ST elevation myocardial infarction histopathology: Difference between revisions
Brian Blank (talk | contribs) No edit summary |
|||
| Line 25: | Line 25: | ||
==Wavy Fiber Phase== | ==Wavy Fiber Phase== | ||
Although earlier changes can be discerned using [[electron microscopy]], one of the earliest changes under a normal microscope are so-called ''wavy fibers''.<ref name= | Although earlier changes can be discerned using [[electron microscopy]], one of the earliest changes under a normal microscope are so-called ''wavy fibers''.<ref name="pmid782705">{{cite journal |author=Eichbaum FW |title='Wavy' myocardial fibers in spontaneous and experimental adrenergic cardiopathies |journal=Cardiology |volume=60 |issue=6 |pages=358–65 |year=1975 |pmid=782705 |doi= |url=}}</ref>Thin wavy myocytes are (the earliest light microscopic finding of acute myocardial infarction) visible as early as one hour following the onset of infarction. <ref name="pmid4359735">{{cite journal |author=Bouchardy B, Majno G |title=Histopathology of early myocardial infarcts. A new approach |journal=Am. J. Pathol. |volume=74 |issue=2 |pages=301–30 |year=1974 |month=February |pmid=4359735 |pmc=1910768 |doi= |url=}}</ref> | ||
==Eosinophilic Phase with Loss of Cell Nucleus== | ==Eosinophilic Phase with Loss of Cell Nucleus== | ||
| Line 31: | Line 31: | ||
==Coagulation Necrosis== | ==Coagulation Necrosis== | ||
Coagulation necrosis, characterized by hypereosinophilia and nuclear [[pyknosis]], followed by karyorrhexis, karyolysis, total loss of nuclei and loss of cytoplasmic cross-striations, is generally first visible in the period from 4-12 hours following infarction.<ref>Schoen FJ. The heart. Chapter 12 in Robbins Pathologic Basis of Disease, fifth edition, 1994, Cotran RS, Kumar V, Schoen FJ, eds., Philadelphia, W.B.Saunders, pp.517-582</ref>. Necrotic myocytes may retain their striations for a long time.<ref>Fishbein MC, Maclean D, Maroko PR | Coagulation necrosis, characterized by hypereosinophilia and nuclear [[pyknosis]], followed by karyorrhexis, karyolysis, total loss of nuclei and loss of cytoplasmic cross-striations, is generally first visible in the period from 4-12 hours following infarction.<ref>Schoen FJ. The heart. Chapter 12 in Robbins Pathologic Basis of Disease, fifth edition, 1994, Cotran RS, Kumar V, Schoen FJ, eds., Philadelphia, W.B.Saunders, pp.517-582</ref>. Necrotic myocytes may retain their striations for a long time.<ref name="pmid657859">{{cite journal |author=Fishbein MC, Maclean D, Maroko PR |title=The histopathologic evolution of myocardial infarction |journal=Chest |volume=73 |issue=6 |pages=843–9 |year=1978 |month=June |pmid=657859 |doi= |url=http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=657859}}</ref> | ||
Neutrophilic infiltration (acute inflammation), edema and hemorrhage are also first visible at 4-12 hours but generally closer to 12 hours. The interstitium at the margin of the infarcted area is initially infiltrated with [[neutrophil]]s, then with [[lymphocyte]]s and [[macrophage]]s, who [[phagocytosis|phagocytose]] ("eat") the myocyte debris. The necrotic area is surrounded and progressively invaded by [[granulation tissue]], which will replace the infarct with a fibrous ([[collagen]]ous) [[scar]] (which are typical steps in [[wound healing]]). The interstitial space (the space between cells outside of blood vessels) may be infiltrated with [[red blood cell]]s.<ref name=rubin546/> | Neutrophilic infiltration (acute inflammation), edema and hemorrhage are also first visible at 4-12 hours but generally closer to 12 hours. The interstitium at the margin of the infarcted area is initially infiltrated with [[neutrophil]]s, then with [[lymphocyte]]s and [[macrophage]]s, who [[phagocytosis|phagocytose]] ("eat") the myocyte debris. The necrotic area is surrounded and progressively invaded by [[granulation tissue]], which will replace the infarct with a fibrous ([[collagen]]ous) [[scar]] (which are typical steps in [[wound healing]]). The interstitial space (the space between cells outside of blood vessels) may be infiltrated with [[red blood cell]]s.<ref name=rubin546/> | ||
Acute inflammation is generally present in a narrow band of the periphery at 24 hours, in a broad band of the periphery at 48 hours and tends to be maximal around 72 hours, with extensive basophilic debris from degenerating neutrophils.<ref>Fishbein MC, Maclean D, Maroko PR | Acute inflammation is generally present in a narrow band of the periphery at 24 hours, in a broad band of the periphery at 48 hours and tends to be maximal around 72 hours, with extensive basophilic debris from degenerating neutrophils.<ref name="pmid657859">{{cite journal |author=Fishbein MC, Maclean D, Maroko PR |title=The histopathologic evolution of myocardial infarction |journal=Chest |volume=73 |issue=6 |pages=843–9 |year=1978 |month=June |pmid=657859 |doi= |url=http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=657859}}</ref> | ||
Infiltration by macrophages, lymphocytes, eosinophils, fibroblasts and capillaries begins around the periphery at 3-10 days. Contraction band necrosis, characterized by hypereosinophilic transverse bands of precipitated myofibrils in dead myocytes is usually seen at the edge of an infarct or with reperfusion (e.g. with thrombolytic therapy).<ref>Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.</ref> | Infiltration by macrophages, lymphocytes, eosinophils, fibroblasts and capillaries begins around the periphery at 3-10 days. Contraction band necrosis, characterized by hypereosinophilic transverse bands of precipitated myofibrils in dead myocytes is usually seen at the edge of an infarct or with reperfusion (e.g. with thrombolytic therapy).<ref>Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.</ref> | ||
| Line 41: | Line 41: | ||
Reperfusion of an infarct is also associated with more hemorrhage, less acute inflammation, less limitation of the acute inflammation to the periphery in the first few days, reactive stromal cells, more macrophage infiltration earlier and a more patchy distribution of necrosis, especially around the periphery.<ref>Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.</ref> | Reperfusion of an infarct is also associated with more hemorrhage, less acute inflammation, less limitation of the acute inflammation to the periphery in the first few days, reactive stromal cells, more macrophage infiltration earlier and a more patchy distribution of necrosis, especially around the periphery.<ref>Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.</ref> | ||
