Thalidomide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
|authorTag= | |authorTag= | ||
{{VP}} | |||
<!--Overview--> | <!--Overview--> | ||
| Line 307: | Line 308: | ||
T4 | T4 | ||
*Other Adverse Events Observed in ENL Patients | |||
:*THALOMID in doses up to 400 mg/day has been administered investigationally in the United States over a 19-year period in 1465 patients with ENL. The published literature describes the treatment of an additional 1678 patients. To provide a meaningful estimate of the proportion of the individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using a modified COSTART dictionary/terminology. These categories are used in the listing below. All reported events are included except those already listed in the previous table. Due to the fact that these data were collected from uncontrolled studies, the incidence rate cannot be determined. No causal relationship between THALOMID and these events can be conclusively determined at this time. These are reports of all adverse events noted by investigators in patients to whom they had administered thalidomide. | |||
=====Body as a Whole===== | =====Body as a Whole===== | ||
Abdomen enlarged, fever, photosensitivity, upper extremity pain. | |||
=====Cardiovascular System===== | |||
Bradycardia, hypertension, hypotension, peripheral vascular disorder, tachycardia, vasodilation. | |||
===== | =====Digestive System===== | ||
Anorexia, appetite increase/weight gain, dry mouth, dyspepsia, enlarged liver, eructation, flatulence, increased liver function tests, intestinal obstruction, vomiting. | |||
=====Hemic and Lymphatic===== | |||
ESR decrease, eosinophilia, granulocytopenia, hypochromic anemia, leukemia, leukocytosis, leukopenia, MCV elevated, RBC abnormal, spleen palpable, thrombocytopenia. | |||
===== | =====Metabolic and Endocrine===== | ||
ADH inappropriate, amyloidosis, bilirubinemia, BUN increased, creatinine increased, cyanosis, diabetes, edema, electrolyte abnormalities, hyperglycemia, hyperkalemia, hyperuricemia, hypocalcemia, hypoproteinemia, LDH increased, phosphorus decreased, SGPT increased. | |||
=====Muscular Skeletal===== | |||
Arthritis, bone tenderness, hypertonia, joint disorder, leg cramps, myalgia, myasthenia, periosteal disorder. | |||
===== | =====Nervous System===== | ||
Abnormal thinking, agitation, amnesia, anxiety, causalgia, circumoral paresthesia, confusion, depression, euphoria, hyperesthesia, insomnia, nervousness, neuralgia, neuritis, neuropathy, paresthesia, peripheral neuritis, psychosis. | |||
=====Respiratory System===== | |||
Cough, emphysema, epistaxis, pulmonary embolus, rales, upper respiratory infection, voice alteration. | |||
===== | =====Skin and Appendages===== | ||
Acne, alopecia, dry skin, eczematous rash, exfoliative dermatitis, ichthyosis, perifollicular thickening, skin necrosis, seborrhea, sweating, urticaria, vesiculobullous rash. | |||
=====Special Senses===== | |||
Amblyopia, deafness, dry eye, eye pain, tinnitus. | |||
===== | =====Urogenital===== | ||
Decreased creatinine clearance, hematuria, orchitis, proteinuria, pyuria, urinary frequency. | |||
*Other Adverse Events Observed in HIV-seropositive Patients | |||
:*In addition to controlled clinical trials, THALOMID has been used in uncontrolled studies in 145 patients. Less frequent adverse events that have been reported in these HIV-seropositive patients treated with THALOMID were grouped into a smaller number of standardized categories using modified COSTART dictionary/terminology and these categories are used in the listing below. Adverse events that have already been included in the tables and narrative above, or that are too general to be informative are not listed. | |||
=====Body as a Whole===== | |||
Ascites, AIDS, allergic reaction, cellulitis, chest pain, chills and fever, cyst, decreased CD4 count, facial edema, flu syndrome, hernia, thyroid hormone level altered, moniliasis, photosensitivity reaction, sarcoma, sepsis, viral infection. | |||
=====Cardiovascular System===== | |||
Angina pectoris, arrhythmia, atrial fibrillation, bradycardia, cerebral ischemia, cerebrovascular accident, congestive heart failure, deep thrombophlebitis, heart arrest, heart failure, hypertension, hypotension, murmur, myocardial infarct, palpitation, pericarditis, peripheral vascular disorder, postural hypotension, syncope, tachycardia, thrombophlebitis, thrombosis. | |||
=====Digestive System===== | |||
Cholangitis, cholestatic jaundice, colitis, dyspepsia, dysphagia, esophagitis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gum disorder, hepatitis, pancreatitis, parotid gland enlargement, periodontitis, stomatitis, tongue discoloration, tooth disorder. | |||
=====Hemic and Lymphatic===== | |||
Aplastic anemia, macrocytic anemia, megaloblastic anemia, microcytic anemia. | |||
