Reye's syndrome: Difference between revisions
No edit summary |
(→Causes) |
||
| (38 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
<div style="-webkit-user-select: none;"> | |||
{|class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right; | |||
|- | |||
| {{#ev:youtube|https://https://www.youtube.com/watch?v=Esgq0C2xjdY|350}} | |||
|- | |||
|} | |||
__NOTOC__ | |||
'''For patient information click [[{{PAGENAME}} (patient information)|here]]''' | '''For patient information click [[{{PAGENAME}} (patient information)|here]]''' | ||
{{Infobox_Disease | | {{Infobox_Disease | | ||
| Line 16: | Line 23: | ||
}} | }} | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} ; {{AE}} {{ADI}} | ||
==Overview== | ==Overview== | ||
| Line 22: | Line 29: | ||
==Historical Perspective== | ==Historical Perspective== | ||
The syndrome is named after Dr R. Douglas Reye, who, along with fellow Australians Dr. [[Graeme Morgan]] and Dr. [[Jim Baral]], published the first study of the syndrome in 1963 in the British medical journal called [[The Lancet]].<ref>{{cite journal|author=Reye RD, Morgan G, Baral J |title=Encephalopathy and fatty degeneration of the viscera. A Disease entity in childhood|journal=Lancet |volume=2 |issue= |pages=749-52 |year=1963 |pmid=14055046 |doi=}}</ref> In retrospect, the occurrence of the syndrome may have first been reported in 1929. Also in 1963, Dr. [[George Johnson]] and colleagues published an investigation of an outbreak of influenza B that described 16 children who developed neurological problems, four of whom had a remarkably similar profile to Reye’s syndrome. Some investigators refer to this disorder as Reye-Johnson syndrome, although it is more commonly called Reye's syndrome. During the late 1970s and early 1980s, studies in Ohio, Michigan and Arizona<ref name=Mortimer/>pointed to the use of aspirin during an upper respiratory tract or chicken pox infection as a possible trigger of the syndrome. Beginning in 1980, the CDC cautioned physicians and parents about the association between Reye’s syndrome and the use of salicylates in children and teenagers with chickenpox or virus-like illnesses. In 1982 the US Surgeon General issued an advisory and in 1986 the Food and Drug Administration required a Reye’s syndrome-related warning label for all aspirin-containing medications. | The syndrome is named after Dr R. Douglas Reye, who, along with fellow Australians Dr. [[Graeme Morgan]] and Dr. [[Jim Baral]], published the first study of the syndrome in 1963 in the British medical journal called [[The Lancet]].<ref>{{cite journal|author=Reye RD, Morgan G, Baral J |title=Encephalopathy and fatty degeneration of the viscera. A Disease entity in childhood|journal=Lancet |volume=2 |issue= |pages=749-52 |year=1963 |pmid=14055046 |doi=}}</ref> In retrospect, the occurrence of the syndrome may have first been reported in 1929. Also in 1963, Dr. [[George Johnson]] and colleagues published an investigation of an outbreak of influenza B that described 16 children who developed neurological problems, four of whom had a remarkably similar profile to Reye’s syndrome. Some investigators refer to this disorder as Reye-Johnson syndrome, although it is more commonly called Reye's syndrome. During the late 1970s and early 1980s, studies in Ohio, Michigan and Arizona<ref name="Mortimer" />pointed to the use of aspirin during an upper respiratory tract or chicken pox infection as a possible trigger of the syndrome. Beginning in 1980, the CDC cautioned physicians and parents about the association between Reye’s syndrome and the use of salicylates in children and teenagers with chickenpox or virus-like illnesses. In 1982 the US Surgeon General issued an advisory and in 1986 the Food and Drug Administration required a Reye’s syndrome-related warning label for all aspirin-containing medications. | ||
==Pathophysiology== | ==Pathophysiology== | ||