These features can be recognized in cases where the perfusion was not restored; reperfused infarcts can have other hallmarks, such as contraction band necrosis.<ref name=" | These features can be recognized in cases where the perfusion was not restored; reperfused infarcts can have other hallmarks, such as contraction band necrosis.<ref name="pmid2182247">{{cite journal |author=Fishbein MC |title=Reperfusion injury |journal=Clin Cardiol |volume=13 |issue=3 |pages=213–7 |year=1990 |month=March |pmid=2182247 |doi= |url=}}</ref> | ||
'''Summary of Time from Onset and Morphologic Findings''' | '''Summary of Time from Onset and Morphologic Findings''' | ||
Revision as of 17:52, 11 February 2009
| Myocardial infarction | |
 | |
|---|---|
| Histopathology of myonecrosis in the emmolition phase. | |
| ICD-10 | I21-I22 |
| ICD-9 | 410 |
| DiseasesDB | 8664 |
| MedlinePlus | 000195 |
| eMedicine | med/1567 emerg/327 ped/2520 |
| Cardiology Network |
 Discuss ST elevation myocardial infarction histopathology further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Template:WikiDoc Cardiology News Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Histopathological examination of the heart may reveal infarction at autopsy. Under the microscope, myocardial infarction presents as a circumscribed area of ischemic, coagulative necrosis (cell death). On gross examination, the infarct is not identifiable within the first 12 hours.[1]
Wavy Fiber Phase
Although earlier changes can be discerned using electron microscopy, one of the earliest changes under a normal microscope are so-called wavy fibers.[2]Thin wavy myocytes are (the earliest light microscopic finding of acute myocardial infarction) visible as early as one hour following the onset of infarction. [3]
Eosinophilic Phase with Loss of Cell Nucleus
Subsequently, the myocyte cytoplasm becomes more eosinophilic (pink) and the cells lose their transversal striations, with typical changes and eventually loss of the cell nucleus.[4]
Coagulation Necrosis
Coagulation necrosis, characterized by hypereosinophilia and nuclear pyknosis, followed by karyorrhexis, karyolysis, total loss of nuclei and loss of cytoplasmic cross-striations, is generally first visible in the period from 4-12 hours following infarction.[5]. Necrotic myocytes may retain their striations for a long time.[6]
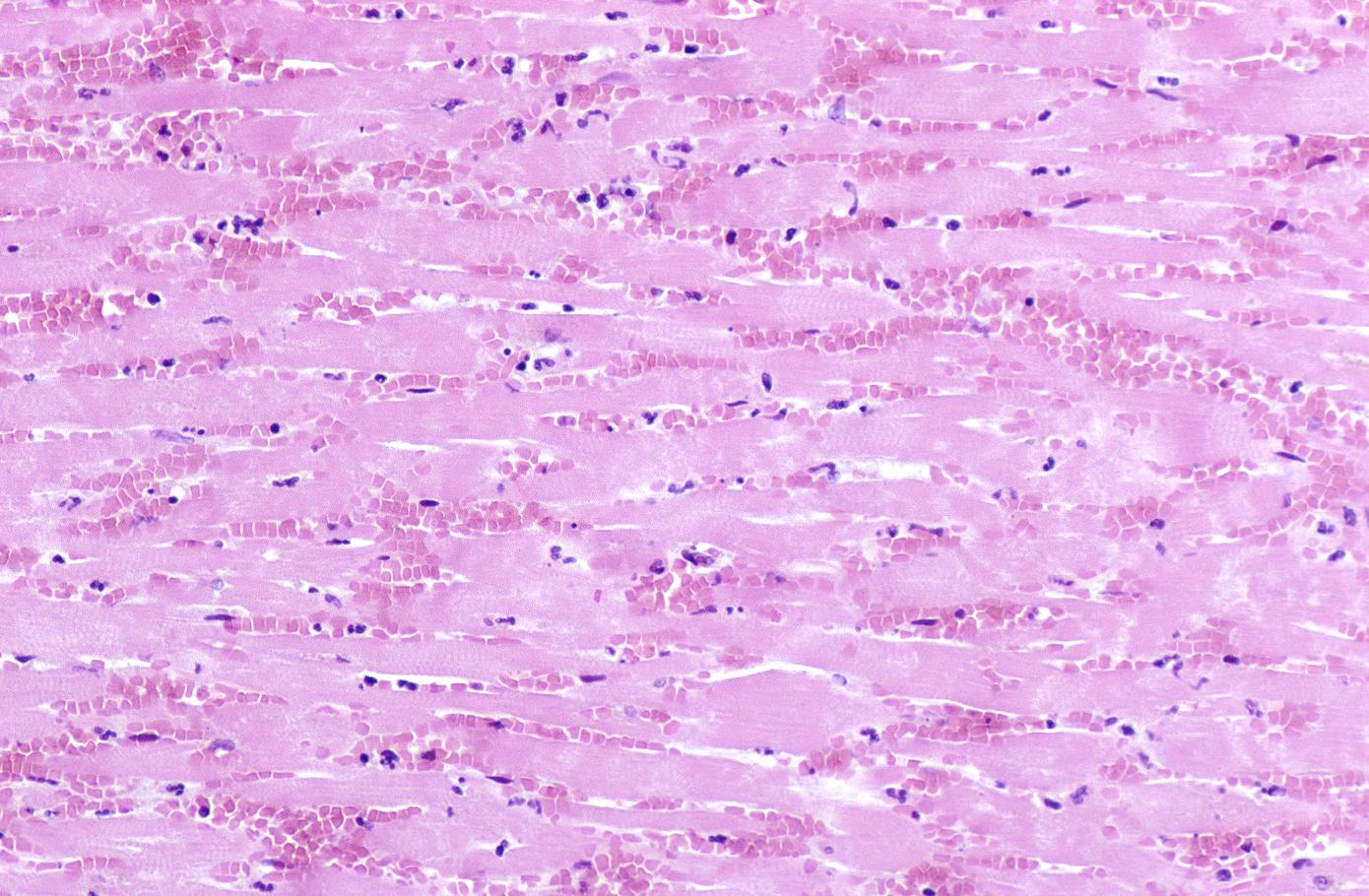
Neutrophilic infiltration (acute inflammation), edema and hemorrhage are also first visible at 4-12 hours but generally closer to 12 hours. The interstitium at the margin of the infarcted area is initially infiltrated with neutrophils, then with lymphocytes and macrophages, who phagocytose ("eat") the myocyte debris. The necrotic area is surrounded and progressively invaded by granulation tissue, which will replace the infarct with a fibrous (collagenous) scar (which are typical steps in wound healing). The interstitial space (the space between cells outside of blood vessels) may be infiltrated with red blood cells.[1]
Acute inflammation is generally present in a narrow band of the periphery at 24 hours, in a broad band of the periphery at 48 hours and tends to be maximal around 72 hours, with extensive basophilic debris from degenerating neutrophils.[6]
Infiltration by macrophages, lymphocytes, eosinophils, fibroblasts and capillaries begins around the periphery at 3-10 days. Contraction band necrosis, characterized by hypereosinophilic transverse bands of precipitated myofibrils in dead myocytes is usually seen at the edge of an infarct or with reperfusion (e.g. with thrombolytic therapy).[7]
Reperfusion of an infarct is also associated with more hemorrhage, less acute inflammation, less limitation of the acute inflammation to the periphery in the first few days, reactive stromal cells, more macrophage infiltration earlier and a more patchy distribution of necrosis, especially around the periphery.[8]
These features can be recognized in cases where the perfusion was not restored; reperfused infarcts can have other hallmarks, such as contraction band necrosis.[9]
Summary of Time from Onset and Morphologic Findings
- 1 - 3 hours: Wavy myocardial fibers
- 2 - 3 hours: Staining defect with tetrazolium or basic fuchsin dye
- 4 - 12 hours: Coagulation necrosis with loss of cross striations, contraction bands, edema, hemorrhage, and early neutrophilic infiltrate
- 18 - 24 hours: Continuing coagulation necrosis, pyknosis of nuclei, and marginal contraction bands
- 24 - 72 hours: Total loss of nuclei and striations along with heavy neutrophilic infiltrate
- 3 - 7 days: Macrophage and mononuclear infiltration begin, fibrovascular response begins
- 10 - 21 days: Fibrovascular response with prominent granulation tissue
- 7 weeks: Fibrosis
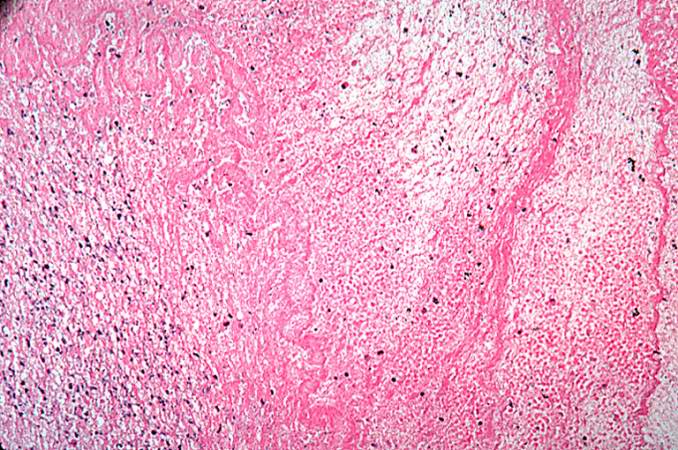
Images of Histopathological Findings
-
Myocardial infarction, subacute, granulation tissue.
-
Myocardial infarction, subendocardial ischemia with swollen myocytes
-
Myocardial infarction, mural thrombus
-
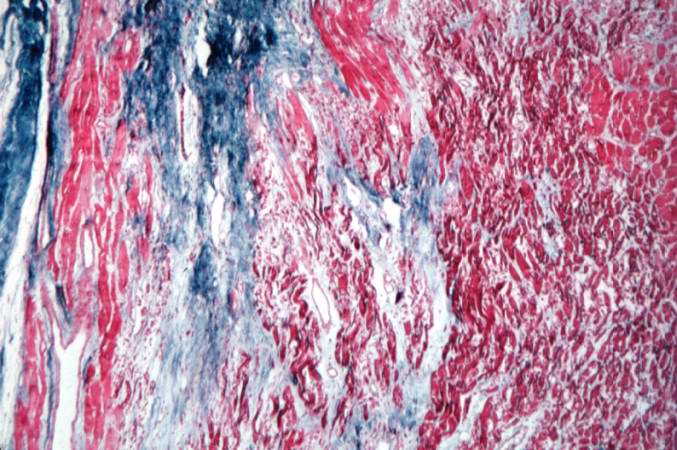
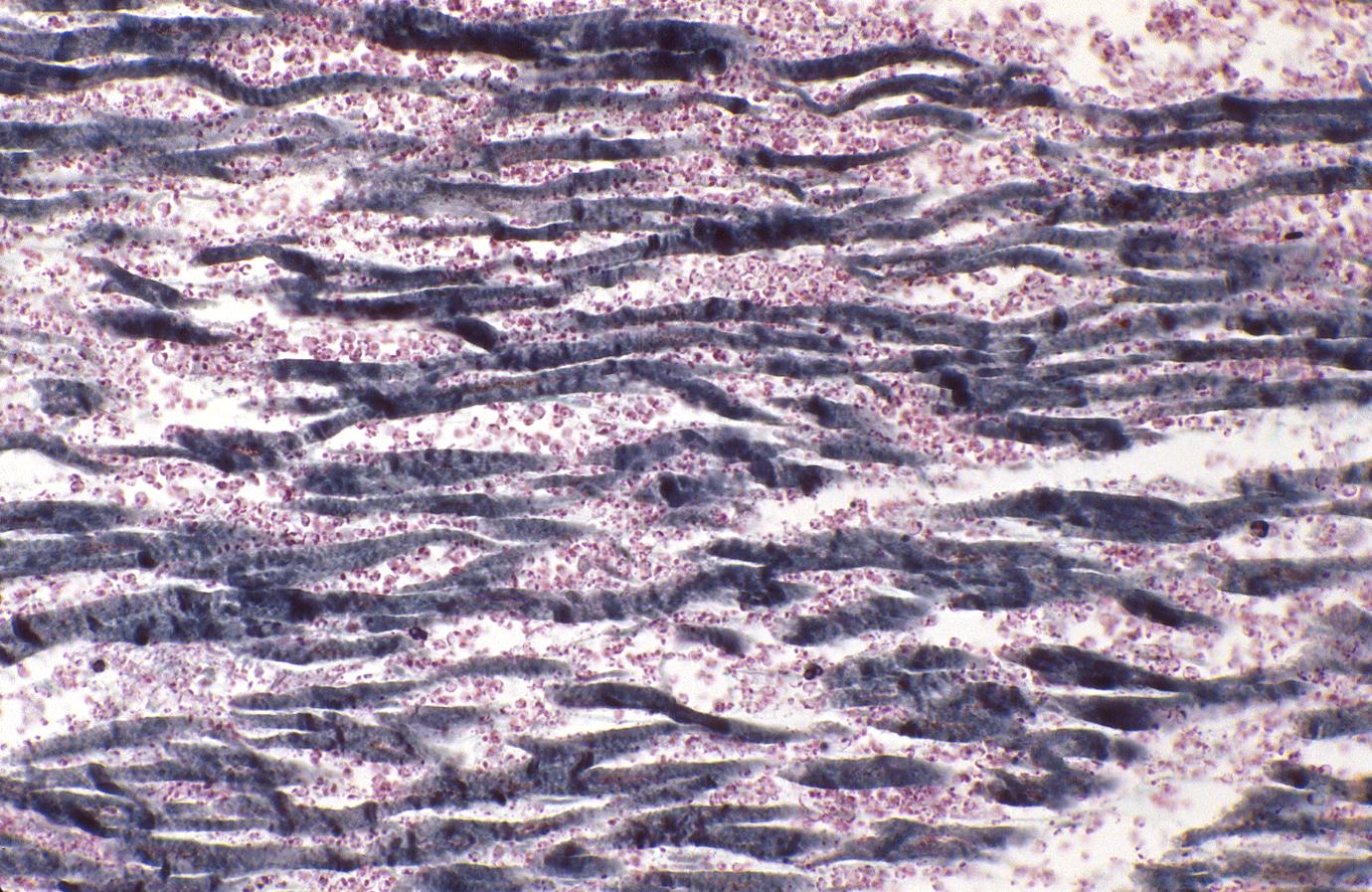
Acute myocardial infarction, ischemic fibers demonstrated by aldehyde fuchsin stain
Case Examples
Case 1
Medical History, Clinical & Laboratory Findings
An 83-year-old male was admitted with the chief complaint of chest pain. He had been awakened the previous night with dull chest pain which was retrosternal and radiated through to his back. The pain was associated with sweating, nausea, and vomiting and could not be relieved by antacids. Nitroglycerin gave prompt relief.
Following admission he developed cardiac arrhythmias.
His AST was found to be 130 IU/L. In the early morning of the day after admission, he developed severe epigastric pain and several episodes of tachycardia (150-160 beats per minute) and later cardiac arrest.
There was a history of hypertension and diabetes.
Autopsy Findings
The heart weighed 500 grams.
There was massive acute myocardial infarction (about 2 days old) involving the posterior left ventricle, interventricular septum, and right ventricle from apex to base.
The infarct was transmural, and there was a small rupture in the soft infarcted area at the apex.
There were 1200 mL of blood within the right pleural cavity, probably secondary to this rupture.
The coronary arteries showed moderate to severe atherosclerosis throughout the coronary tree.
Histopathologic Findings
-
This is a gross photograph of thrombosed coronary artery (arrows).
-
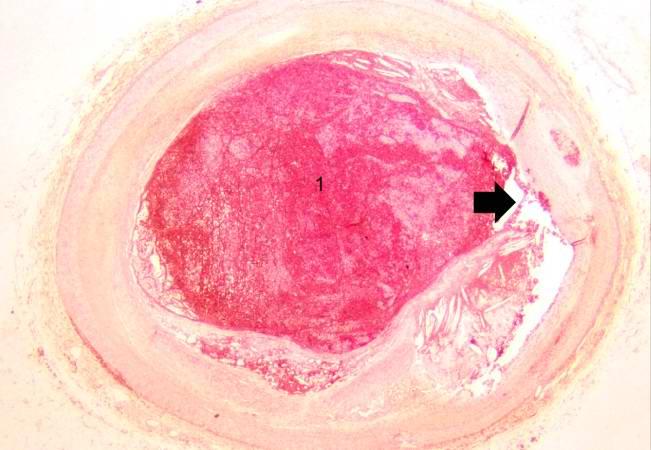
This is a low-power photomicrograph of thrombosed coronary artery. The thrombus (1) completely occludes the vessel. Note the layering of the thrombus. The fibrous cap is ruptured (arrow) and there is hemorrhage into the atherosclerotic plaque. Note the cholesterol crystals in the plaque.