=====Metabolic and Endocrine===== | |||
Avitaminosis, bilirubinemia, dehydration, hypercholesteremia, hypoglycemia, increased alkaline phosphatase, increased lipase, increased serum creatinine, peripheral edema. | |||
=====Muscular Skeletal===== | |||
Myalgia, myasthenia. | |||
=====Nervous System===== | |||
Abnormal gait, ataxia, decreased libido, decreased reflexes, dementia, dysesthesia, dyskinesia, emotional lability, hostility, hypalgesia, hyperkinesia, incoordination, meningitis, neurologic disorder, tremor, vertigo. | |||
=====Respiratory System===== | |||
Apnea, bronchitis, lung disorder, lung edema, pneumonia (including Pneumocystis carinii pneumonia), rhinitis. | |||
=====Skin and Appendages===== | |||
Angioedema, benign skin neoplasm, eczema, herpes simplex, incomplete Stevens-Johnson syndrome, nail disorder, pruritus, psoriasis, skin discoloration, skin disorder. | |||
=====Special Senses===== | =====Special Senses===== | ||
Conjunctivitis, eye disorder, lacrimation disorder, retinitis, taste perversion. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 377: | Line 402: | ||
|postmarketing= | |postmarketing= | ||
*The following adverse reactions have been identified during post approval use of THALOMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
=====Cardiovascular System===== | |||
Cardiac arrhythmias including atrial fibrillation, bradycardia, tachycardia, sick sinus syndrome, EKG abnormalities, myocardial infarction. | |||
=====Digestive System===== | |||
Intestinal perforation, gastrointestinal perforations, intestinal obstruction. | |||
===== | =====Metabolic and Endocrine===== | ||
Electrolyte imbalance including hypercalcemia or hypocalcemia, hyperkalemia and hypokalemia, hyponatremia, hypothyroidism, increased alkaline phosphatase, tumor lysis syndrome. | |||
=====Nervous System===== | |||
Changes in mental status or mood including depression and suicide attempts, disturbances in consciousness including lethargy, syncope, loss of consciousness or stupor, seizures including grand mal convulsions and status epilepticus, Parkinson’s disease, stroke. | |||
=====Skin and Appendages===== | |||
Erythema multiforme, toxic epidermal necrolysis. | |||
===== | =====Hemic and Lymphatic===== | ||
Decreased white blood cell counts including neutropenia and febrile neutropenia, changes in prothrombin time, pancytopenia. | |||
=====Respiratory System===== | |||
Pleural effusion. | |||
=====Reproductive System and Breast Disorders===== | |||
Amenorrhea, sexual dysfunction. | |||
===== | =====Immune System Disorders===== | ||
Hypersensitivity, angioedema/urticaria. | |||
=====Ear and Labyrinthine Disorders===== | |||
Hearing impairment/deafness. | |||
===== | =====Renal and Urinary Disorders===== | ||
Renal failure. | |||
*Other Adverse Events in the Published Literature or Reported from Other Sources | |||
:*The following additional events have been identified either in the published literature or from spontaneous reports from other sources: acute renal failure, amenorrhea, aphthous stomatitis, bile duct obstruction, carpal tunnel, chronic myelogenous leukemia, diplopia, dysesthesia, dyspnea, enuresis, erythema nodosum, erythroleukemia, foot drop, galactorrhea, gynecomastia, hangover effect, hypomagnesemia, hypothyroidism, lymphedema, lymphopenia, metrorrhagia, migraine, myxedema, nodular sclerosing Hodgkin’s disease, nystagmus, oliguria, pancytopenia, petechiae, purpura, Raynaud’s syndrome, stomach ulcer, suicide attempt, interstitial lung disease and severe infections (e.g., fatal sepsis including septic shock). | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
Revision as of 17:15, 10 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY AND VENOUS THROMBOEMBOLISM
See full prescribing information for complete Boxed Warning.
|
Overview
Thalidomide is a that is FDA approved for the {{{indicationType}}} of newly diagnosed multiple myeloma in combination with dexamethasone and cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). There is a Black Box Warning for this drug as shown here. Common adverse reactions include fatigue, hypocalcemia, edema, constipation, neuropathy-sensory, dyspnea, muscle weakness, leukopenia, neutropenia, rash/desquamation, confusion, anorexia, nausea, anxiety/agitation, asthenia, tremor, fever, weight loss, thrombosis/embolism, neuropathy-motor, weight gain, dizziness, and dry skin.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Multiple Myeloma
- THALOMID is administered in combination with dexamethasone in 28-day treatment cycles. The dose of THALOMID is 200 mg administered orally once daily with water, preferably at bedtime and at least 1 hour after the evening meal. The dose of dexamethasone is 40 mg daily administered orally on days 1-4, 9-12, and 17-20 every 28 days.