The disease causes [[hepatic steatosis|fatty liver]] with minimal inflammation, and severe [[encephalopathy]] (with swelling of the brain). The [[liver]] may become slightly enlarged and firm, and there is a change in the appearance of the [[kidney]]s. [[Jaundice]] is not usually present.<ref name="Suchy">{{cite book |last=Suchy| first= FJ, el al.| last2 =Sokol | first2 = RJ| last3 =Balistreri | first3 = WF|chapter= |title=Liver Disease in Children |publisher=[[Cambridge University Press]]| location=Cambridge |year=2007|pages=| isbn=0-5218-5657-4}}</ref> | The disease causes [[hepatic steatosis|fatty liver]] with minimal inflammation, and severe [[encephalopathy]] (with swelling of the brain). The [[liver]] may become slightly enlarged and firm, and there is a change in the appearance of the [[kidney]]s. [[Jaundice]] is not usually present.<ref name="Suchy">{{cite book |last=Suchy| first= FJ, el al.| last2 =Sokol | first2 = RJ| last3 =Balistreri | first3 = WF|chapter= |title=Liver Disease in Children |publisher=[[Cambridge University Press]]| location=Cambridge |year=2007|pages=| isbn=0-5218-5657-4}}</ref> | ||
The precise mechanism by which Reye's syndrome occurs remains unknown. However, the major form of Reye’s syndrome reported in the United States is characteristically preceded by a viral-like flu illness or chickenpox. Many studies have demonstrated a strong association between aspirin taken for these viral illnesses and the development of Reye’s syndrome. | |||
The precise mechanism by which Reye's syndrome occurs remains unknown. | |||
The serious symptoms of Reye's syndrome appear to result from damage to cellular [[mitochondria]], at least in the liver, and there are a number of ways that aspirin could cause or exacerbate mitochondrial damage. An increased risk of contracting Reye's syndrome is one of the main reasons that aspirin has not been recommended for use in children and teenagers, the age group for which the risk of lasting serious effects is highest. | |||
===Microscopic Pathology=== | |||
Diffuse panlobular steatosis and microvesicular fatty droplets with central nuclei are consistent with typical Reye's syndrome. Few patients may have multifocal spotty necrosis or centrilobular necrosis. | |||
[[Image:Reye's syndrome liver-histology.jpg|thumb|center|300px| Histopathology of liver in a child with Reye's syndrome. Hepatocytes are pale-staining due to intracellular fat droplets]] | |||
==Causes== | |||
At least five epidemiologic studies published in US medical journals,<ref name="Mortimer">{{cite journal |author=Mortimer EA |title=Reye's syndrome, salicylates, epidemiology, and public health policy |journal=JAMA |volume=257 |issue=14 |pages=941 |year=1987 |pmid=3820516 |doi=}}</ref> including one study that was supported by funds from the [[aspirin]] industry,<ref>{{cite journal |author=Forsyth BW, Horwitz RI, Acampora D, ''et al'' |title=New epidemiologic evidence confirming that bias does not explain the aspirin/Reye's syndrome association |journal=JAMA |volume=261 |issue=17 |pages=2517-24 |year=1989 |pmid=2704111 |doi=}}</ref> have confirmed an association between the development of Reye's syndrome and the use of [[aspirin]] (a [[salicylate]] compound) for treating the symptoms of [[influenza]]-like illnesses or [[chicken pox]].<ref name="Mortimer" /> | |||
==Differentiating Reye syndrome from other Diseases== | ==Differentiating Reye syndrome from other Diseases== | ||
Reye's is often mistaken for diagnosis of other conditions due to the symptoms of [[encephalopathy]]. | |||
* Various inborn [[metabolic disorders]] | * Various inborn [[metabolic disorders]] - specific metabolic tests are done and any co-morbidity will help in differentiating from Reye's syndrome. | ||
* [[Encephalitis|Viral encephalitis]] | * [[Encephalitis|Viral encephalitis]] - presence of meningeal signs and symptoms may exclude the diagnosis. | ||
* [[Drug overdose]] or [[Catalyst poisoning|poisoning]] | * [[Drug overdose]] or [[Catalyst poisoning|poisoning]] - careful and detailed history helps in identifying the drug or poison. | ||
* [[Head trauma]] | * [[Head trauma]] - history of trauma and abrasion will provide clue to diagnosis. | ||
* [[Hepatic failure]] due to other causes | * [[Hepatic failure]] due to other causes - associated co-morbid conditions. | ||
* [[Meningitis]] | * [[Meningitis]] - presence of [[meningeal signs]] will exclude the diagnosis | ||
* [[Renal failure]] | * [[Renal failure]] - symptoms like increase in [[blood pressure]], [[electrolyte abnormalities]], [[pedal edema]] help in the diagnosis. | ||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
