Smoking cessation: Difference between revisions
Usama Talib (talk | contribs) No edit summary |
|||
| Line 7: | Line 7: | ||
[[Tobacco]] use is the leading cause of preventable disease, disability, and death in the United States. Each year, nearly half a million Americans die prematurely of smoking or exposure to secondhand smoke and 16 million live with a serious illness caused by [[smoking]]. [[Smoking]] can cause repairable damage to various organs including the [[heart]], [[lungs]], [[kidneys]], [[stomach]] and [[intestines]]. [[Smoking]] is associated with the causation of various cancers in the humans. Quitting [[smoking]] cuts [[cardiovascular]] risks, reduces risk for stroke to about half that of a nonsmoker’s, reduces risks for cancers of the [[mouth]], [[throat]], [[esophagus]], and [[bladder]] by half within 5 years and ten years after quitting [[smoking]], the risk for lung cancer drops by half. [[Smoking cessation]] can be achieved by some general, non-pharmacological and pharmacological strategies. | [[Tobacco]] use is the leading cause of preventable disease, disability, and death in the United States. Each year, nearly half a million Americans die prematurely of smoking or exposure to secondhand smoke and 16 million live with a serious illness caused by [[smoking]]. [[Smoking]] can cause repairable damage to various organs including the [[heart]], [[lungs]], [[kidneys]], [[stomach]] and [[intestines]]. [[Smoking]] is associated with the causation of various cancers in the humans. Quitting [[smoking]] cuts [[cardiovascular]] risks, reduces risk for stroke to about half that of a nonsmoker’s, reduces risks for cancers of the [[mouth]], [[throat]], [[esophagus]], and [[bladder]] by half within 5 years and ten years after quitting [[smoking]], the risk for lung cancer drops by half. [[Smoking cessation]] can be achieved by some general, non-pharmacological and pharmacological strategies. | ||
==Clinical practice guidelines== | |||
Clinical practice guidelines by the [[USPSTF]] recommend<ref>USPSTF (2015). [https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1?ds=1&s=smoking%20cessation Tobacco Smoking Cessation in Adults, Including Pregnant Women: Behavioral and Pharmacotherapy Interventions]</ref>: | |||
* "Adults who are not pregnant: The USPSTF recommends that clinicians ask all adults about tobacco use, advise them to stop using tobacco, and provide behavioral interventions and U.S. Food and Drug Administration – approved pharmacotherapy for cessation to adults who use tobacco. GRADE A" | |||
* "Pregnant women: The USPSTF recommends that clinicians ask all pregnant woment about tobacco use, advise them to stop using tobacco, and provide behavioral interventions for cessation to pregnant women who use tobacco. GRADE A" | |||
* "Pregnant women: The USPSTF concludes that the current evidence is insufficient ot assess the balance of benefits and harms of pharmacotherpay interventions for tobacco cessation in pregnant women. GRADE I" | |||
* "All adults, including pregnant women: The USPSTF concludes that the current evidence is insufficient to recommend electronic nicotine delivery systems for tobacco cessation in adults, incuding pregnant women. The USPSTF recommends that clinicians direct patients who smoke tobacco to other cessation interventions with established effectiveness and safety. GRADE I" | |||
==Smoking and Health== | ==Smoking and Health== | ||
Revision as of 01:09, 26 March 2019
|
WikiDoc Resources for Smoking cessation |
|
Articles |
|---|
|
Most recent articles on Smoking cessation Most cited articles on Smoking cessation |
|
Media |
|
Powerpoint slides on Smoking cessation |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Smoking cessation |
|
Clinical Trials |
|
Ongoing Trials on Smoking cessation at Clinical Trials.gov Trial results on Smoking cessation Clinical Trials on Smoking cessation at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Smoking cessation NICE Guidance on Smoking cessation
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Smoking cessation Discussion groups on Smoking cessation Patient Handouts on Smoking cessation Directions to Hospitals Treating Smoking cessation Risk calculators and risk factors for Smoking cessation
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Smoking cessation |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Seyedmahdi Pahlavani, M.D. [2],Usama Talib, BSc, MD [3],Aravind Kuchkuntla, M.B.B.S[4]
To review the wikidoc chapter on smoking, click here.
Overview
Tobacco use is the leading cause of preventable disease, disability, and death in the United States. Each year, nearly half a million Americans die prematurely of smoking or exposure to secondhand smoke and 16 million live with a serious illness caused by smoking. Smoking can cause repairable damage to various organs including the heart, lungs, kidneys, stomach and intestines. Smoking is associated with the causation of various cancers in the humans. Quitting smoking cuts cardiovascular risks, reduces risk for stroke to about half that of a nonsmoker’s, reduces risks for cancers of the mouth, throat, esophagus, and bladder by half within 5 years and ten years after quitting smoking, the risk for lung cancer drops by half. Smoking cessation can be achieved by some general, non-pharmacological and pharmacological strategies.