-
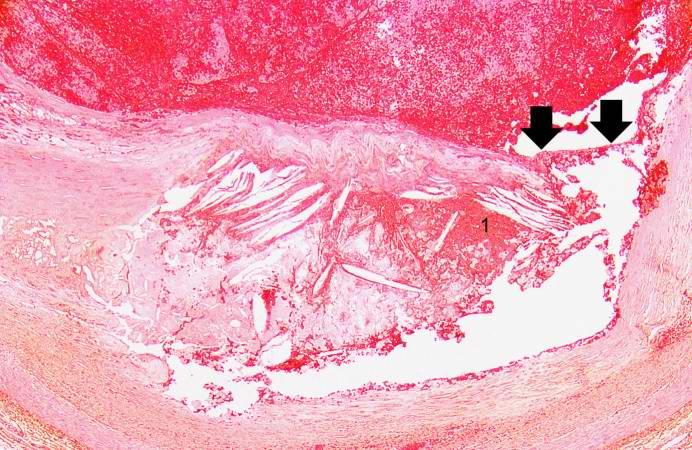
This is a higher-power photomicrograph of the ruptured fibrous cap (arrows) with hemorrhage (1) into the atherosclerotic plaque.
-
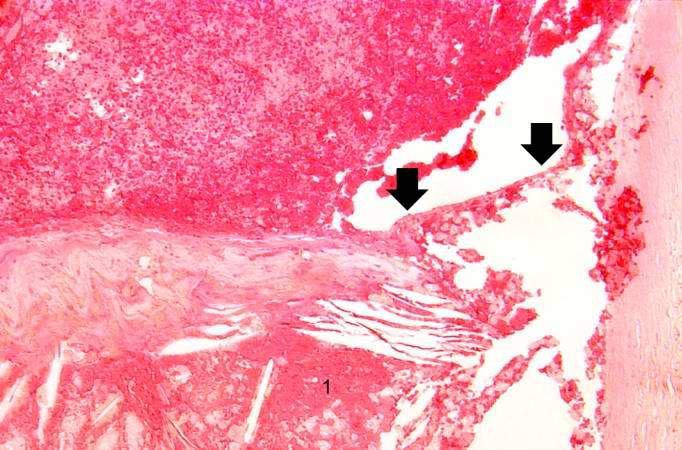
This is another high-power photomicrograph of the ruptured fibrous cap (arrows) with hemorrhage (1) into the atherosclerotic plaque. Note the presence of cholesterol crystals.
-
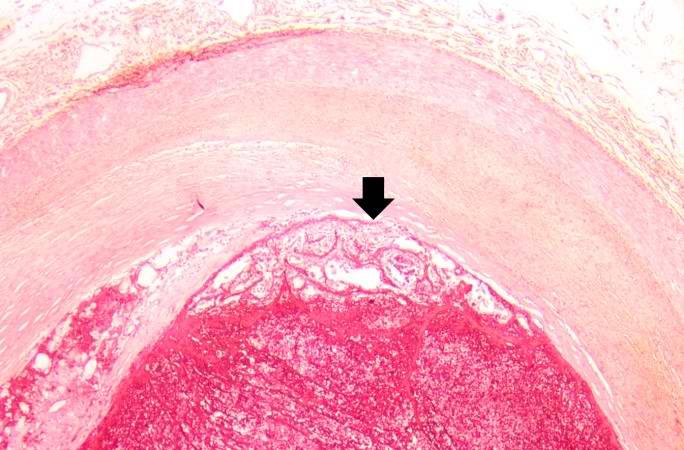
This is a high-power photomicrograph of thrombus attached to the wall of the vessel. There is early organization of the thrombus (arrow).
-
This is a higher-power photomicrograph of thrombus attached to the wall of the vessel. Note the early organization with in-growth of fibroblasts and small blood vessels from the wall of the artery (arrows).
-
In this low-power photomicrograph of another coronary artery from this patient, a mural thrombus has undergone re-organization. The mural thrombus has been invaded by the in-growth of fibroblasts and small blood vessels from the wall of the artery. The thrombotic material has been phagocytosed and removed by macrophages and is replaced by fibrous connective tissue and blood vessels. This re-organized thrombus still compromises the lumen of this vessel.
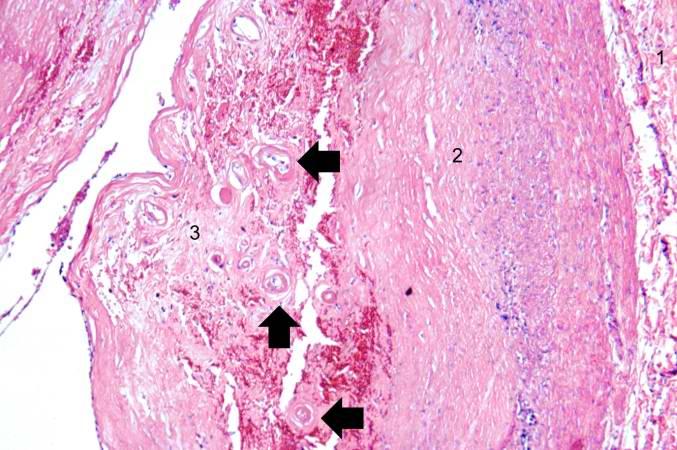
-
This is a higher-power photomicrograph of the vessel wall. The adventitia (1) and the media (2) contain inflammatory cells. The recanalized portion of the vessel is composed of fibrous connective tissue and contains numerous small blood vessels. There is a small area of hemorrhage (arrow) in the central portion of this image.
-
This is a higher-power photomicrograph of another region of the vessel wall. The adventitia (1) and the media (2) contain inflammatory cells. The recanalized portion of the vessel (3) is composed of fibrous connective tissue and contains numerous small blood vessels (arrows).
-
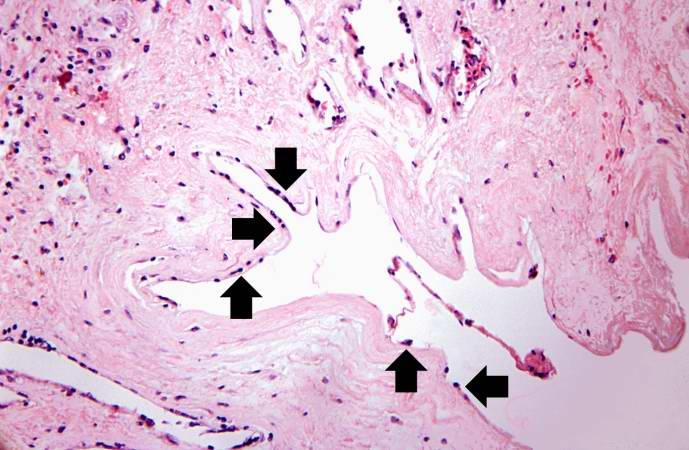
This is a high-power photomicrograph of the luminal surface of a re-canalized vessel. Note that the vessel lumen is lined by endothelial cells (arrows).
Case 2
Medical History, Clinical & Laboratory Findings
A 78-year-old male experienced a posterior myocardial infarction six years prior to this admission.
Recently, he had begun to experience occasional angina.
Four days prior to death, he experienced anterior chest pain and discomfort which he regarded as not too distressing.
However, EKGs showed a classic acute anterior myocardial infarction in addition to the healed posterior infarct.
The patient progressively deteriorated with left ventricular failure and died with arrhythmias and pulmonary edema. Pertinent laboratory data are:
- Aspartate aminotransferase (AST) 60 IU/L.
- Total Creatine phosphokinase (CPK) 165 IU/L. All of the activity was due to CPK III (MM) isoenzyme fraction; no CPK (MB) activity was detectable.
- Lactate dehydrogenase (LDH) 720 IU/L. LD1 fraction was greater than LD2.
Autopsy Findings
Examination of the heart showed a healed posterior infarct. The right coronary artery was completely occluded but partially recanalized. The left main coronary artery had severe atherosclerotic stenosis and a thrombus filling the lumen. The entire anterolateral aspect of the left ventricle was soft with variegated areas appearing hyperemic or pale.
There was extensive mural thrombosis and reactive pericarditis.
Histopathologic Findings
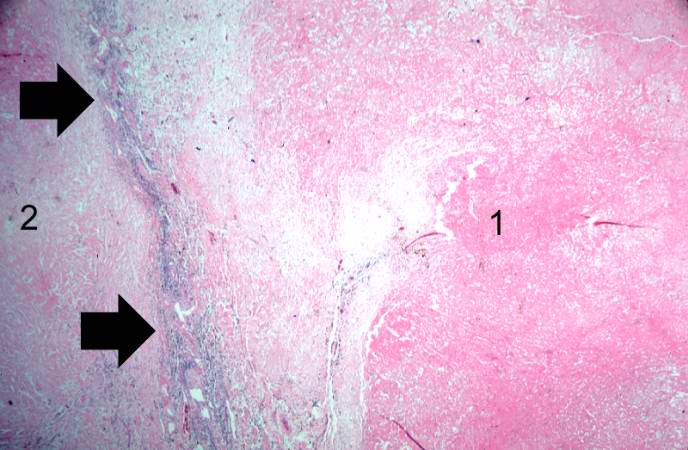
-
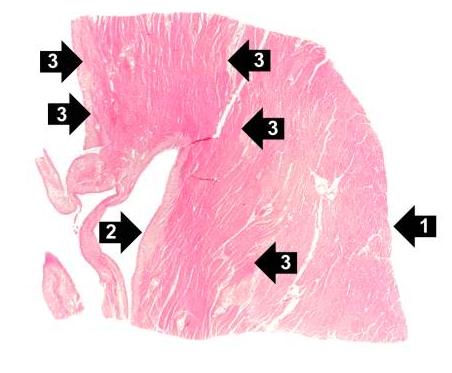
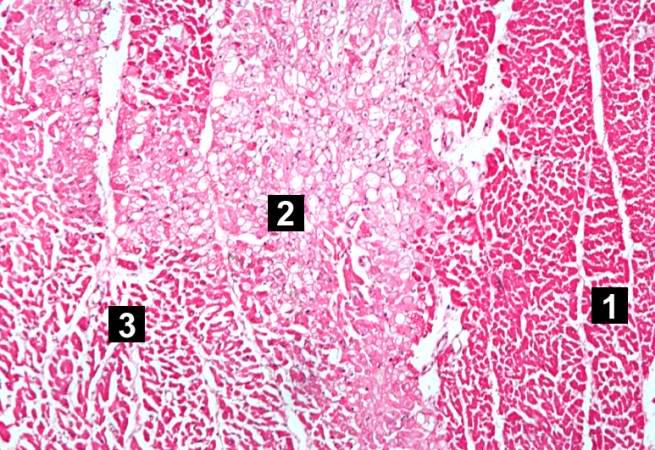
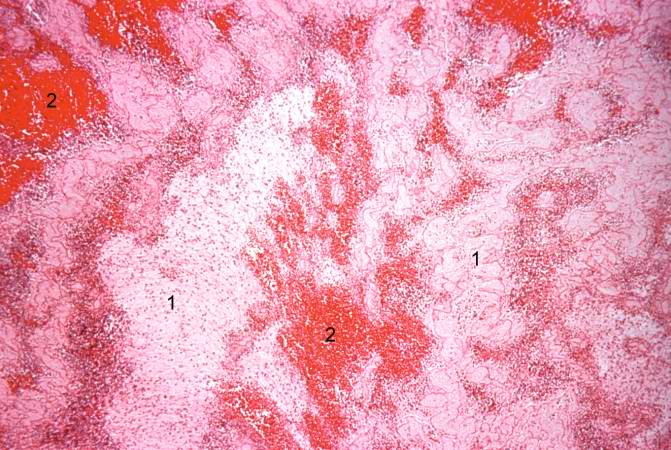
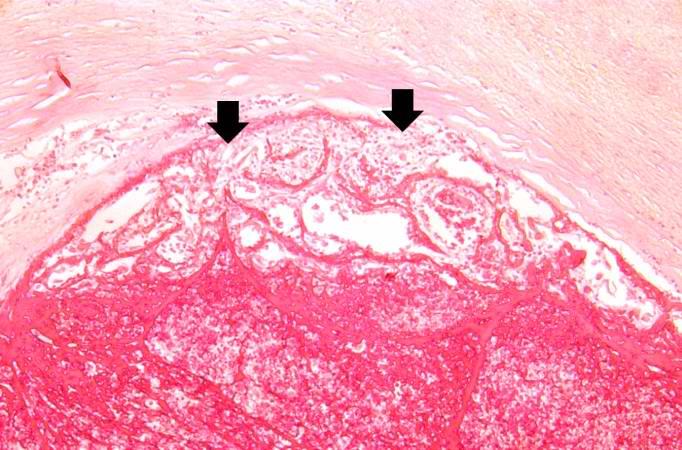
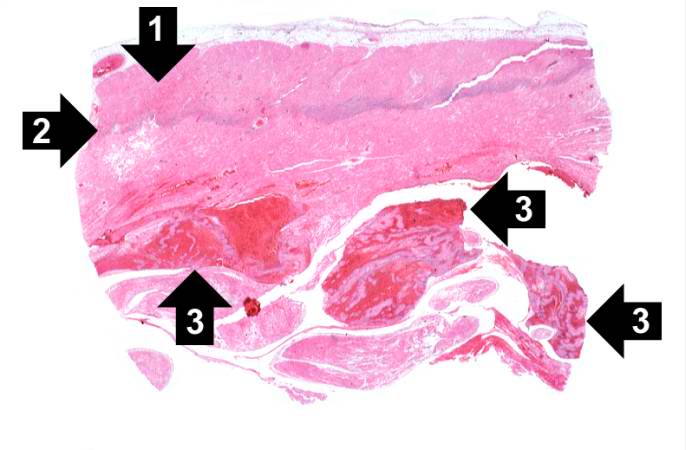
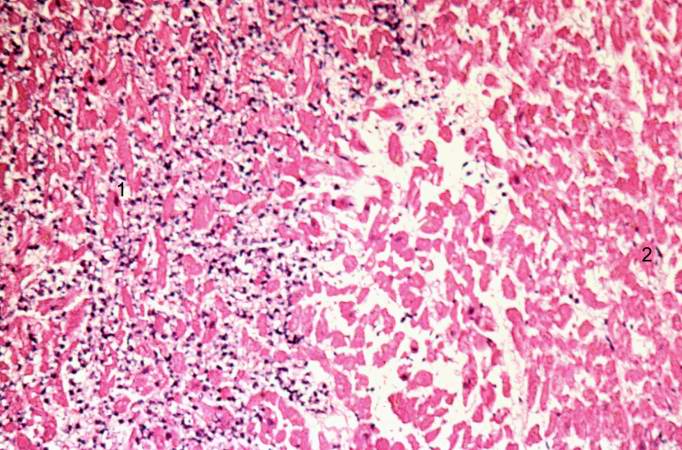
This is a low-power photomicrograph of infarcted heart. There is a layer of surviving myocardial tissue (1) along the epicardium and then a blue line (2) which represents the accumulation of inflammatory cells at the border of the infarct. There is thrombotic material (3) adherent to the endocardial surface.