- Patients who develop adverse reactions such as constipation, somnolence, or peripheral neuropathy may benefit by either temporarily discontinuing the drug or continuing at a lower dose. With the abatement of these adverse reactions, the drug may be started at a lower dose or at the previous dose based on clinical judgment.
Erythema Nodosum Leprosum
- For an episode of cutaneous ENL, THALOMID dosing should be initiated at 100 to 300 mg/day, administered once daily with water, preferably at bedtime and at least 1 hour after the evening meal. Patients weighing less than 50 kilograms should be started at the low end of the dose range.
- In patients with a severe cutaneous ENL reaction, or in those who have previously required higher doses to control the reaction, THALOMID dosing may be initiated at higher doses up to 400 mg/day once daily at bedtime or in divided doses with water, at least 1 hour after meals.
- In patients with moderate to severe neuritis associated with a severe ENL reaction, corticosteroids may be started concomitantly with THALOMID. Steroid usage can be tapered and discontinued when the neuritis has ameliorated.
- Dosing with THALOMID should usually continue until signs and symptoms of active reaction have subsided, usually a period of at least 2 weeks. Patients may then be tapered off medication in 50 mg decrements every 2 to 4 weeks.
- Patients who have a documented history of requiring prolonged maintenance treatment to prevent the recurrence of cutaneous ENL or who flare during tapering should be maintained on the minimum dose necessary to control the reaction. Tapering off medication should be attempted every 3 to 6 months, in decrements of 50 mg every 2 to 4 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Thalidomide in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thalidomide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Thalidomide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Thalidomide in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thalidomide in pediatric patients.
Contraindications
- Pregnancy
- THALOMID can cause fetal harm when administered to a pregnant female. Thalidomide is contraindicated in females who are pregnant. Thalidomide is a powerful human teratogen, inducing a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. If pregnancy occurs during thalidomide treatment, the drug should be discontinued immediately.
- Hypersensitivity
- THALOMID is contraindicated in patients who have demonstrated hypersensitivity to the drug or its components.
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY AND VENOUS THROMBOEMBOLISM
See full prescribing information for complete Boxed Warning.
|
Precautions
- Embryo-Fetal Toxicity
- Thalidomide is a powerful human teratogen that induces a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. When there is no satisfactory alternative treatment, females of reproductive potential may be treated with thalidomide provided adequate precautions are taken to avoid pregnancy. THALOMID® (thalidomide) is only available through the THALOMID REMS™ program (formerly known as the “S.T.E.P.S.® program”), [see Warnings and Precautions (5.2)].
- Oral ingestion is the only type of maternal thalidomide exposure known to result in drug-associated birth defects. There are no specific data available regarding the reproductive risks of cutaneous absorption or inhalation of thalidomide; however, females of reproductive potential should avoid contact with THALOMID® (thalidomide) Capsules. THALOMID Capsules should be stored in blister packs until ingestion. If there is contact with non-intact thalidomide capsules or the powder contents, the exposed area should be washed with soap and water.
- If healthcare providers or other care givers are exposed to body fluids from patients receiving THALOMID (thalidomide) the exposed area should be washed with soap and water. Appropriate precautions should be utilized, such as wearing gloves to prevent the potential cutaneous exposure to THALOMID.
- Females of Reproductive Potential
- Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning THALOMID therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.
- Females must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control, beginning 4 weeks prior to initiating treatment with THALOMID, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of THALOMID therapy.
- Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours prior to prescribing THALOMID therapy and then weekly during the first month, then monthly thereafter in women with regular menstrual cycles or every 2 weeks in women with irregular menstrual cycles [see Use in Specific Populations (8.6)].
- Males
- Thalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy. Male patients taking THALOMID must not donate sperm [see Use in Specific Populations (8.6)].
- Males
- Blood Donation
- Patients must not donate blood during treatment with THALOMID and for 1 month following discontinuation of the drug because the blood might be given to a pregnant female patient whose fetus must not be exposed to THALOMID.
- Blood Donation
- THALOMID REMS™ Program
- Because of the embryo-fetal risk [see Warnings and Precautions (5.1)], THALOMID is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), the THALOMID REMS™ program (formerly known as the “S.T.E.P.S.®” program).
- Required components of the THALOMID REMS™ program include the following:
- Prescribers must be certified with the THALOMID REMS™ program by enrolling and complying with the REMS requirements.
