer, J., Miklossy, J., Relkin, N., St George-Hyslop, P. H., Gandy, S. E., Martins, R. N. (2002), Association between presenilin-1 Glu318Gly mutation and familial Alzheimer's disease in the Australian population. Molecular Psychiatry, vol. 7, no. 7, pp. 776-781.
Tanzi, R and Parson A. "Decoding Darkness: The Search for the Genetics Causes of Alzheimer's Disease" Perseus Press, 2000.
- Tomita, T., Maruyama, K., Saido, T. C., Kume, H., Shinozaki, K., Tokuhiro, S., Capell, A., Walter, J., Grunberg, J., Haass, C., Iwatsubo, T., Obata, K. (1997), The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proceedings of the National Academy of Science, vol. 94, pp. 2025-2030.
- Van Broeckhoven C, Backhovens H, Cruts M, De Winter G, Bruyland M, Cras P, Martin JJ., Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3, Nat Genet. 1992 Dec;2(4):335-9.
- Yoshikai, S., Sasaki, H., Doh-ura, K., Furuya, H., Sakaki, Y. (1990), Genomic organization of the human amyloid beta-protein precursor gene. Gene, vol. 87, pp. 257-263.
- Zubenko, G. S., Hughes, H. B., III, Stiffler, J. S. (2001), D10S1423 identifies a susceptibility locus for Alzheimer's disease in a prospective, longitudinal, double-blind study of asymptomatic individuals. Molecular Psychiatry, vol. 6, pp. 413-419.
External links
- Laboratory for Alzheimer's and Parkinson's Disease Research - Prof. Dr. Christian Haass
- Alzforum - Early-Onset Familial AD