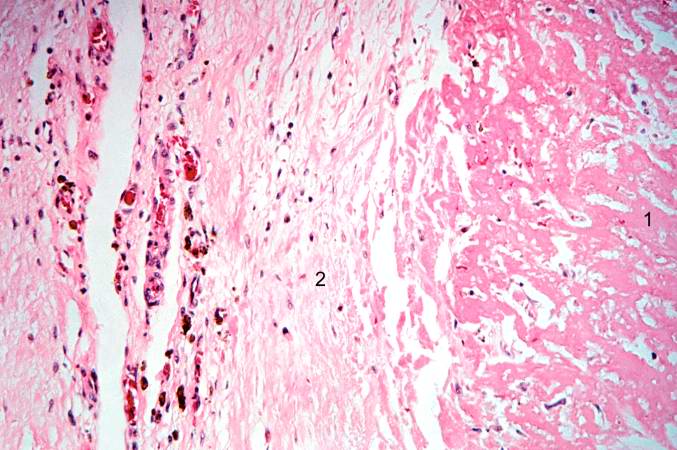
-
This is a higher-power photomicrograph which shows more clearly the viable tissue along the epicardium (1), the blue line of inflammatory cells (2), and the infarcted myocardium (3).
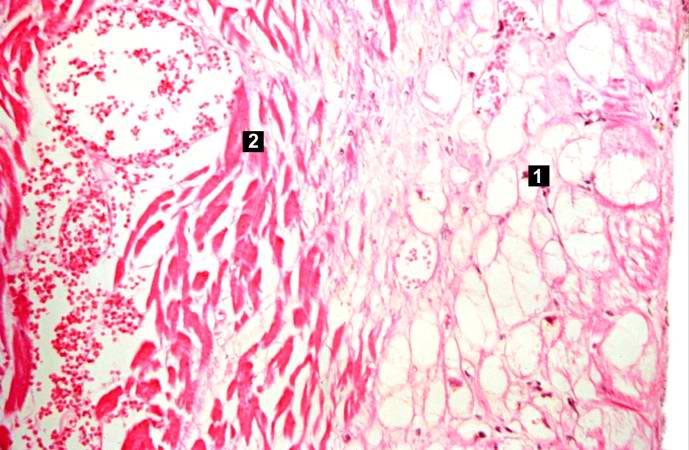
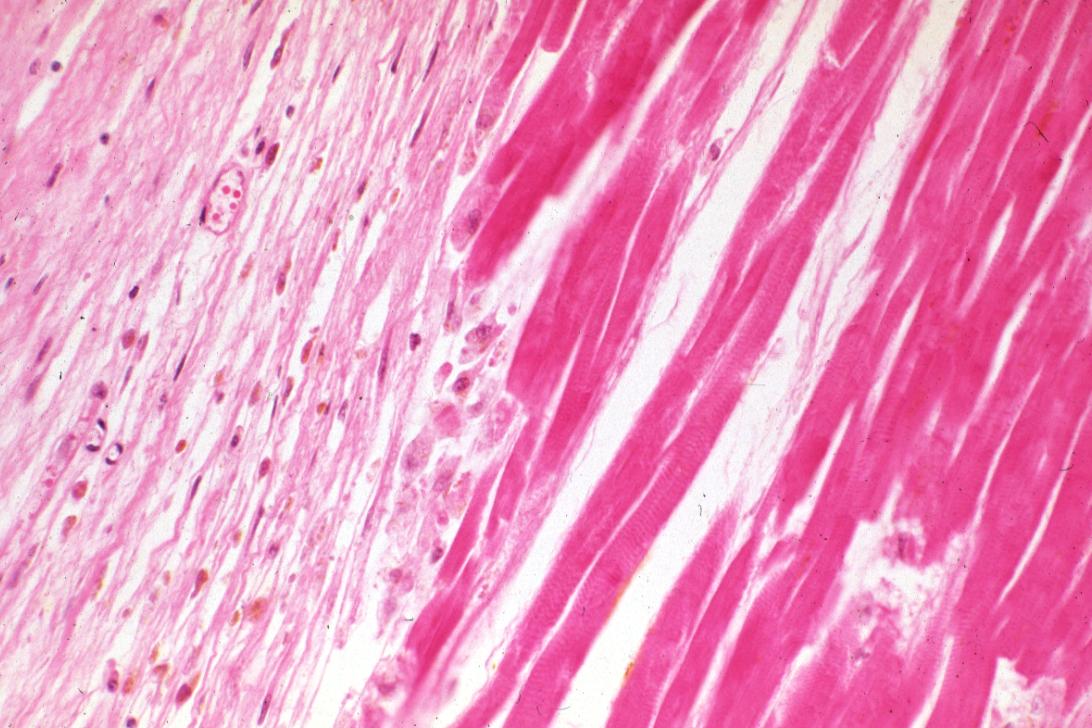
-
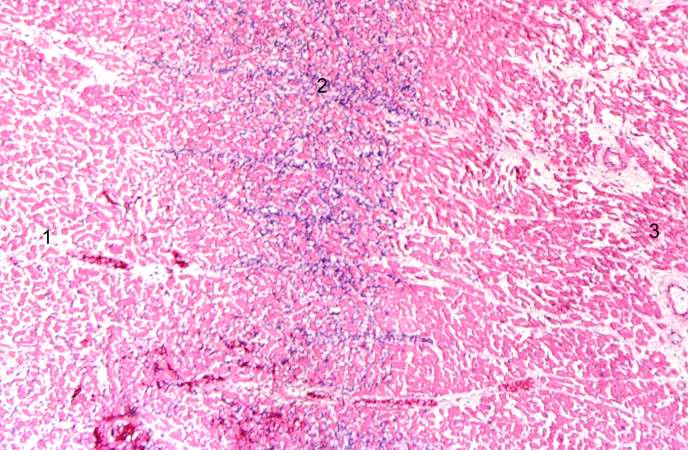
This is a photomicrograph of the edge of the infarct with normal tissue on the left (1). The accumulation of inflammatory cells (2) is at the edge of the infarcted tissue (3).
-
This is a higher-power photomicrograph of the edge of the infarct. The accumulation of inflammatory cells is on the left (1) and the infarcted tissue is on the right (2). Note that intact cells can be seen in the infarct but there are no nuclei.
-
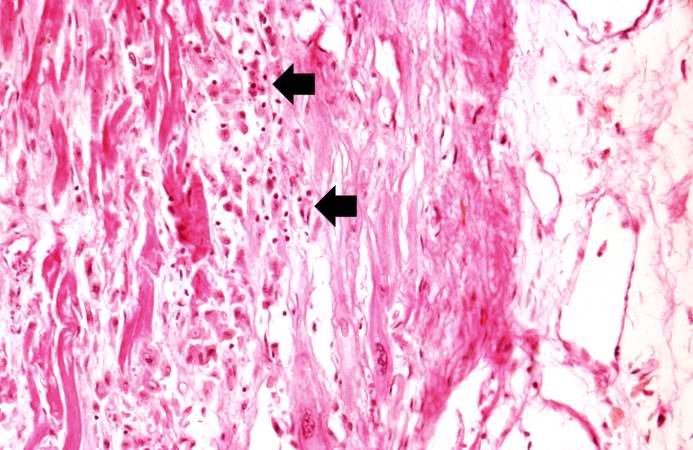
This is a high-power photomicrograph of another area of this section. There are several hypereosinophilic cells within this section (arrows).