In 1980, after CDC began cautioning physicians and parents about the association between Reye’s syndrome and the use of salicylates in children with chickenpox or virus-like illnesses, the incidence of Reye's syndrome in the United States began to decline. In the United States between 1980 and 1997, the number of reported cases of Reye’s syndrome decreased from 555 cases in 1980 to about 2 cases per year since 1994. During this time period 93% of reported cases for which racial data were available occurred in whites and the median age was six years. A viral illness occurred in 93% of cases in the preceding three week period. For the period 1991-1994, the annual rate of hospitalizations due to Reye’s syndrome in the US was estimated to be between 0.2 and 1.1 per million population less than 18 years of age. | In 1980, after CDC began cautioning physicians and parents about the association between Reye’s syndrome and the use of salicylates in children with chickenpox or virus-like illnesses, the incidence of Reye's syndrome in the United States began to decline. In the United States between 1980 and 1997, the number of reported cases of Reye’s syndrome decreased from 555 cases in 1980 to about 2 cases per year since 1994. During this time period 93% of reported cases for which racial data were available occurred in whites and the median age was six years. A viral illness occurred in 93% of cases in the preceding three week period. For the period 1991-1994, the annual rate of hospitalizations due to Reye’s syndrome in the US was estimated to be between 0.2 and 1.1 per million population less than 18 years of age. | ||
| Line 59: | Line 63: | ||
From November 1995 to November 1996 in France, a national survey of pediatric departments for children under 15 years of age with unexplained [[encephalopathy]] and a three fold (or greater) increase in serum aminotransferase and/or ammonia led to the identification of nine definite cases of Reye’s syndrome (0.79 cases per million children). Eight of the nine children with Reye’s syndrome were found to have been exposed to aspirin. In part because of this survey result, the French Medicines Agency reinforced the international attention to the relationship between aspirin and Reye’s syndrome by issuing its own public and professional warnings about this relationship.<ref>{{cite journal |author=Autret-Leca E, Jonville-Béra AP, Llau ME, ''et al''|title=Incidence of Reye's syndrome in France: a hospital-based survey |journal=Journal of clinical epidemiology |volume=54|issue=8 |pages=857-62 |year=2001 |pmid=11470397 |doi=}}</ref> | From November 1995 to November 1996 in France, a national survey of pediatric departments for children under 15 years of age with unexplained [[encephalopathy]] and a three fold (or greater) increase in serum aminotransferase and/or ammonia led to the identification of nine definite cases of Reye’s syndrome (0.79 cases per million children). Eight of the nine children with Reye’s syndrome were found to have been exposed to aspirin. In part because of this survey result, the French Medicines Agency reinforced the international attention to the relationship between aspirin and Reye’s syndrome by issuing its own public and professional warnings about this relationship.<ref>{{cite journal |author=Autret-Leca E, Jonville-Béra AP, Llau ME, ''et al''|title=Incidence of Reye's syndrome in France: a hospital-based survey |journal=Journal of clinical epidemiology |volume=54|issue=8 |pages=857-62 |year=2001 |pmid=11470397 |doi=}}</ref> | ||
Some studies indicate that a significant percentage of cases, particularly in very young children, are later re-categorized as other disorders or conditions -- as high as 25% in the UK and 50% in Australia. These re-categorized disorders, unlike the characteristic Reye’s syndrome, are not strongly linked to exposure to [[aspirin]]. | |||
===Age=== | |||
* Reye’s syndrome occurs almost exclusively in children although it has been reported to occur in adults. | |||
* However, adults do not appear to be as vulnerable to permanent neural or [[liver damage]]. | |||
* The surveillance for Reye’s syndrome in the US is focused on patients under 18 years of age. | |||
==Risk Factors== | |||
* Reye's syndrome is most often seen in children ages 4 - 12. | |||
* A few infections like [[chicken pox]], [[influenza]] and other upper respiratory tract infections. | |||
==Natural History, Complications and Prognosis== | ==Natural History, Complications and Prognosis== | ||
Reye's syndrome progresses through five stages, explained below: | Reye's syndrome progresses through five stages, explained below: | ||
* Stage I | * Stage I | ||
** Persistent, heavy [[vomiting]] that is not relieved by eating | ** Persistent, heavy [[vomiting]] that is not relieved by eating | ||
| Line 71: | Line 85: | ||
