Ropinirole: Difference between revisions
Gerald Chi (talk | contribs) m (Changed protection level for "Ropinirole" ([Edit=Allow only autoconfirmed users] (expires 15:10, 18 June 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 15:10, 18 June 2014 (UTC)))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag= | ||
| | |||
| | {{VP}} | ||
| | |||
| | <!--Overview--> | ||
| | |||
| | |genericName= | ||
| | |||
| | |||
| | |||
|aOrAn= | |||
| | |||
| | a | ||
|drugClass= | |||
| | dopamine agonist | ||
|indication= | |||
}} | |||
parkinson’s disease (PD) and primary restless legs syndrome (RLS) | |||
|hasBlackBoxWarning= | |||
|adverseReactions= | |||
[[nausea]], [[somnolence]], [[dizziness]], [[syncope]], asthenic condition, viral infection, leg [[edema]], [[vomiting]], and [[dyspepsia]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Parkinson's Disease===== | |||
* Dosing Information | |||
:*The recommended starting dose for Parkinson’s disease is 0.25 mg three times daily. Based on individual patient therapeutic response and tolerability, if necessary, the dose should then be titrated with weekly increments as described in Table 1. After Week 4, if necessary, the daily dose may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly up to a maximum recommended total daily dose of 24 mg/day (8 mg three times daily). Doses greater than 24 mg/day have not been tested in clinical trials. | |||
T1 | |||
:*REQUIP should be discontinued gradually over a 7-day period in patients with Parkinson’s disease. The frequency of administration should be reduced from three times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of REQUIP. | |||
*Renal Impairment | |||
:*No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg three times a day. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of REQUIP in patients with severe renal impairment without regular dialysis has not been studied. | |||
=====Restless leg syndrome===== | |||
* Dosing Information | |||
:*The recommended adult starting dose for RLS is 0.25 mg once daily 1 to 3 hours before bedtime. After 2 days, if necessary, the dose can be increased to 0.5 mg once daily, and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 2 as needed to achieve efficacy. Titration should be based on individual patient therapeutic response and tolerability, up to a maximum recommended dose of 4 mg daily. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established. | |||
T2 | |||
:*In clinical trials of patients treated for RLS with doses up to 4 mg once daily, REQUIP was discontinued without a taper. | |||
*Renal Impairment | |||
:*No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg once daily. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 3 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of REQUIP in patients with severe renal impairment without regular dialysis has not been studied. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*REQUIP is contraindicated in patients known to have a [[hypersensitivity]]/[[allergic]] reaction (including [[urticaria]], [[angioedema]], [[rash]], [[pruritus]]) to ropinirole or to any of the excipients. | |||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
*Falling Asleep during Activities of Daily Living and Somnolence | |||
:*Patients treated with REQUIP have reported falling asleep while engaged in activities of daily living, including driving or operating machinery, which sometimes resulted in accidents. Although many of these patients reported somnolence while on REQUIP, some perceived that they had no warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some have reported these events more than 1 year after initiation of treatment. | |||
:*In controlled clinical trials, somnolence was commonly reported in patients receiving REQUIP and was more frequent in Parkinson's disease (up to 40% REQUIP, 6% placebo) than in Restless Legs Syndrome (12% REQUIP, 6% placebo)[see Adverse Reactions (6.1)]. | |||
:*It has been reported that falling asleep while engaged in activities of daily living usually occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. | |||
:*Before initiating treatment with REQUIP, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with REQUIP such as concomitant sedating medications, the presence of sleep disorders (other than RLS), and concomitant medications that increase ropinirole plasma levels (e.g., ciprofloxacin) [see Drug Interactions (7.1)]. If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., driving a motor vehicle, conversations, eating), REQUIP should ordinarily be discontinued [see Dosage and Administration (2.2, 2.3)]. If a decision is made to continue REQUIP, patients should be advised to not drive and to avoid other potentially dangerous activities. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living. | |||