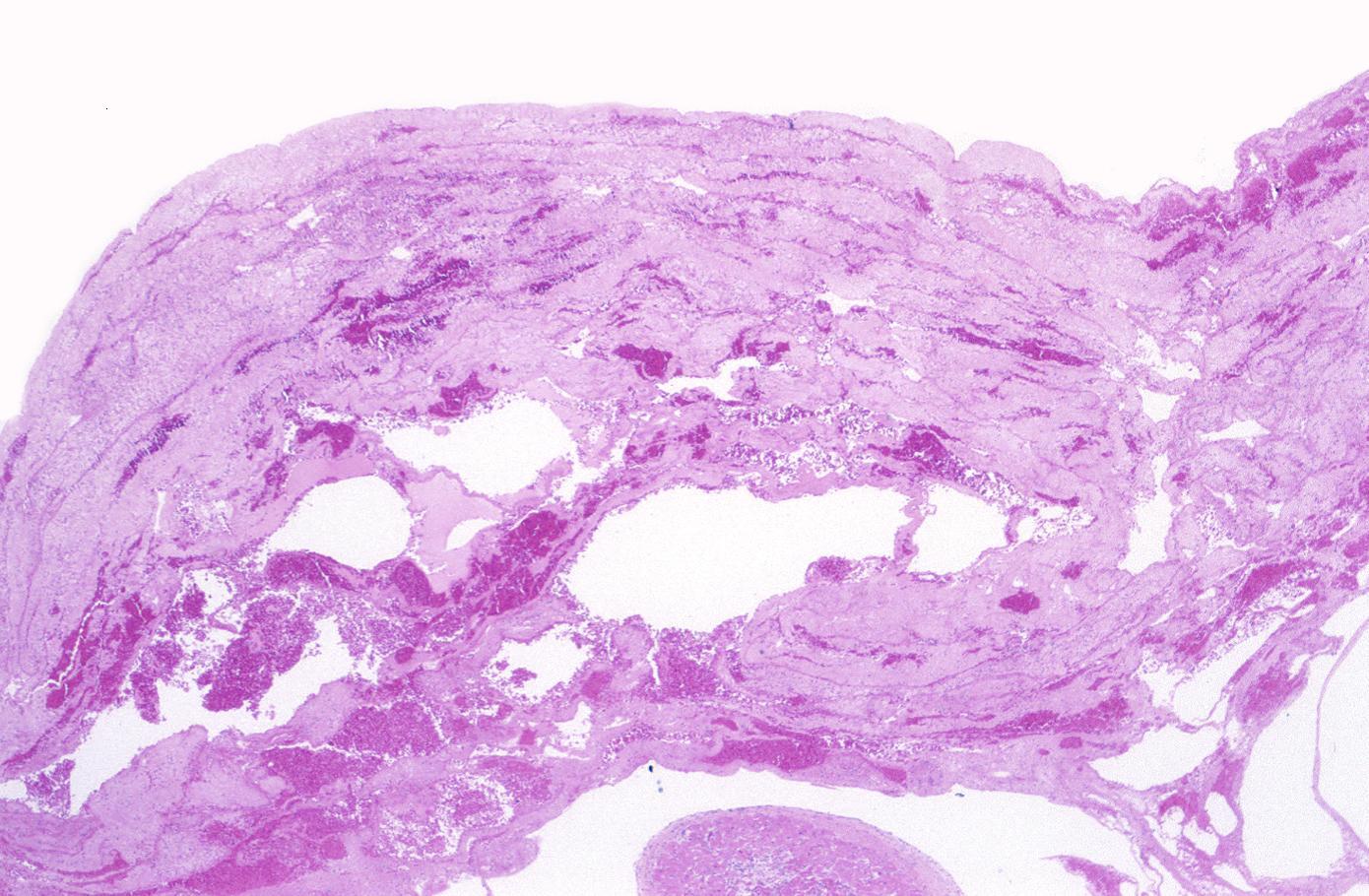
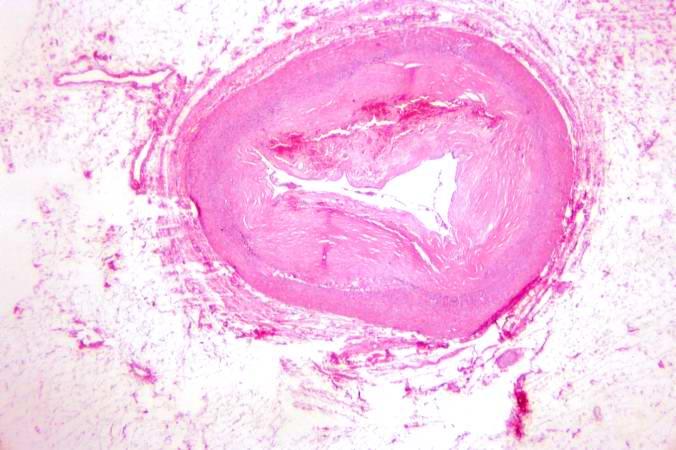
-
This is a low-power photomicrograph of a mural thrombus (1) adherent to the endocardial surface (arrows).
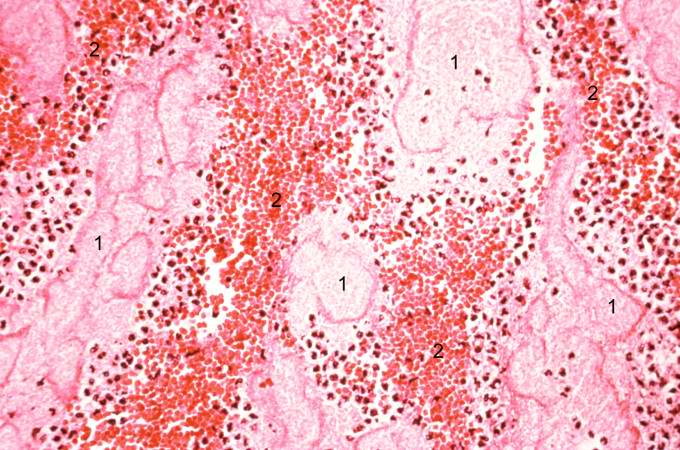
-
This is a photomicrograph of the lines of Zahn. Pale areas (1) represent platelets with some fibrin and the darker lines (2) represent RBCs and ppleukocyte]]s enmeshed in fibrin strands.
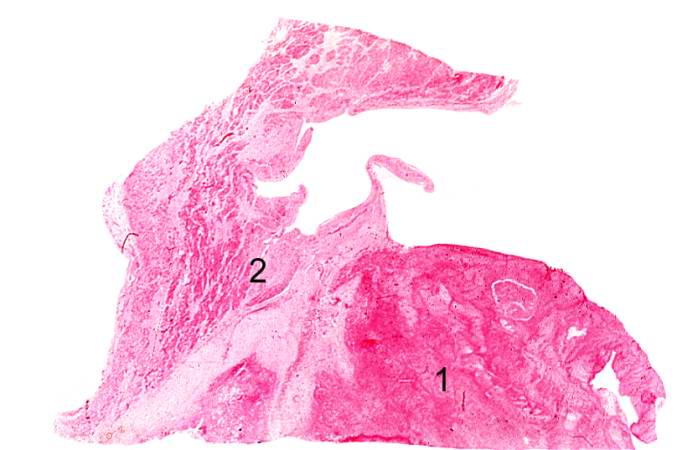
Case 3: Healed Myocardial Infarction
Medical History
A 37-year-old female with a 22 year history of insulin-dependent diabetes mellitus was admitted to the hospital 10 hours prior to death complaining of chest pain and shortness of breath.
Three months before, she had begun to experience progressive weakness and for the previous 3 weeks she noticed increasing dyspnea on exertion and worsening of a chronic cough.
Autopsy Findings
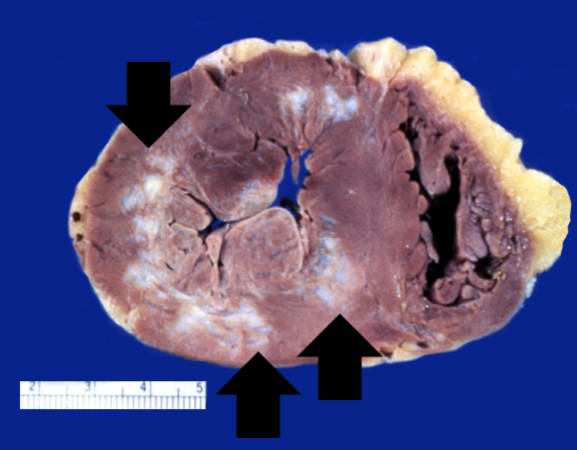
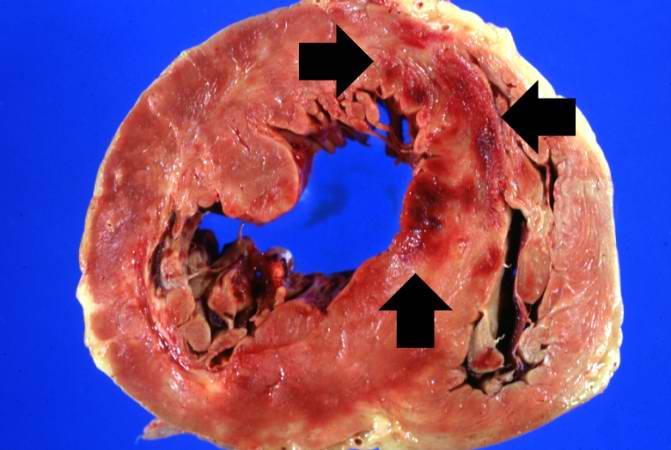
Autopsy showed a 340-gram heart with extensive transmural reddish discoloration of the anterolateral portion of the myocardium of the left ventricle.
There was severe atherosclerotic narrowing of all coronary arteries especially the left anterior descending artery.
The lungs showed pulmonary edema and early bronchopneumonia.
Histopathologic Findings
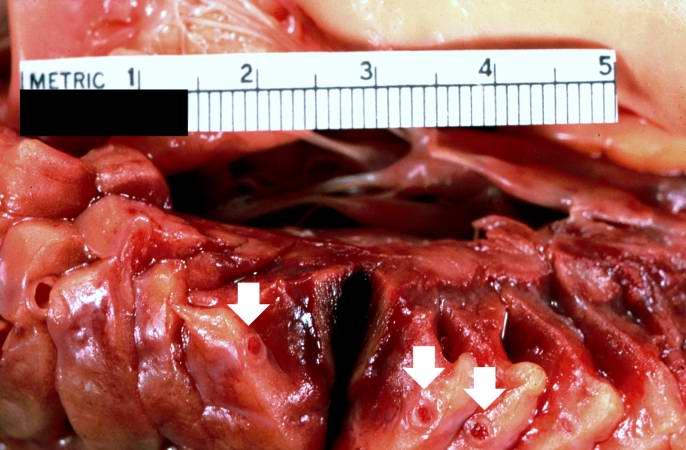
-
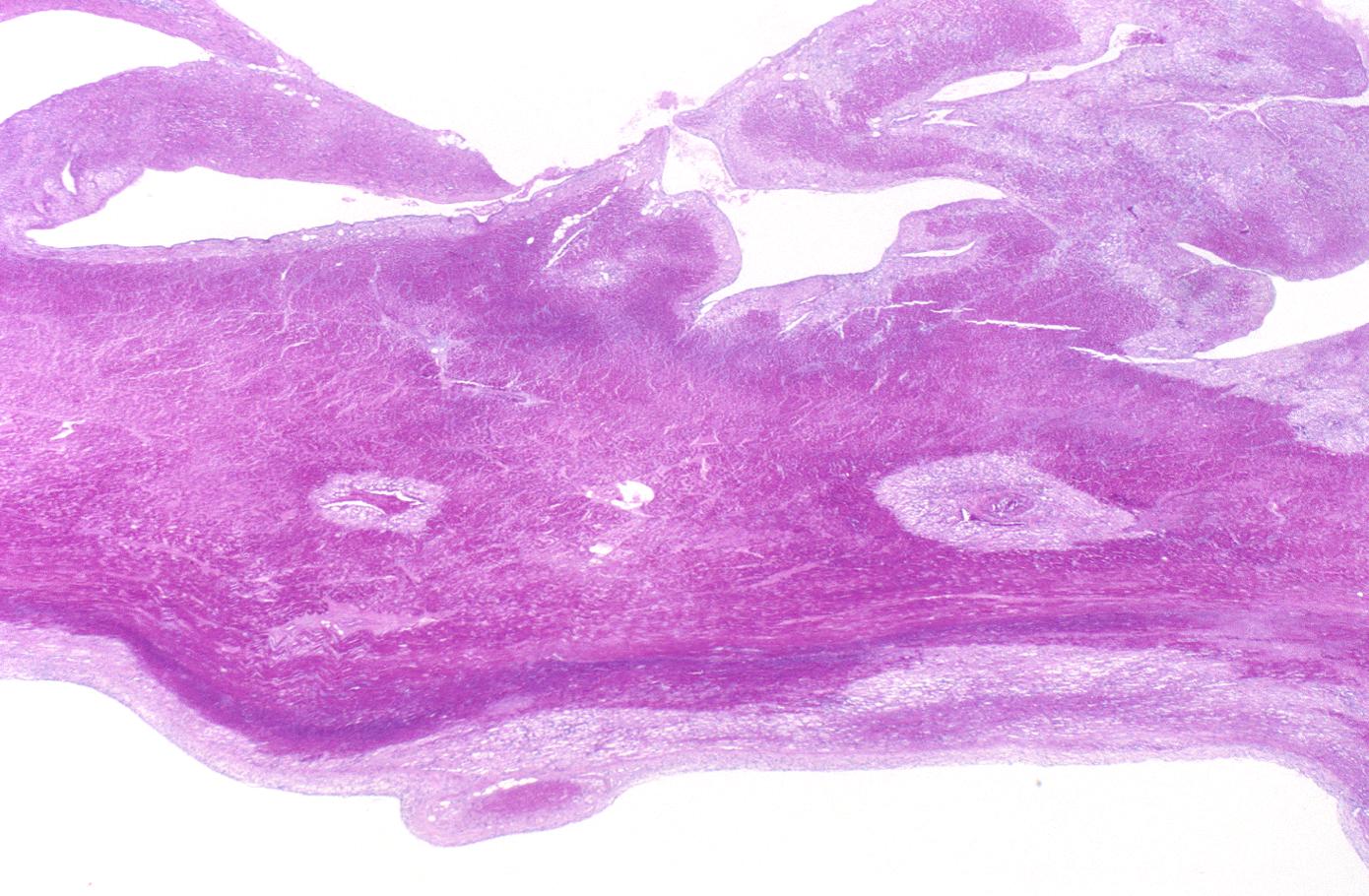
This is a gross photograph of a heart with areas of old healed myocardial infarction (scars) outlined by arrows.