- Patients must sign a Patient-Physician Agreement Form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements [see Use in Specific Populations (8.6)] and males must comply with contraception requirements [see Use in Specific Populations (8.6)].
- Pharmacies must be certified with the THALOMID REMS™ program, must only dispense to patients who are authorized to receive THALOMID and comply with REMS requirements.
- Further information about the THALOMID REMS™ program is available at www.celgeneriskmanagement.com or by telephone at 1-888-423-5436.
- Venous and Arterial Thromboembolism
- The use of THALOMID in patients with MM results in an increased risk of venous thromboembolism, such as deep venous thrombosis and pulmonary embolism. This risk increases significantly when thalidomide is used in combination with standard chemotherapeutic agents including dexamethasone. In one controlled trial, the rate of venous thromboembolism was 22.5% in patients receiving thalidomide in combination with dexamethasone compared to 4.9% in patients receiving dexamethasone alone (p = 0.002).
- Ischemic heart disease (11.1%), including myocardial infarction (1.3%), and stroke (cerebrovascular accident, 2.6%) have also occurred in patients with previously untreated MM treated with THALOMID and dexamethasone compared to placebo and dexamethasone (4.7%, 1.7%, and 0.9%, respectively) in one clinical trial [see Adverse Reactions (6.1)].
- Consider thromboprophylaxis based on an assessment of individual patients’ underlying risk factors. Patients and physicians should be observant for the signs and symptoms of thromboembolism. Advise patients to seek immediate medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling [see Boxed Warning]. Agents that also may increase the risk of thromboembolism should be used with caution in patients receiving THALOMID [see Drug Interactions (7.7)].
- Drowsiness and Somnolence
- Thalidomide frequently causes drowsiness and somnolence. Patients should be instructed to avoid situations where drowsiness may be a problem and not to take other medications that may cause drowsiness without adequate medical advice [see Drug Interactions (7.1)]. Advise patients as to the possible impairment of mental and/or physical abilities required for the performance of hazardous tasks, such as driving a car or operating other complex or dangerous machinery. Dose reductions may be required.
- Peripheral Neuropathy
- Thalidomide is known to cause nerve damage that may be permanent. Peripheral neuropathy is a common (≥10%) and potentially severe adverse reaction of treatment with thalidomide that may be irreversible. Peripheral neuropathy generally occurs following chronic use over a period of months; however, peripheral neuropathy following relatively short-term use has been reported. The correlation with cumulative dose is unclear. Symptoms may occur some time after thalidomide treatment has been stopped and may resolve slowly or not at all.
- Few reports of neuropathy have arisen in the treatment of ENL despite long-term thalidomide treatment. However, the inability clinically to differentiate thalidomide neuropathy from the neuropathy often seen in Hansen’s disease makes it difficult to determine accurately the incidence of thalidomide-related neuropathy in ENL patients treated with thalidomide.
- Patients should be examined at monthly intervals for the first 3 months of thalidomide therapy to enable the clinician to detect early signs of neuropathy, which include numbness, tingling or pain in the hands and feet. Patients should be evaluated periodically thereafter during treatment. Patients should be regularly counseled, questioned, and evaluated for signs or symptoms of peripheral neuropathy. Consideration should be given to electrophysiological testing, consisting of measurement of sensory nerve action potential (SNAP) amplitudes at baseline and thereafter every 6 months in an effort to detect asymptomatic neuropathy. If symptoms of drug-induced neuropathy develop, thalidomide should be discontinued immediately to limit further damage, if clinically appropriate. Usually, treatment with thalidomide should only be reinitiated if the neuropathy returns to baseline status.
- Medications known to be associated with neuropathy should be used with caution in patients receiving thalidomide [see Drug Interactions (7.3)].
- Dizziness and Orthostatic Hypotension
- Patients should also be advised that thalidomide may cause dizziness and orthostatic hypotension and that, therefore, they should sit upright for a few minutes prior to standing up from a recumbent position.
- Neutropenia
- Decreased white blood cell counts, including neutropenia, have been reported in association with the clinical use of thalidomide. Treatment should not be initiated with an absolute neutrophil count (ANC) of <750/mm3. White blood cell count and differential should be monitored on an ongoing basis, especially in patients who may be more prone to neutropenia, such as patients who are HIV-seropositive. If ANC decreases to below 750/mm3 while on treatment, the patient’s medication regimen should be re-evaluated and, if the neutropenia persists, consideration should be given to withholding thalidomide if clinically appropriate.