*Syncope | |||
:*Syncope, sometimes associated with bradycardia, was observed in association with ropinirole in both patients with Parkinson’s disease and patients with RLS. In controlled clinical trials in patients with Parkinson’s disease, syncope was observed more frequently in patients receiving REQUIP than in patients receiving placebo (early Parkinson’s disease without L-dopa: REQUIP 12%, placebo 1%; advanced Parkinson’s disease: REQUIP 3%, placebo 2%). Syncope was reported in 1% of patients treated with REQUIP for RLS in 12-week, placebo-controlled clinical trials compared with 0.2% of patients treated with placebo[see Adverse Reactions (6.1)]. Most cases occurred more than 4 weeks after initiation of therapy with REQUIP, and were usually associated with a recent increase in dose. | |||
:*Because the trials of REQUIP excluded patients with significant cardiovascular disease, patients with significant cardiovascular disease should be treated with caution. | |||
:*Approximately 4% of patients with Parkinson’s disease enrolled in Phase 1 trials had syncope following a 1-mg dose of REQUIP. In two trials in patients with RLS that used a forced-titration regimen and orthostatic challenge with intensive blood pressure monitoring, 2% of RLS patients treated with REQUIP compared with 0% of patients receiving placebo reported syncope. | |||
:*In Phase 1 trials including healthy volunteers, the incidence of syncope was 2%. Of note, 1 subject with syncope developed hypotension, bradycardia, and sinus arrest; the subject recovered spontaneously without intervention. | |||
*Hypotension/Orthostatic Hypotension | |||
:*Dopamine agonists in clinical trials and clinical experience appear to impair the systemic regulation of blood pressure, with resulting orthostatic hypotension, especially during dose escalation. In addition, patients with Parkinson’s disease appear to have an impaired capacity to respond to a postural challenge. For these reasons, patients should be monitored for signs and symptoms of orthostatic hypotension, especially during dose escalation, and patients should be informed of the risk for syncope and hypotension [see Patient Counseling Information (17)]. | |||
:*Although the clinical trials were not designed to systematically monitor blood pressure, there were individual reported cases of orthostatic hypotension in early Parkinson’s disease (without L-dopa) in patients treated with REQUIP. Most of these cases occurred more than 4 weeks after initiation of therapy with REQUIP and were usually associated with a recent increase in dose. | |||
:*In 12-week, placebo-controlled trials of patients with RLS, the adverse event orthostatic hypotension was reported by 4 of 496 patients (0.8%) treated with REQUIP compared with 2 of 500 patients (0.4%) receiving placebo. | |||
:*In two Phase 2 studies in patients with RLS, 14 of 55 patients (25%) receiving REQUIP experienced an adverse event of hypotension or orthostatic hypotension compared with none of the 27 patients receiving placebo. In these studies, 11 of the 55 patients (20%) receiving REQUIP and 3 of the 26 patients (12%) who had post-dose blood pressure assessments following placebo, experienced an orthostatic blood pressure decrease of at least 40 mm Hg systolic and/or at least 20 mm Hg diastolic. | |||
:*In Phase 1 trials of REQUIP with healthy volunteers who received single doses on morethan one occasion without titration, 7% had documented symptomatic orthostatic hypotension. These episodes appeared mainly at doses above 0.8 mg and these doses are higher than the starting doses recommended for patients with either Parkinson’s disease or with RLS. In most of these individuals, the hypotension was accompanied by bradycardia but did not develop into syncope [see Warnings and Precautions (5.2)]. | |||