Clinical practice guidelines
Clinical practice guidelines by the USPSTF recommend[1]:
- "Adults who are not pregnant: The USPSTF recommends that clinicians ask all adults about tobacco use, advise them to stop using tobacco, and provide behavioral interventions and U.S. Food and Drug Administration – approved pharmacotherapy for cessation to adults who use tobacco. GRADE A"
- "Pregnant women: The USPSTF recommends that clinicians ask all pregnant woment about tobacco use, advise them to stop using tobacco, and provide behavioral interventions for cessation to pregnant women who use tobacco. GRADE A"
- "Pregnant women: The USPSTF concludes that the current evidence is insufficient ot assess the balance of benefits and harms of pharmacotherpay interventions for tobacco cessation in pregnant women. GRADE I"
- "All adults, including pregnant women: The USPSTF concludes that the current evidence is insufficient to recommend electronic nicotine delivery systems for tobacco cessation in adults, incuding pregnant women. The USPSTF recommends that clinicians direct patients who smoke tobacco to other cessation interventions with established effectiveness and safety. GRADE I"
Smoking and Health
The impact of smoking on the health can be summarized as follows:[2][3][4]
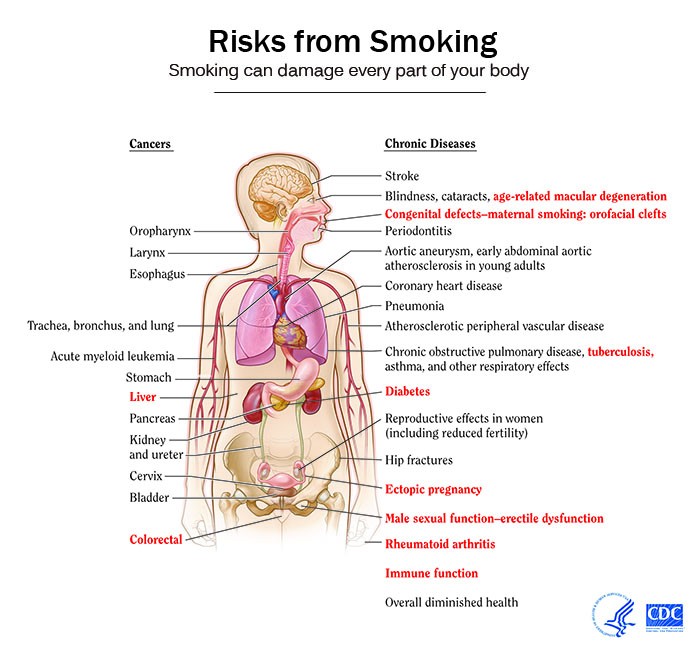
Effect of Smoking Cessation on various Risks
- Quitting smoking cuts cardiovascular risks. Just 1 year after quitting smoking, your risk for a heart attack drops sharply.
- Within 2 to 5 years after quitting smoking, your risk for stroke may reduce to about that of a nonsmoker’s.
- If you quit smoking, your risks for cancers of the mouth, throat, esophagus, and bladder drop by half within 5 years.
- Ten years after you quit smoking, your risk for lung cancer drops by half.
Smoking cessation
General Principles
The 5As are an evidence-based framework for structuring smoking cessation in health care settings. The 5As include: Ask, Assess, Advise, Assist and Arrange follow-up.
|
Pharmacological
First-line pharmacotherapy includes the multiple forms of nicotine replacement therapy (patch, nasal spray, losenge, gum, inhaler), sustained- release bupropion hydrochloride, and varenicline. Second line therapy includes clonidine and nortriptyline and have been found to be efficacious.[5]
The following is a description of the various treatment modalities available:[6]
- Sustained release bupropion hydrochloride:
- Nicotine gum:
- Dose: 1–24 cigarettes/day: 2mg gum (up to 24 pieces/day). ≥ 25 cigarettes/day: 4 mg gum (up to 24 pieces/day).
- Duration: Up to 12 weeks
- Adverse effects: Mouth soreness and dyspepsia
- Nicotine inhaler:
- Dose: 6–16 cartridges/day
- Duration: Up to 6 months
- Adverse effects: Local irritation of mouth and throat
- Nicotine lozenges:
- Nicotine nasal spray:
- Dose: 8–40 doses/day
- Duration: 3–6 months
- Adverse effects: Nasal irritation
- Varenicline:
- Dose: 0.5 mg/day for 3 days followed by 0.5 mg twice/day for 4 days. Then, 1 mg twice/day
- Duration: 3–6 months
- Adverse effects: Nausea, trouble sleeping, vivid/strange dreams and depressed mood
See also
- Nicotine Anonymous
- Health promotion
- NicVAX
- Nicotine replacement therapy
- Tobacco cessation clinic
- Tobacco and health
References
- ↑ USPSTF (2015). Tobacco Smoking Cessation in Adults, Including Pregnant Women: Behavioral and Pharmacotherapy Interventions
- ↑ "CDC - 2010 Surgeon General's Report - Consumer Booklet - Smoking & Tobacco Use".
- ↑ "QuickStats: Number of Deaths from 10 Leading Causes — National Vital Statistics System, United States, 2010".
- ↑ "CDC - 2014 Surgeon General's Report - Smoking & Tobacco Use".
- ↑ "www.vapremier.com" (PDF).
- ↑ Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff (2008). "A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report". Am J Prev Med. 35 (2): 158–76. doi:10.1016/j.amepre.2008.04.009. PMC 4465757. PMID 18617085.