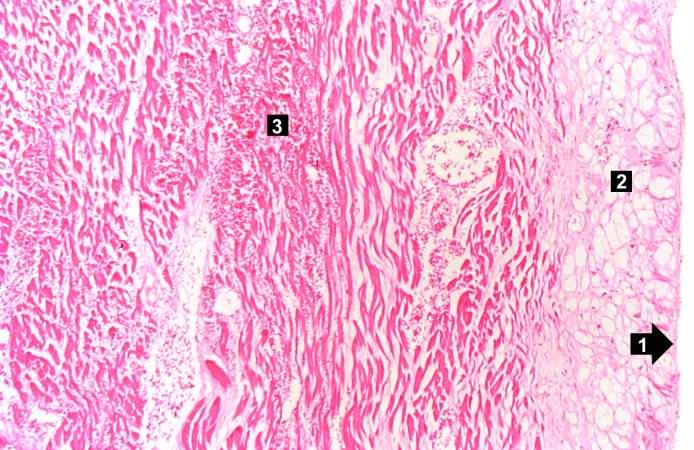
-
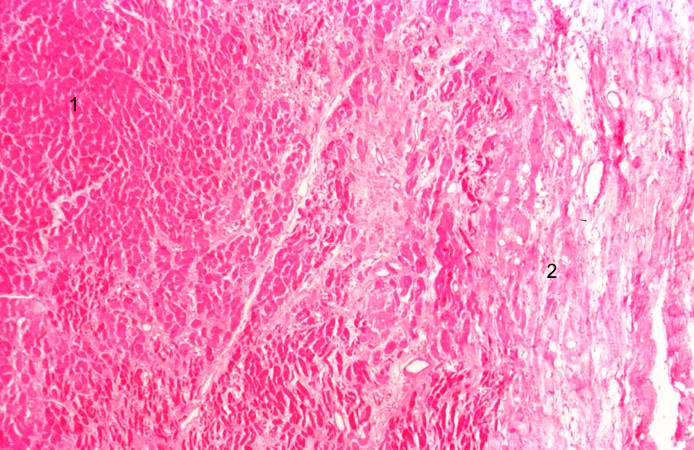
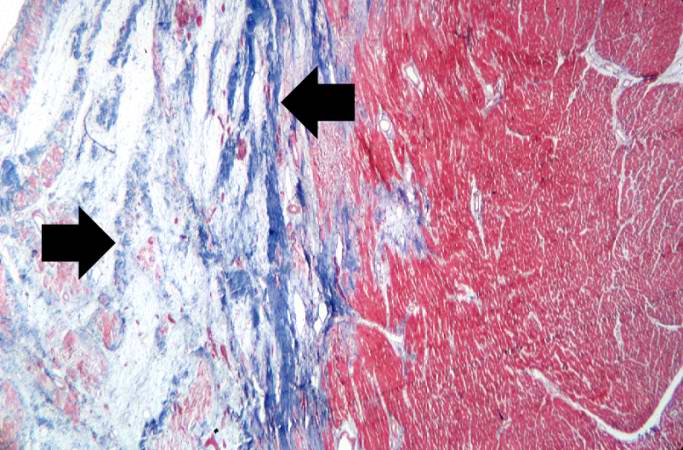
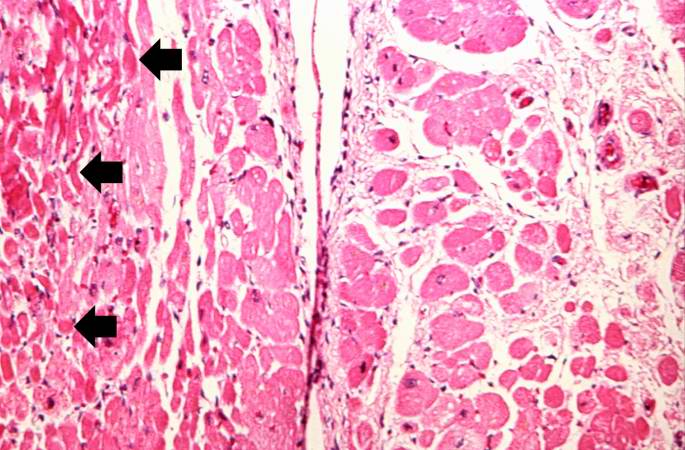
This is a low-power photomicrograph of a healed myocardial infarction with a fibrous scar. Remaining normal tissue is on the left (1) and the fibrous connective tissue scar is on the right (2).
-
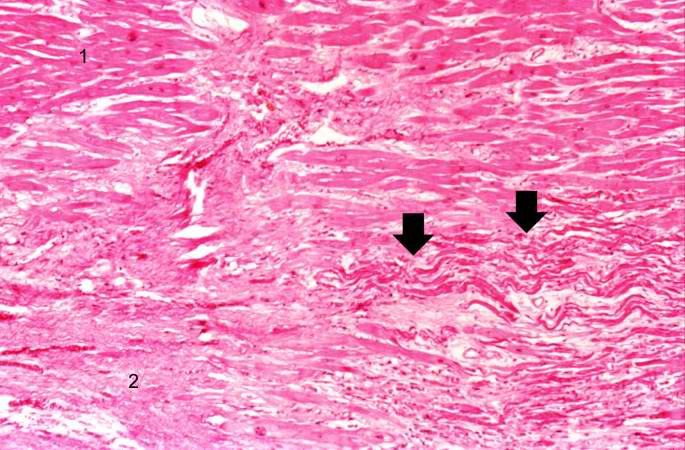
This is a higher-power photomicrograph of a healed myocardial infarction with a fibrous scar. Remaining normal tissue is at the top (1) and the fibrous connective tissue scar is at the bottom (2). Note the presence of occasional hypereosinophilic myocytes indicating recent acute ischemic injury to this region of old scar tissue (arrows).
-
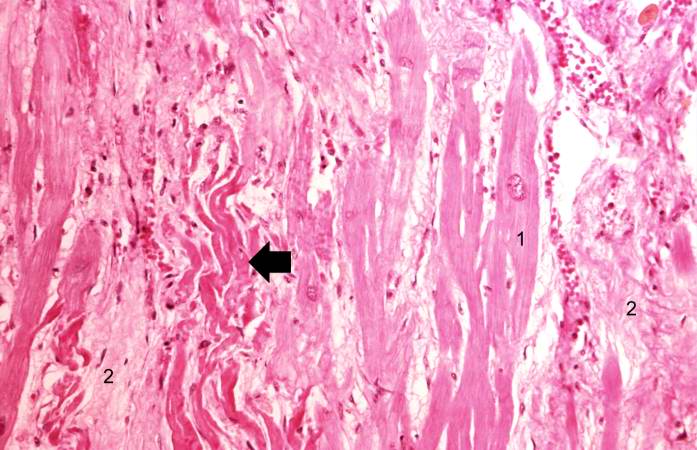
This is another high-power photomicrograph of a healed myocardial infarction. Note the remaining normal myocytes (1), the fibrous connective tissue (2), and occasional hypereosinophilic myocytes indicating recent acute ischemic injury (arrow).
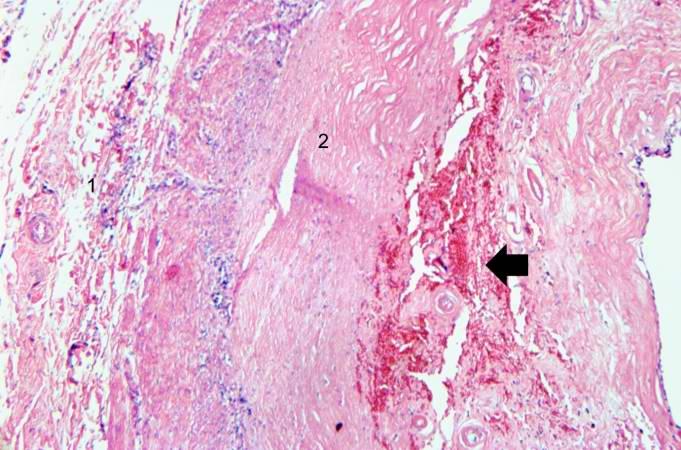
-
This is a high-power photomicrograph of a different region of this healed MI. Note the chronic inflammatory reaction (arrows) in this region suggesting that there had been ischemic injury to this area within the last several weeks to months.
-
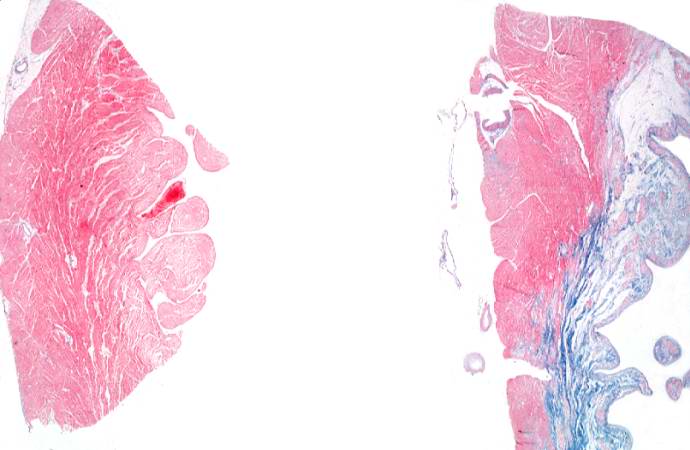
This is a low-power photomicrograph of two sections of myocardial tissue stained with a trichrome stain to demonstrate fibrous connective tissue (blue). The section on the left is from a heart with a recent myocardial infarction. Notice the absence of fibrous connective tissue. The section on the right is from a heart with an old healed infarct and it contains extensive fibrous connective tissue scars.