:*Although dizziness is not a specific manifestation of hypotension or orthostatic hypotension, patients with hypotension or orthostatic hypotension frequently reported dizziness. In controlled clinical trials, dizziness was a common adverse reaction in patients receiving REQUIP and was more frequent in patients with Parkinson’s disease or with RLS receiving REQUIP than in patients receiving placebo (early Parkinson’s disease without L-dopa: REQUIP 40%, placebo 22%; advanced Parkinson’s disease: REQUIP 26%, placebo 16%; RLS: REQUIP 11%, placebo 5%). Dizziness of sufficient severity to cause trial discontinuation of REQUIP was 4% in patients with early Parkinson’s disease without L-dopa, 3% in patients with advanced Parkinson’s disease, and 1% in patients with RLS. [See Adverse Reactions (6.1).] | |||
*Hallucinations/Psychotic-like Behavior | |||
:*In double-blind, placebo-controlled, early-therapy trials in patients with Parkinson’s disease who were not treated with L-dopa, 5.2% (8 of 157) of patients treated with REQUIP reported hallucinations, compared with 1.4% of patients on placebo (2 of 147). Among those patients receiving both REQUIP and L-dopa in advanced Parkinson’s disease studies, 10.1% (21 of 208) were reported to experience hallucinations, compared with 4.2% (5 of 120) of patients treated with placebo and L-dopa. | |||
:*The incidence of hallucination was increased in elderly patients (i.e., older than 65 years) treated with extended-release REQUIP [see Use in Specific Populations (8.5)]. | |||
:*Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with REQUIP or after starting or increasing the dose of REQUIP. Other drugs prescribed to improve the symptoms of Parkinson’s disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium. | |||
:*Patients with a major psychotic disorder should ordinarily not be treated with REQUIP because of the risk of exacerbating the psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of REQUIP [seeDrug Interactions (7.3)]. | |||
*Dyskinesia | |||
:*REQUIP may potentiate the dopaminergic side effects of L-dopa and may cause and/or exacerbate pre-existing dyskinesia in patients treated with L-dopa for Parkinson’s disease. In double-blind, placebo-controlled trials in advanced Parkinson’s disease, dyskinesia was much more common in patients treated with REQUIP than in those treated with placebo. Among those patients receiving both REQUIP and L-dopa in advanced Parkinson’s disease trials, 34% were reported to experience dyskinesia, compared with 13% of patients treated with placebo [see Adverse Reactions (6.1)].Decreasing the dose of the dopaminergic drug may ameliorate this adverse reaction. | |||
*Impulse Control/Compulsive Behaviors | |||
:*Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spendmoney, binge or compulsive eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including REQUIP, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease and RLS. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with REQUIP. Physiciansshould consider dose reduction or stopping the medication if a patient develops such urges while taking REQUIP. | |||
*Withdrawal-emergent Hyperpyrexia and Confusion | |||
:*A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy. Therefore, it is recommended that the dose be tapered at the end of treatment with REQUIP for Parkinson’s disease as a prophylactic measure [see Dosage and Administration (2.2)]. | |||
*Melanoma | |||
:*Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear. | |||
:*For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using REQUIP for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists). | |||
*Augmentation and Early-morning Rebound in Restless Legs Syndrome | |||
:*Reports in the literature indicate treatment of RLS with dopaminergic medications can result in recurrence of symptoms in the early morning hours, referred to as rebound. Augmentation has also been described during therapy for RLS. Augmentation refers to the earlier onset of symptoms in the evening (or even the afternoon), increase in symptoms, and spread of symptoms to involve other extremities. Rebound refers to new onset of symptoms in the early morning hours. Augmentation and/or early-morning rebound have been observed in a postmarketing trial. If augmentation or early-morning rebound occurs, the use of REQUIP should be reviewed and dosage adjustment or discontinuation of treatment should be considered. | |||
*Fibrotic Complications | |||
:*Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, pericarditis, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur. | |||
:*Although these adverse reactions are believed to be related to the ergoline structure of these compounds, whether other, non-ergot‑derived dopamine agonists such as ropinirole can cause them is unknown. | |||