- Increased HIV Viral Load
- In a randomized, placebo-controlled trial of thalidomide in an HIV-seropositive patient population, plasma HIV RNA levels were found to increase (median change = 0.42 log10 copies HIV RNA/mL, p = 0.04 compared to placebo). A similar trend was observed in a second, unpublished study conducted in patients who were HIV-seropositive. The clinical significance of this increase is unknown. Both studies were conducted prior to availability of highly active antiretroviral therapy. Until the clinical significance of this finding is further understood, in HIV-seropositive patients, viral load should be measured after the first and third months of treatment and every 3 months thereafter.
- Bradycardia
- Bradycardia in association with thalidomide use has been reported. Cases of bradycardia have been reported, some required medical interventions. The clinical significance and underlying etiology of the bradycardia noted in some thalidomide-treated patients are presently unknown. Monitor patients for bradycardia and syncope. Dose reduction or discontinuation may be required.
- Medications known to decrease heart rate should be used with caution in patients receiving thalidomide [see Drug Interactions (7.2)].
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
- Serious dermatologic reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis, which may be fatal, have been reported. THALOMID should be discontinued if a skin rash occurs and only resumed following appropriate clinical evaluation. If the rash is exfoliative, purpuric, or bullous or if Stevens-Johnson syndrome or toxic epidermal necrolysis is suspected, use of THALOMID should not be resumed.
- Seizures
- Although not reported from pre-marketing controlled clinical trials, seizures, including grand mal convulsions, have been reported during post-approval use of THALOMID in clinical practice. Because these events are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. Most patients had disorders that may have predisposed them to seizure activity, and it is not currently known whether thalidomide has any epileptogenic influence. During therapy with thalidomide, patients with a history of seizures or with other risk factors for the development of seizures should be monitored closely for clinical changes that could precipitate acute seizure activity.
- Tumor Lysis Syndrome
- Monitor patients at risk of tumor lysis syndrome (e.g., patients with high tumor burden prior to treatment) and take appropriate precautions.
- Contraceptive Risks
- Some contraceptive methods may pose a higher risk of adverse effects or may be medically contraindicated in some patients treated with THALOMID. Because some patients may develop sudden, severe neutropenia and/or thrombocytopenia, use of an intrauterine device (IUD) or implantable contraception in these patients may carry an increased risk for infection or bleeding either at insertion, removal or during use. Treatment with THALOMID, the presence of an underlying malignancy, and/or use of an estrogen-containing contraceptive can each increase the risk of thromboembolism. It is not known if these risks of thromboembolism are additive. However, they should be taken into consideration when choosing contraceptive methods.
- Hypersensitivity
- Hypersensitivity to THALOMID has been reported. Signs and symptoms have included the occurrence of erythematous macular rash, possibly associated with fever, tachycardia, and hypotension, and if severe, may necessitate interruption of therapy. If the reaction recurs when dosing is resumed, THALOMID should be discontinued.
Adverse Reactions
Clinical Trials Experience
- Teratogenicity
- The most serious toxicity associated with thalidomide is its documented human teratogenicity. The risk of severe birth defects, primarily phocomelia or death to the fetus, is extremely high during the critical period of pregnancy. The critical period is estimated, depending on the source of information, to range from 35 to 50 days after the last menstrual period. The risk of other potentially severe birth defects outside this critical period is unknown, but may be significant. Based on present knowledge, thalidomide must not be used at any time during pregnancy.
- Because thalidomide is present in the semen of patients receiving the drug, males receiving thalidomide must always use a latex or synthetic condom during any sexual contact with females of reproductive potential, even if he has undergone a successful vasectomy.
- Venous and Arterial Thromboembolism
- An increased risk of venous thromboembolism (such as deep vein thrombosis and pulmonary embolism), ischemic heart disease (including myocardial infarction), and stroke have been reported in patients with multiple myeloma treated with thalidomide [See Venous and Arterial Thromboembolism (5.3)].
- Peripheral Neuropathy
- Peripheral Neuropathy is a very common, potentially severe, adverse reaction of treatment with thalidomide that may result in irreversible damage. Peripheral neuropathy generally occurs following chronic use over a period of months. However, reports following relatively short-term use also exist. Incidence of neuropathy events leading to discontinuation, dose reduction or interruption increases with cumulative dose and duration of therapy. Symptoms may occur some time after thalidomide treatment has been stopped and may resolve slowly or not at all.
- Somnolence, dizziness, and rash are the most commonly observed adverse reactions associated with the use of thalidomide. Adverse event profiles from clinical trials are summarized in the sections that follow.