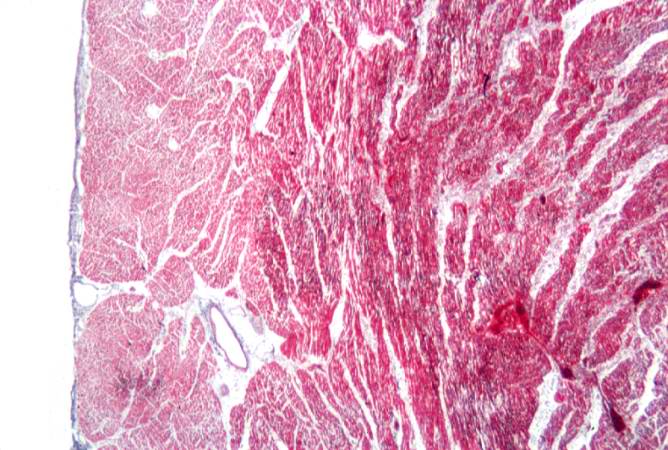
-
This is a photomicrograph of a trichrome-stained section from a heart with an acute myocardial infarction. Note that there is little fibrous connective tissue. It is too early for scar formation to have taken place in this acute lesion.
-
This is a photomicrograph of a trichrome-stained section of heart containing an old healed myocardial infarction. The scar is composed of mature fibrous connective tissue (arrows).
-
This is a higher-power photomicrograph of a trichrome-stained section of heart containing an old healed MI. The scar tissue (mature fibrous connective tissue) is stained blue.
Case 4: Coagulative Necrosis
Clinical Summary
This was a 57-year-old male whose hospital course following abdominal surgery was characterized by progressive deterioration and hypotension.
Four days post-operatively, the patient sustained an anterior myocardial infarction and died the next day.
Autopsy Findings
The patient's heart weighed 410 grams.
Examination of the coronary arteries revealed marked atherosclerotic narrowing of all three vessels with focal occlusion by a thrombus of the left anterior descending artery.
Fresh necrosis of the anterior wall of the left ventricle and anterior portion of the septum was present, extending from the endocardium to the inner half of the ventricular wall.
Histopathological Findings
-
In this gross photograph of the heart from this case, note the area of fresh myocardial infarction (arrows) in the anterior portion of the left ventricle and extending into the anterior portion of the interventricular septum. Note that the walls of the left and right ventricle are slightly thicker than normal.
-
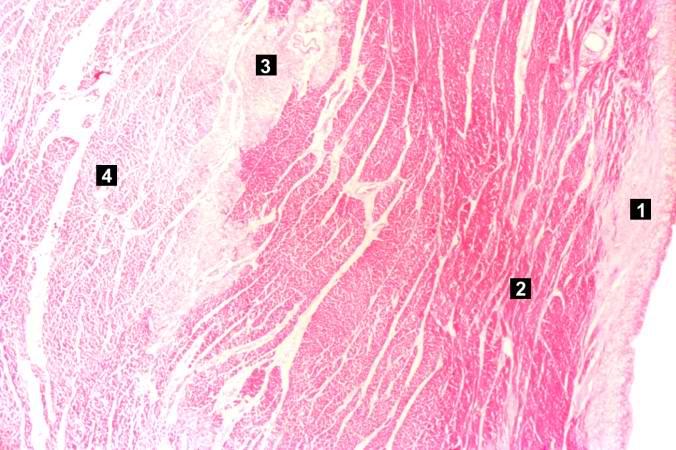
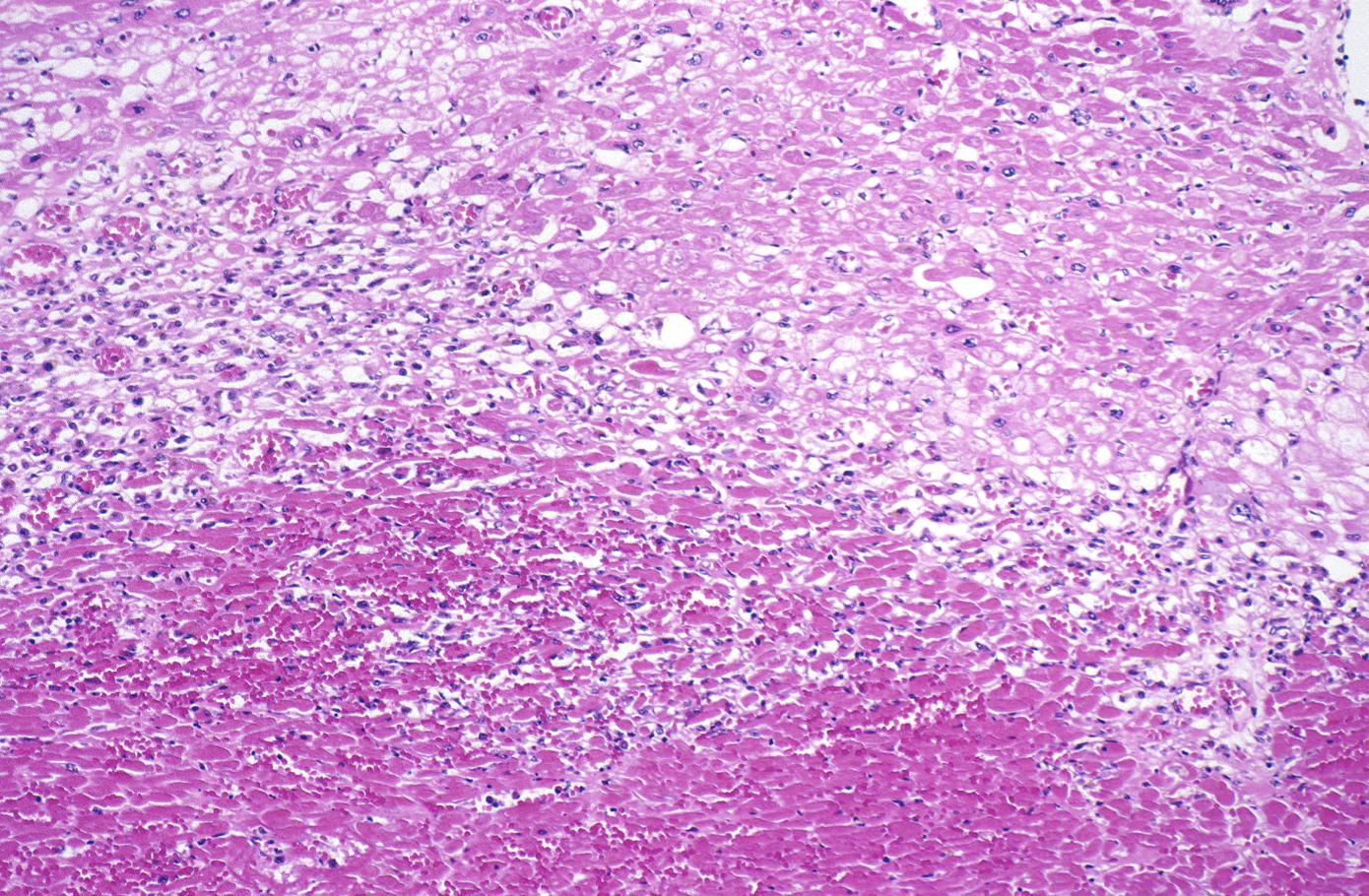
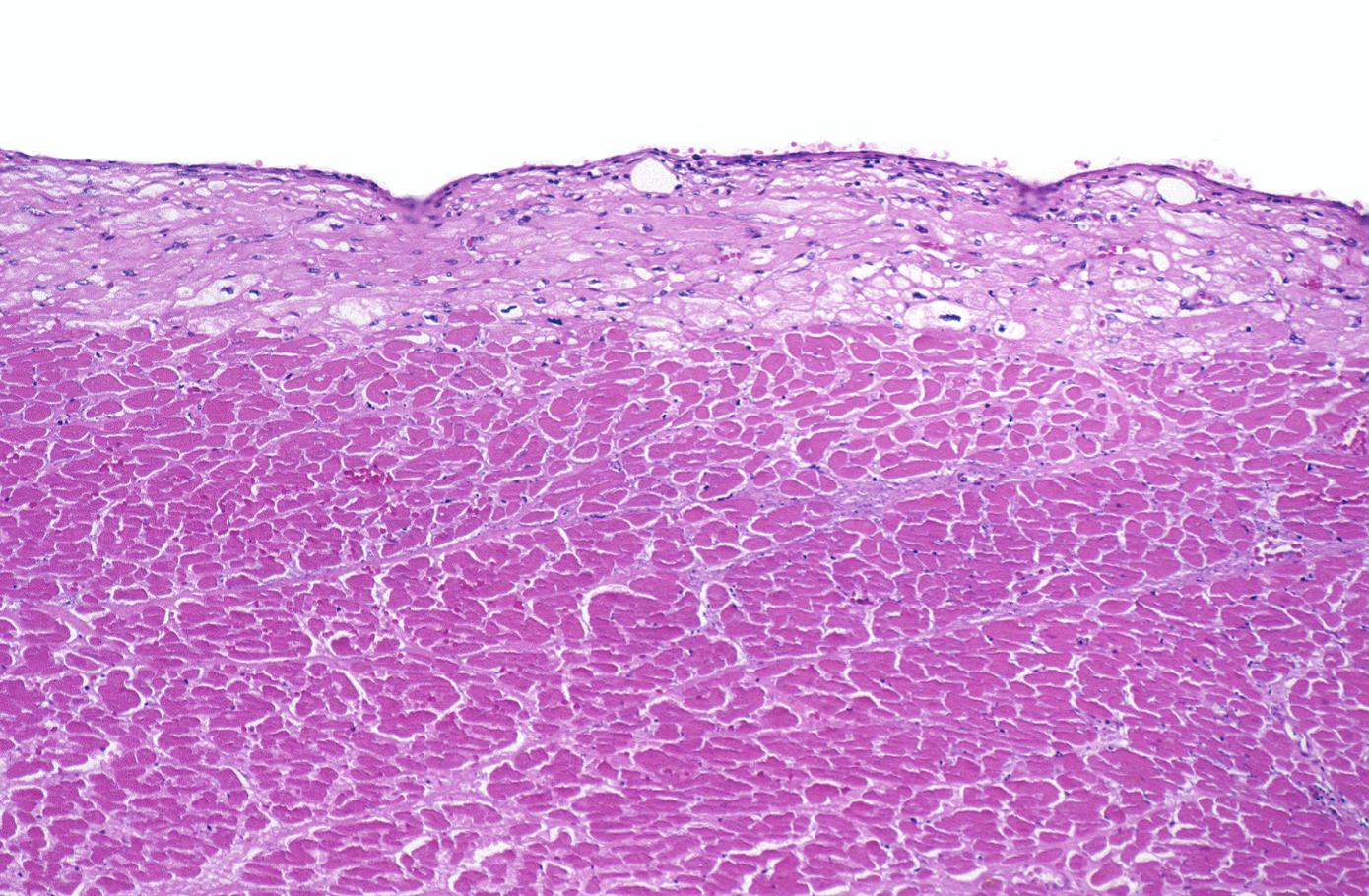
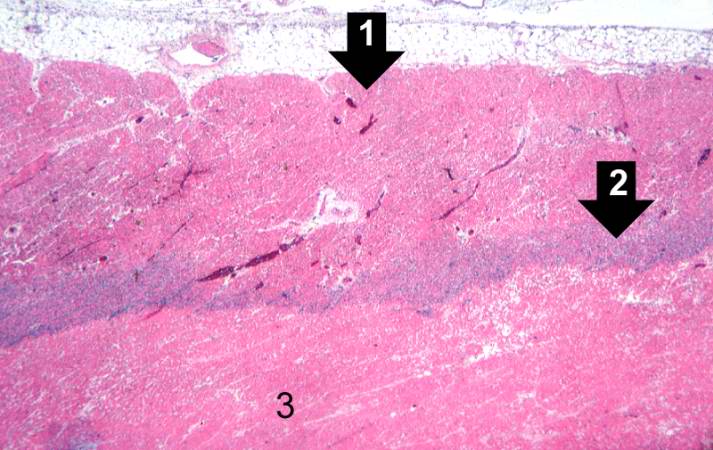
This is a low-power photomicrograph of the left ventricular free wall extending from the epicardium (1) to the endocardium (2). The area of infarction is the darker red (hypereosinophilic area) along the subendocardium (3).