:*Cases of possible fibrotic complications, including pleural effusion, pleural fibrosis, interstitial lung disease, and cardiac valvulopathy have been reported in the development program and postmarketing experience for ropinirole. While the evidence is not sufficient to establish a causal relationship between ropinirole and these fibrotic complications, a contribution of ropinirole cannot be excluded. | |||
*Retinal Pathology | |||
:*Retinal degeneration was observed in albino rats in the 2-year carcinogenicity study at all doses tested (equivalent to 0.6 to 20 times the maximum recommended human dose [MRHD] for Parkinson’s disease [24 mg/day] on a mg/m2 basis), but was statistically significant at the highest dose (50 mg/kg/day). Retinal degeneration was not observed in a 3-month study in pigmented rats, in a 2-year carcinogenicity study in albino mice, or in 1‑year studies in monkeys or albino rats. The significance of this effect for humans has not been established but involves disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding). | |||
:*Ocular electroretinogram (ERG) assessments were conducted during a 2-year, double-blind, multicenter, flexible dose, L-dopa-controlled clinical trial of ropinirole in patients with Parkinson’s disease; 156 patients (78 on ropinirole, mean dose: 11.9 mg/day, and 78 on L-dopa, mean dose: 555.2 mg/day) were evaluated for evidence of retinal dysfunction through electroretinograms. There was no clinically meaningful difference between the treatment groups in retinal function over the duration of the trial. | |||
*Binding to Melanin | |||
:*Ropinirole binds to melanin-containing tissues (i.e., eyes, skin) in pigmented rats. After a single dose, long-term retention of drug was demonstrated, with a half-life in the eye of 20 days | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* Drug | |||
:* Description | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intravenous | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
* Description | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Description | |||
====Management==== | |||
* Description | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[ | [[Category:Drug]] | ||
Revision as of 13:56, 20 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ropinirole is a dopamine agonist that is FDA approved for the {{{indicationType}}} of parkinson’s disease (PD) and primary restless legs syndrome (RLS). Common adverse reactions include nausea, somnolence, dizziness, syncope, asthenic condition, viral infection, leg edema, vomiting, and dyspepsia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Parkinson's Disease
- Dosing Information
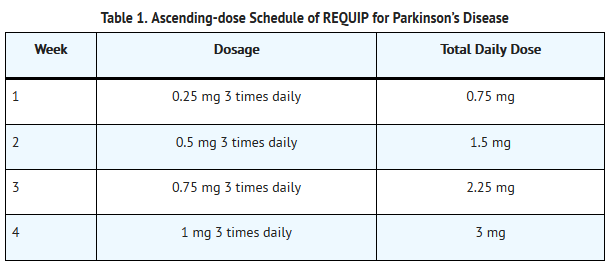
- The recommended starting dose for Parkinson’s disease is 0.25 mg three times daily. Based on individual patient therapeutic response and tolerability, if necessary, the dose should then be titrated with weekly increments as described in Table 1. After Week 4, if necessary, the daily dose may be increased by 1.5 mg/day on a weekly basis up to a dose of 9 mg/day, and then by up to 3 mg/day weekly up to a maximum recommended total daily dose of 24 mg/day (8 mg three times daily). Doses greater than 24 mg/day have not been tested in clinical trials.
T1
- REQUIP should be discontinued gradually over a 7-day period in patients with Parkinson’s disease. The frequency of administration should be reduced from three times daily to twice daily for 4 days. For the remaining 3 days, the frequency should be reduced to once daily prior to complete withdrawal of REQUIP.
- Renal Impairment
- No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg three times a day. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 18 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of REQUIP in patients with severe renal impairment without regular dialysis has not been studied.
Restless leg syndrome
- Dosing Information
- The recommended adult starting dose for RLS is 0.25 mg once daily 1 to 3 hours before bedtime. After 2 days, if necessary, the dose can be increased to 0.5 mg once daily, and to 1 mg once daily at the end of the first week of dosing, then as shown in Table 2 as needed to achieve efficacy. Titration should be based on individual patient therapeutic response and tolerability, up to a maximum recommended dose of 4 mg daily. For RLS, the safety and effectiveness of doses greater than 4 mg once daily have not been established.
T2
- In clinical trials of patients treated for RLS with doses up to 4 mg once daily, REQUIP was discontinued without a taper.