- Adverse Reactions in Multiple Myeloma Controlled Clinical Trials
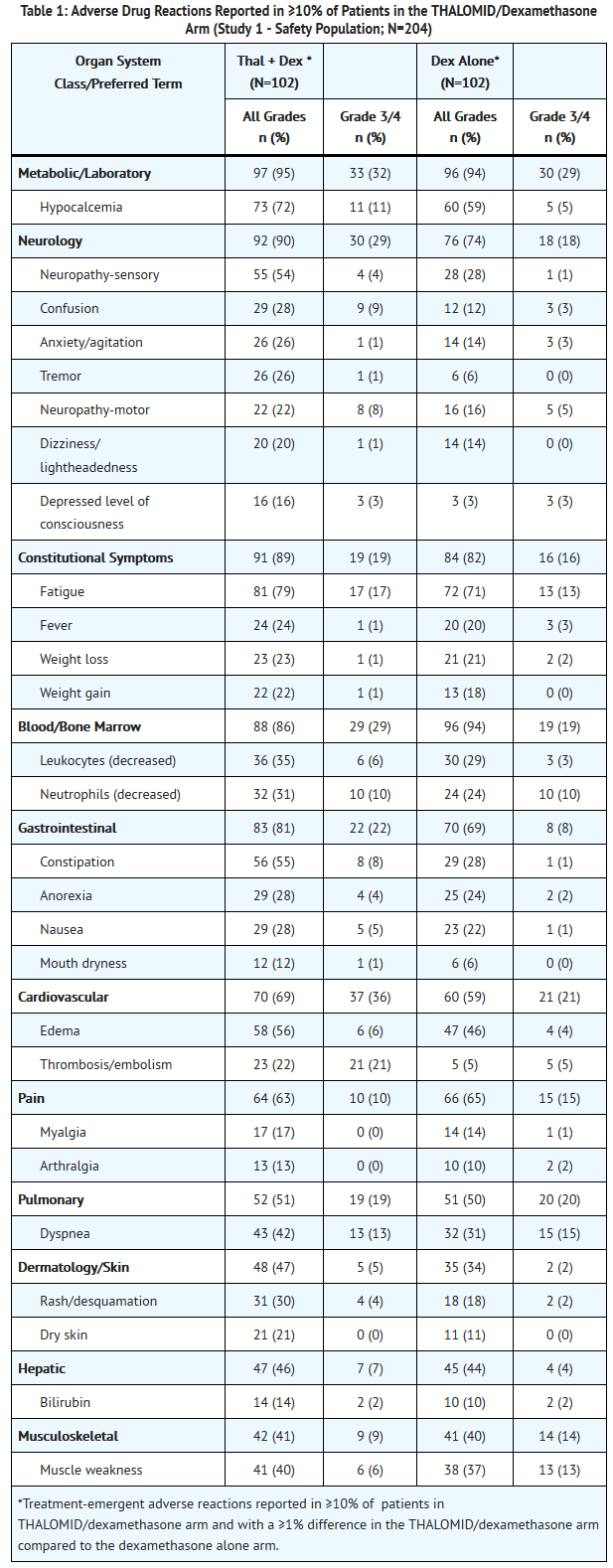
- The safety analyses were conducted in two controlled clinical studies (Study 1 and Study 2). The safety analysis in Study 1 was conducted on 204 patients who received treatment. Table 1 lists the most common adverse drug reactions (≥ 10%). The most frequently reported adverse reactions were fatigue, hypocalcemia, edema, constipation, sensory neuropathy, dyspnea, muscle weakness, leukopenia, neutropenia, rash/desquamation, confusion, anorexia, nausea, anxiety/agitation , tremor, fever, weight loss, thrombosis/embolism, neuropathy-motor, weight gain, dizziness, and dry skin .
- Twenty-three percent of patients (47/204) discontinued due to adverse reactions; 30% (31/102) from the THALOMID/dexamethasone arm and 16% (16/102) from the dexamethasone alone arm.
T1
- The safety analysis in Study 2 was conducted on 466 patients who received treatment. Table 2 lists the most common adverse drug reactions (≥ 10%) that were observed. Table 3 lists the most common Grade 3/4 adverse drug reactions (occurring at > 2%) that were observed. The adverse reactions most often reported by patients treated with THALOMID/dexamethasone were constipation, peripheral edema, tremor, asthenia, dizziness and fatigue. Adverse reactions with a frequency at least 2-fold higher in the THALOMID/dexamethasone group than in the placebo/dexamethasone group include constipation, tremor, deep vein thrombosis and peripheral sensory neuropathy.
- Twenty-six percent of patients (121/466) discontinued due to adverse events; 37% (86/234) from the THALOMID/dexamethasone arm and 15% (35/232) from the placebo/dexamethasone arm.