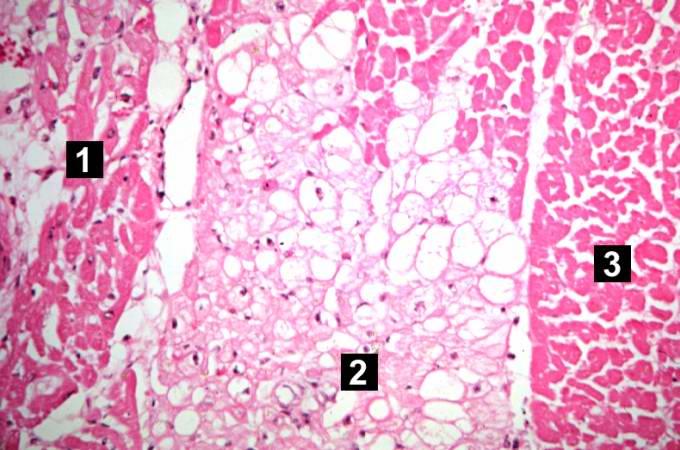
-
This higher-power photomicrograph shows endocardium on the right side of this image. Directly beneath the endocardium is a pale area consisting of cardiac myocytes exhibiting vacuolar degeneration (1). The area of infarction is visible as a hypereosinophilic area (2) and there is a second zone of vacuolated myocytes (3) between the infarct and the normal myocardium (4).
-
This high-power photomicrograph shows the area of infarction on the right (1). There is an area of vacuolated myocytes (2) adjacent to the infarcted myocytes and then normal cardiac muscle to the left (3).
-
This high-power photomicrograph shows the endocardium (1) and the area of subendocardial vacuolar degeneration (2). The area of infarction (3) contains some red blood cells.
-
This high-power photomicrograph demonstrates the border between the vacuolated subendocardial myocytes (1) and the infarcted myocytes (2).
-
This high-power photomicrograph contains normal myocytes (1), vacuolated myocytes (2), and infarcted myocytes (3).
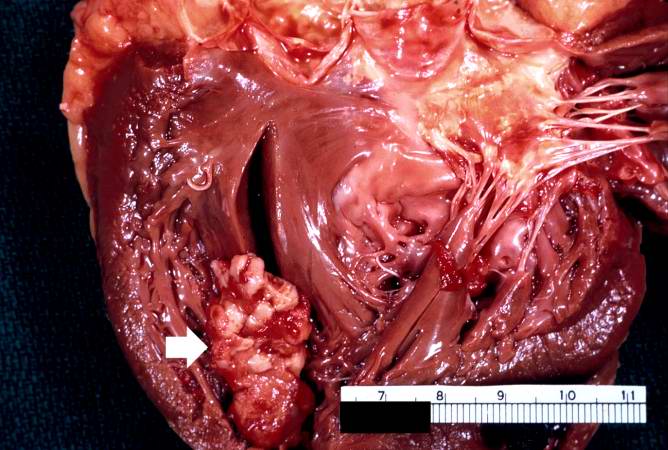
Case 5: Mural Thrombus
Clinical Summary
A 67-year-old female was transferred to the hospital from a nursing home in a comatose state.
Physical findings on examination were compatible with brain stem infarction.
On the fourth hospital day, an electrocardiogram revealed changes compatible with anterior myocardial infarction.
The patient remained comatose with quadriplegia and died on the 16th day of hospital stay.
Autopsy Findings
Examination of the brain revealed extensive infarction involving the midbrain and cerebellum with complete occlusion of the upper one-half of the basilar artery; there was also extensive coronary artery atherosclerosis.
A large aneurysm of the left ventricle was present; this was filled with mural thrombus.
Extensive infarction of the lateral and posterior portions of the left ventricular wall toward the base of the heart was also found.
Histopathological Findings
-
This is a gross photograph of the heart from this case demonstrating the well-formed thrombus (arrow) tightly attached to the myocardium near the apex of the left ventricle.
-
This is a low-power photomicrograph of the thrombus (1) attached to the myocardium (2).
-
This higher-power photomicrograph shows the border between the thrombus on the right (1) and the endocardium on the left (2). There is a line of inflammatory cells at this interface (arrow).
-
This is a high-power photomicrograph of the border zone between the thrombus (1) and the endocardium (2). In this region there is less inflammation at the border zone.
-
This photomicrograph illustrates the layered effect of the thrombus.
-
This is a higher-power photomicrograph of the thrombus. Note the pale regions which contain primarily platelets (degranulated platelets) with some fibrin (1), and the red areas which contain RBCs, some leukocytes, and fibrin(2).
-
This high-power photomicrograph of thrombus demonstrates more clearly the components of the layers--the pale regions which contain primarily platelets (degranulated platelets) with some fibrin (1), and the red areas which contain RBCs, some leukocytes, and fibrin (2).
Virtual Microscopic Images
References
- ↑ 1.0 1.1 Rubin's Pathology - Clinicopathological Foundations of Medicine. Maryland: Lippincott Williams & Wilkins. 2001. pp. p. 546. ISBN 0-7817-4733-3. Unknown parameter
|coauthors=ignored (help) - ↑ Eichbaum FW (1975). "'Wavy' myocardial fibers in spontaneous and experimental adrenergic cardiopathies". Cardiology. 60 (6): 358–65. PMID 782705.
- ↑ Bouchardy B, Majno G (1974). "Histopathology of early myocardial infarcts. A new approach". Am. J. Pathol. 74 (2): 301–30. PMC 1910768. PMID 4359735. Unknown parameter
|month=ignored (help) - ↑ S Roy. Myocardial infarction. Retrieved November 28, 2006.
- ↑ Schoen FJ. The heart. Chapter 12 in Robbins Pathologic Basis of Disease, fifth edition, 1994, Cotran RS, Kumar V, Schoen FJ, eds., Philadelphia, W.B.Saunders, pp.517-582
- ↑ 6.0 6.1 Fishbein MC, Maclean D, Maroko PR (1978). "The histopathologic evolution of myocardial infarction". Chest. 73 (6): 843–9. PMID 657859. Unknown parameter
|month=ignored (help) - ↑ Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.
- ↑ Reichenbach D, Cowan MJ. Healing of myocardial infarction with and without reperfusion. Chapter 5, in Cardiovascular Pathology, 1991, Virmani R, Atkinson JB, Fenoglio JJ, eds., Philadelphia, W.B.Saunders, pp. 86-98.
- ↑ Fishbein MC (1990). "Reperfusion injury". Clin Cardiol. 13 (3): 213–7. PMID 2182247. Unknown parameter
|month=ignored (help)
External links
- The MD TV: Comments on Hot Topics, State of the Art Presentations in Cardiovascular Medicine, Expert Reviews on Cardiovascular Research
- Clinical Trial Results: An up to dated resource of Cardiovascular Research
- Risk Assessment Tool for Estimating Your 10-year Risk of Having a Heart Attack - based on information of the Framingham Heart Study, from the United States National Heart, Lung and Blood Institute
- Heart Attack - overview of resources from MedlinePlus.
- Heart Attack Warning Signals from the Heart and Stroke Foundation of Canada
- Regional PCI for STEMI Resource Center - Evidence based online resource center for the development of regional PCI networks for acute STEMI
- STEMI Systems - Articles, profiles, and reviews of the latest publications involved in STEMI care. Quarterly newsletter.
- American College of Cardiology (ACC) Door to Balloon (D2B) Initiative.
- American Heart Association's Heart Attack web site - Information and resources for preventing, recognizing and treating heart attack.
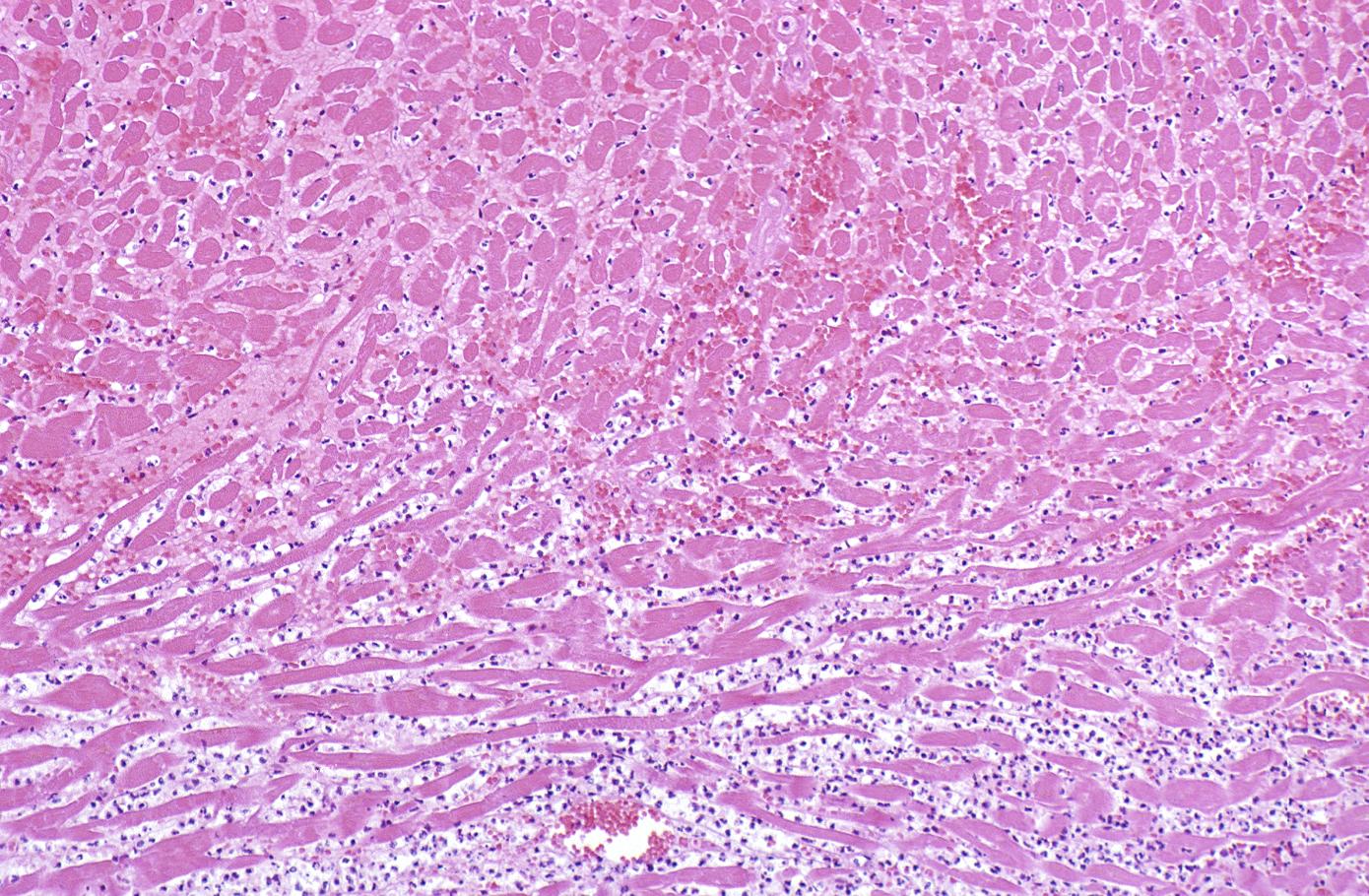

![This is a photomicrograph of the lines of Zahn. Pale areas (1) represent platelets with some fibrin and the darker lines (2) represent RBCs and ppleukocyte]]s enmeshed in fibrin strands.](/images/7/74/Coronary_thrombosis_case_2.7.jpg)