- Renal Impairment
- No dose adjustment is necessary in patients with moderate renal impairment (creatinine clearance of 30 to 50 mL/min). The recommended initial dose of ropinirole for patients with end-stage renal disease on hemodialysis is 0.25 mg once daily. Further dose escalations should be based on tolerability and need for efficacy. The recommended maximum total daily dose is 3 mg/day in patients receiving regular dialysis. Supplemental doses after dialysis are not required. The use of REQUIP in patients with severe renal impairment without regular dialysis has not been studied.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ropinirole in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ropinirole in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Ropinirole in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ropinirole in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ropinirole in pediatric patients.
Contraindications
- REQUIP is contraindicated in patients known to have a hypersensitivity/allergic reaction (including urticaria, angioedema, rash, pruritus) to ropinirole or to any of the excipients.
Warnings
Precautions
- Falling Asleep during Activities of Daily Living and Somnolence
- Patients treated with REQUIP have reported falling asleep while engaged in activities of daily living, including driving or operating machinery, which sometimes resulted in accidents. Although many of these patients reported somnolence while on REQUIP, some perceived that they had no warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some have reported these events more than 1 year after initiation of treatment.
- In controlled clinical trials, somnolence was commonly reported in patients receiving REQUIP and was more frequent in Parkinson's disease (up to 40% REQUIP, 6% placebo) than in Restless Legs Syndrome (12% REQUIP, 6% placebo)[see Adverse Reactions (6.1)].
- It has been reported that falling asleep while engaged in activities of daily living usually occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
- Before initiating treatment with REQUIP, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with REQUIP such as concomitant sedating medications, the presence of sleep disorders (other than RLS), and concomitant medications that increase ropinirole plasma levels (e.g., ciprofloxacin) [see Drug Interactions (7.1)]. If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., driving a motor vehicle, conversations, eating), REQUIP should ordinarily be discontinued [see Dosage and Administration (2.2, 2.3)]. If a decision is made to continue REQUIP, patients should be advised to not drive and to avoid other potentially dangerous activities. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
- Syncope
- Syncope, sometimes associated with bradycardia, was observed in association with ropinirole in both patients with Parkinson’s disease and patients with RLS. In controlled clinical trials in patients with Parkinson’s disease, syncope was observed more frequently in patients receiving REQUIP than in patients receiving placebo (early Parkinson’s disease without L-dopa: REQUIP 12%, placebo 1%; advanced Parkinson’s disease: REQUIP 3%, placebo 2%). Syncope was reported in 1% of patients treated with REQUIP for RLS in 12-week, placebo-controlled clinical trials compared with 0.2% of patients treated with placebo[see Adverse Reactions (6.1)]. Most cases occurred more than 4 weeks after initiation of therapy with REQUIP, and were usually associated with a recent increase in dose.
- Because the trials of REQUIP excluded patients with significant cardiovascular disease, patients with significant cardiovascular disease should be treated with caution.
- Approximately 4% of patients with Parkinson’s disease enrolled in Phase 1 trials had syncope following a 1-mg dose of REQUIP. In two trials in patients with RLS that used a forced-titration regimen and orthostatic challenge with intensive blood pressure monitoring, 2% of RLS patients treated with REQUIP compared with 0% of patients receiving placebo reported syncope.
- In Phase 1 trials including healthy volunteers, the incidence of syncope was 2%. Of note, 1 subject with syncope developed hypotension, bradycardia, and sinus arrest; the subject recovered spontaneously without intervention.
- Hypotension/Orthostatic Hypotension
- Dopamine agonists in clinical trials and clinical experience appear to impair the systemic regulation of blood pressure, with resulting orthostatic hypotension, especially during dose escalation. In addition, patients with Parkinson’s disease appear to have an impaired capacity to respond to a postural challenge. For these reasons, patients should be monitored for signs and symptoms of orthostatic hypotension, especially during dose escalation, and patients should be informed of the risk for syncope and hypotension [see Patient Counseling Information (17)].