T2
T3
- Less Common Adverse Drug Reactions in Multiple Myeloma Controlled Clinical Trials
- In Study 2, THALOMID in combination with dexamethasone in patients with multiple myeloma, the following adverse drug reactions not described above were reported*:
Gastrointestinal disorders
Vomiting NOS, dry mouth, peritonitis, diverticular perforation
Nervous system disorders
Somnolence, hypoesthesia, polyneuropathy NOS, transient ischemic attack
Respiratory, thoracic, and mediastinal disorders
Bronchitis NOS
Psychiatric disorders
Mood alteration NOS
Vascular disorders
Hypotension NOS, orthostatic hypotension
Cardiac disorders
Bradycardia NOS
Eye disorders
Blurred vision
- All adverse reactions with ≥3% of patients in THALOMID/dexamethasone arm and with a ≥1% difference in proportion of patients between the THALOMID/dexamethasone arm compared to the placebo/dexamethasone arm. All grade 3/4 and serious adverse reactions reported >2 patients in THALOMID/dexamethasone arm and with a percentage higher in the THALOMID/dexamethasone arm compared to the placebo/dexamethasone arm have been considered for possible inclusion. In any cases medical judgment has been applied for consideration of causality assessment.
- Adverse Reactions in Erythema Nodosum Leprosum (ENL) Clinical Trials
- Table 4 lists treatment-emergent signs and symptoms that occurred in THALOMID-treated patients in clinical trials in ENL. The most common adverse reactions (≥10%) reported in patients with ENL were somnolence, rash, headache. Doses ranged from 50 to 300 mg/day. All adverse reactions were mild to moderate in severity, and none resulted in discontinuation.
T4
- Other Adverse Events Observed in ENL Patients
- THALOMID in doses up to 400 mg/day has been administered investigationally in the United States over a 19-year period in 1465 patients with ENL. The published literature describes the treatment of an additional 1678 patients. To provide a meaningful estimate of the proportion of the individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using a modified COSTART dictionary/terminology. These categories are used in the listing below. All reported events are included except those already listed in the previous table. Due to the fact that these data were collected from uncontrolled studies, the incidence rate cannot be determined. No causal relationship between THALOMID and these events can be conclusively determined at this time. These are reports of all adverse events noted by investigators in patients to whom they had administered thalidomide.
Body as a Whole
Abdomen enlarged, fever, photosensitivity, upper extremity pain.
Cardiovascular System
Bradycardia, hypertension, hypotension, peripheral vascular disorder, tachycardia, vasodilation.
Digestive System
Anorexia, appetite increase/weight gain, dry mouth, dyspepsia, enlarged liver, eructation, flatulence, increased liver function tests, intestinal obstruction, vomiting.
Hemic and Lymphatic
ESR decrease, eosinophilia, granulocytopenia, hypochromic anemia, leukemia, leukocytosis, leukopenia, MCV elevated, RBC abnormal, spleen palpable, thrombocytopenia.
Metabolic and Endocrine
ADH inappropriate, amyloidosis, bilirubinemia, BUN increased, creatinine increased, cyanosis, diabetes, edema, electrolyte abnormalities, hyperglycemia, hyperkalemia, hyperuricemia, hypocalcemia, hypoproteinemia, LDH increased, phosphorus decreased, SGPT increased.
Muscular Skeletal
Arthritis, bone tenderness, hypertonia, joint disorder, leg cramps, myalgia, myasthenia, periosteal disorder.
Nervous System
Abnormal thinking, agitation, amnesia, anxiety, causalgia, circumoral paresthesia, confusion, depression, euphoria, hyperesthesia, insomnia, nervousness, neuralgia, neuritis, neuropathy, paresthesia, peripheral neuritis, psychosis.
Respiratory System
Cough, emphysema, epistaxis, pulmonary embolus, rales, upper respiratory infection, voice alteration.
Skin and Appendages
Acne, alopecia, dry skin, eczematous rash, exfoliative dermatitis, ichthyosis, perifollicular thickening, skin necrosis, seborrhea, sweating, urticaria, vesiculobullous rash.
Special Senses
Amblyopia, deafness, dry eye, eye pain, tinnitus.
Urogenital
Decreased creatinine clearance, hematuria, orchitis, proteinuria, pyuria, urinary frequency.
- Other Adverse Events Observed in HIV-seropositive Patients
- In addition to controlled clinical trials, THALOMID has been used in uncontrolled studies in 145 patients. Less frequent adverse events that have been reported in these HIV-seropositive patients treated with THALOMID were grouped into a smaller number of standardized categories using modified COSTART dictionary/terminology and these categories are used in the listing below. Adverse events that have already been included in the tables and narrative above, or that are too general to be informative are not listed.
Body as a Whole
Ascites, AIDS, allergic reaction, cellulitis, chest pain, chills and fever, cyst, decreased CD4 count, facial edema, flu syndrome, hernia, thyroid hormone level altered, moniliasis, photosensitivity reaction, sarcoma, sepsis, viral infection.