- Although the clinical trials were not designed to systematically monitor blood pressure, there were individual reported cases of orthostatic hypotension in early Parkinson’s disease (without L-dopa) in patients treated with REQUIP. Most of these cases occurred more than 4 weeks after initiation of therapy with REQUIP and were usually associated with a recent increase in dose.
- In 12-week, placebo-controlled trials of patients with RLS, the adverse event orthostatic hypotension was reported by 4 of 496 patients (0.8%) treated with REQUIP compared with 2 of 500 patients (0.4%) receiving placebo.
- In two Phase 2 studies in patients with RLS, 14 of 55 patients (25%) receiving REQUIP experienced an adverse event of hypotension or orthostatic hypotension compared with none of the 27 patients receiving placebo. In these studies, 11 of the 55 patients (20%) receiving REQUIP and 3 of the 26 patients (12%) who had post-dose blood pressure assessments following placebo, experienced an orthostatic blood pressure decrease of at least 40 mm Hg systolic and/or at least 20 mm Hg diastolic.
- In Phase 1 trials of REQUIP with healthy volunteers who received single doses on morethan one occasion without titration, 7% had documented symptomatic orthostatic hypotension. These episodes appeared mainly at doses above 0.8 mg and these doses are higher than the starting doses recommended for patients with either Parkinson’s disease or with RLS. In most of these individuals, the hypotension was accompanied by bradycardia but did not develop into syncope [see Warnings and Precautions (5.2)].
- Although dizziness is not a specific manifestation of hypotension or orthostatic hypotension, patients with hypotension or orthostatic hypotension frequently reported dizziness. In controlled clinical trials, dizziness was a common adverse reaction in patients receiving REQUIP and was more frequent in patients with Parkinson’s disease or with RLS receiving REQUIP than in patients receiving placebo (early Parkinson’s disease without L-dopa: REQUIP 40%, placebo 22%; advanced Parkinson’s disease: REQUIP 26%, placebo 16%; RLS: REQUIP 11%, placebo 5%). Dizziness of sufficient severity to cause trial discontinuation of REQUIP was 4% in patients with early Parkinson’s disease without L-dopa, 3% in patients with advanced Parkinson’s disease, and 1% in patients with RLS. [See Adverse Reactions (6.1).]
- Hallucinations/Psychotic-like Behavior
- In double-blind, placebo-controlled, early-therapy trials in patients with Parkinson’s disease who were not treated with L-dopa, 5.2% (8 of 157) of patients treated with REQUIP reported hallucinations, compared with 1.4% of patients on placebo (2 of 147). Among those patients receiving both REQUIP and L-dopa in advanced Parkinson’s disease studies, 10.1% (21 of 208) were reported to experience hallucinations, compared with 4.2% (5 of 120) of patients treated with placebo and L-dopa.
- The incidence of hallucination was increased in elderly patients (i.e., older than 65 years) treated with extended-release REQUIP [see Use in Specific Populations (8.5)].
- Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with REQUIP or after starting or increasing the dose of REQUIP. Other drugs prescribed to improve the symptoms of Parkinson’s disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium.
- Patients with a major psychotic disorder should ordinarily not be treated with REQUIP because of the risk of exacerbating the psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson’s disease and may decrease the effectiveness of REQUIP [seeDrug Interactions (7.3)].
- Dyskinesia
- REQUIP may potentiate the dopaminergic side effects of L-dopa and may cause and/or exacerbate pre-existing dyskinesia in patients treated with L-dopa for Parkinson’s disease. In double-blind, placebo-controlled trials in advanced Parkinson’s disease, dyskinesia was much more common in patients treated with REQUIP than in those treated with placebo. Among those patients receiving both REQUIP and L-dopa in advanced Parkinson’s disease trials, 34% were reported to experience dyskinesia, compared with 13% of patients treated with placebo [see Adverse Reactions (6.1)].Decreasing the dose of the dopaminergic drug may ameliorate this adverse reaction.