Cardiovascular System
Angina pectoris, arrhythmia, atrial fibrillation, bradycardia, cerebral ischemia, cerebrovascular accident, congestive heart failure, deep thrombophlebitis, heart arrest, heart failure, hypertension, hypotension, murmur, myocardial infarct, palpitation, pericarditis, peripheral vascular disorder, postural hypotension, syncope, tachycardia, thrombophlebitis, thrombosis.
Digestive System
Cholangitis, cholestatic jaundice, colitis, dyspepsia, dysphagia, esophagitis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gum disorder, hepatitis, pancreatitis, parotid gland enlargement, periodontitis, stomatitis, tongue discoloration, tooth disorder.
Hemic and Lymphatic
Aplastic anemia, macrocytic anemia, megaloblastic anemia, microcytic anemia.
Metabolic and Endocrine
Avitaminosis, bilirubinemia, dehydration, hypercholesteremia, hypoglycemia, increased alkaline phosphatase, increased lipase, increased serum creatinine, peripheral edema.
Muscular Skeletal
Myalgia, myasthenia.
Nervous System
Abnormal gait, ataxia, decreased libido, decreased reflexes, dementia, dysesthesia, dyskinesia, emotional lability, hostility, hypalgesia, hyperkinesia, incoordination, meningitis, neurologic disorder, tremor, vertigo.
Respiratory System
Apnea, bronchitis, lung disorder, lung edema, pneumonia (including Pneumocystis carinii pneumonia), rhinitis.
Skin and Appendages
Angioedema, benign skin neoplasm, eczema, herpes simplex, incomplete Stevens-Johnson syndrome, nail disorder, pruritus, psoriasis, skin discoloration, skin disorder.
Special Senses
Conjunctivitis, eye disorder, lacrimation disorder, retinitis, taste perversion.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of THALOMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular System
Cardiac arrhythmias including atrial fibrillation, bradycardia, tachycardia, sick sinus syndrome, EKG abnormalities, myocardial infarction.
Digestive System
Intestinal perforation, gastrointestinal perforations, intestinal obstruction.
Metabolic and Endocrine
Electrolyte imbalance including hypercalcemia or hypocalcemia, hyperkalemia and hypokalemia, hyponatremia, hypothyroidism, increased alkaline phosphatase, tumor lysis syndrome.
Nervous System
Changes in mental status or mood including depression and suicide attempts, disturbances in consciousness including lethargy, syncope, loss of consciousness or stupor, seizures including grand mal convulsions and status epilepticus, Parkinson’s disease, stroke.
Skin and Appendages
Erythema multiforme, toxic epidermal necrolysis.
Hemic and Lymphatic
Decreased white blood cell counts including neutropenia and febrile neutropenia, changes in prothrombin time, pancytopenia.
Respiratory System
Pleural effusion.
Reproductive System and Breast Disorders
Amenorrhea, sexual dysfunction.
Immune System Disorders
Hypersensitivity, angioedema/urticaria.
Ear and Labyrinthine Disorders
Hearing impairment/deafness.
Renal and Urinary Disorders
Renal failure.
- Other Adverse Events in the Published Literature or Reported from Other Sources
- The following additional events have been identified either in the published literature or from spontaneous reports from other sources: acute renal failure, amenorrhea, aphthous stomatitis, bile duct obstruction, carpal tunnel, chronic myelogenous leukemia, diplopia, dysesthesia, dyspnea, enuresis, erythema nodosum, erythroleukemia, foot drop, galactorrhea, gynecomastia, hangover effect, hypomagnesemia, hypothyroidism, lymphedema, lymphopenia, metrorrhagia, migraine, myxedema, nodular sclerosing Hodgkin’s disease, nystagmus, oliguria, pancytopenia, petechiae, purpura, Raynaud’s syndrome, stomach ulcer, suicide attempt, interstitial lung disease and severe infections (e.g., fatal sepsis including septic shock).
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Thalidomide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Thalidomide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Thalidomide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Thalidomide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Thalidomide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Thalidomide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Thalidomide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Thalidomide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Thalidomide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Thalidomide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Thalidomide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Thalidomide in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Thalidomide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Thalidomide in the drug label.
Pharmacology
There is limited information regarding Thalidomide Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Thalidomide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Thalidomide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Thalidomide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Thalidomide in the drug label.
How Supplied
Storage
There is limited information regarding Thalidomide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Thalidomide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Thalidomide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Thalidomide in the drug label.
Precautions with Alcohol
- Alcohol-Thalidomide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Thalidomide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide11.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide11.png
}}