- Impulse Control/Compulsive Behaviors
- Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spendmoney, binge or compulsive eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including REQUIP, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease and RLS. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with REQUIP. Physiciansshould consider dose reduction or stopping the medication if a patient develops such urges while taking REQUIP.
- Withdrawal-emergent Hyperpyrexia and Confusion
- A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy. Therefore, it is recommended that the dose be tapered at the end of treatment with REQUIP for Parkinson’s disease as a prophylactic measure [see Dosage and Administration (2.2)].
- Melanoma
- Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
- For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using REQUIP for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
- Augmentation and Early-morning Rebound in Restless Legs Syndrome
- Reports in the literature indicate treatment of RLS with dopaminergic medications can result in recurrence of symptoms in the early morning hours, referred to as rebound. Augmentation has also been described during therapy for RLS. Augmentation refers to the earlier onset of symptoms in the evening (or even the afternoon), increase in symptoms, and spread of symptoms to involve other extremities. Rebound refers to new onset of symptoms in the early morning hours. Augmentation and/or early-morning rebound have been observed in a postmarketing trial. If augmentation or early-morning rebound occurs, the use of REQUIP should be reviewed and dosage adjustment or discontinuation of treatment should be considered.
- Fibrotic Complications
- Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, pericarditis, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur.
- Although these adverse reactions are believed to be related to the ergoline structure of these compounds, whether other, non-ergot‑derived dopamine agonists such as ropinirole can cause them is unknown.
- Cases of possible fibrotic complications, including pleural effusion, pleural fibrosis, interstitial lung disease, and cardiac valvulopathy have been reported in the development program and postmarketing experience for ropinirole. While the evidence is not sufficient to establish a causal relationship between ropinirole and these fibrotic complications, a contribution of ropinirole cannot be excluded.
- Retinal Pathology
- Retinal degeneration was observed in albino rats in the 2-year carcinogenicity study at all doses tested (equivalent to 0.6 to 20 times the maximum recommended human dose [MRHD] for Parkinson’s disease [24 mg/day] on a mg/m2 basis), but was statistically significant at the highest dose (50 mg/kg/day). Retinal degeneration was not observed in a 3-month study in pigmented rats, in a 2-year carcinogenicity study in albino mice, or in 1‑year studies in monkeys or albino rats. The significance of this effect for humans has not been established but involves disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding).
- Ocular electroretinogram (ERG) assessments were conducted during a 2-year, double-blind, multicenter, flexible dose, L-dopa-controlled clinical trial of ropinirole in patients with Parkinson’s disease; 156 patients (78 on ropinirole, mean dose: 11.9 mg/day, and 78 on L-dopa, mean dose: 555.2 mg/day) were evaluated for evidence of retinal dysfunction through electroretinograms. There was no clinically meaningful difference between the treatment groups in retinal function over the duration of the trial.
- Binding to Melanin
- Ropinirole binds to melanin-containing tissues (i.e., eyes, skin) in pigmented rats. After a single dose, long-term retention of drug was demonstrated, with a half-life in the eye of 20 days
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Ropinirole in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ropinirole in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ropinirole in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ropinirole during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ropinirole with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Ropinirole with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Ropinirole with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Ropinirole with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ropinirole with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ropinirole in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ropinirole in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ropinirole in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ropinirole in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Ropinirole in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Ropinirole in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Ropinirole in the drug label.
Pharmacology
There is limited information regarding Ropinirole Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ropinirole in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ropinirole in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ropinirole in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ropinirole in the drug label.
How Supplied
Storage
There is limited information regarding Ropinirole Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ropinirole |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ropinirole |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Ropinirole in the drug label.
Precautions with Alcohol
- Alcohol-Ropinirole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ropinirole |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Ropinirole |Label Name=Ropinirole11.png
}}
{{#subobject:
|Label Page=Ropinirole |Label Name=Ropinirole11.png
}}