* Stage II | * Stage II | ||
** [[Stupor]] caused by minor [[brain]] inflammation | ** [[Stupor]] caused by minor [[brain]] inflammation | ||
** Hyperventilation | ** [[Hyperventilation]] | ||
** [[Fatty liver]] (found by [[biopsy]]) | ** [[Fatty liver]] (found by [[biopsy]]) | ||
** [[Hyperreflexia|Hyperactive reflexes]] | ** [[Hyperreflexia|Hyperactive reflexes]] | ||
| Line 78: | Line 92: | ||
** Possible [[coma]] | ** Possible [[coma]] | ||
** Possible cerebral [[edema]] | ** Possible cerebral [[edema]] | ||
** Rarely, respiratory arrest | ** Rarely, [[respiratory arrest]] | ||
* Stage IV | * Stage IV | ||
** Deepening coma | ** Deepening [[coma]] | ||
** Large pupils with minimal response to light | ** [[Large pupils]] with minimal response to light | ||
** Minimal but still present [[hepatic]] dysfunction | ** Minimal but still present [[hepatic]] dysfunction | ||
* Stage V | * Stage V | ||
** Very rapid onset following stage IV | ** Very rapid onset following stage IV | ||
** Deep coma | ** Deep [[coma]] | ||
** [[Seizures]] | ** [[Seizures]] | ||
** Respiratory failure | ** [[Respiratory failure]] | ||
** [[Flaccidity]] | ** [[Flaccidity]] | ||
** Extremely high blood [[ammonia]] (above 300mg per 100mL of blood | ** Extremely high blood [[ammonia]] (above 300mg per 100mL of blood | ||
** Death | ** Death | ||
| Line 95: | Line 109: | ||
Documented cases of Reye’s syndrome in adults have only been very rarely reported. The recovery of adults with the syndrome is generally complete, with liver and brain function returning to normal within two weeks of the illness. In children however, mild to severe permanent brain damage is possible, especially in infants. Over thirty percent of the cases reported in the United States from 1981 through 1997 died. Early diagnosis is vital, otherwise death or severe brain damage may follow. | Documented cases of Reye’s syndrome in adults have only been very rarely reported. The recovery of adults with the syndrome is generally complete, with liver and brain function returning to normal within two weeks of the illness. In children however, mild to severe permanent brain damage is possible, especially in infants. Over thirty percent of the cases reported in the United States from 1981 through 1997 died. Early diagnosis is vital, otherwise death or severe brain damage may follow. | ||
==Diagnosis== | |||
===History=== | |||
A directed history should be obtained to ascertain the duration of [[infection]], use of [[aspirin]] and any other associated co-morbid conditions ([[diarrhea]] or [[vomiting]]). | |||
===Symptoms=== | |||
* [[Lethargy]] | |||
* [[Diarrhea]] with or without [[vomiting]] | |||
* [[Nightmare]]s | |||
* [[Confusion]] | |||
* [[Seizures]] | |||
===Physical Examination=== | |||
====Appearance of the patient==== | |||
* The child may be [[lethargic]] or in a unusual posture([[Decerebrate rigidity]]) | |||
====Vital Signs==== | |||
* [[Temperature]] - may be increased due to infection | |||
* [[Blood pressure]] - may be low due to [[volume loss]] from [[diarrhea]] or [[vomiting]] | |||
* [[Pulse]] - may be in high rate and low in volume | |||
====Skin==== | |||
* Loss of [[skin turgor]] may be seen in [[dehydration]] | |||
====Eyes==== | |||
* Staring gaze may be seen | |||
* [[Double vision]] may be present | |||
====Lungs==== | |||
* [[Hyperventilation]] may be seen | |||
====Extremities==== | |||
* [[Extensor spasm]]s may be seen | |||
====Neurologic==== | |||
All these findings may not be seen in the patient but if present they are significant | |||
* [[Stupor]] | |||
* [[Delirium]] | |||
* [[Decerebrate rigidity]] | |||
* [[Hearing loss]] | |||
* [[Speech difficulties]] | |||
* [[Flaccidity]] / [[weakness]] of arms or legs | |||
* [[Seizures]] | |||
===Laboratory Findings=== | |||
====Blood Studies==== | |||
* [[Complete blood count]] and differential count to establish infectious cause. | |||
* [[ESR]] is elevated as a result of infection. | |||
* [[Arterial blood gas]] analysis to monitor acid base status. | |||
====Electrolyte and Biomarker Studies==== | |||
* [[Liver enzymes]] are elevated due to damage to liver | |||
* [[Hyperammonemia|Blood ammonia level is elevated]]. | |||
* [[Hypoglycemia]] may be noticed. | |||
====CT and MRI Scans==== | |||
* To differentiate from other causes of [[encephalopathy]]. | |||
====Biopsy==== | |||
* Liver biopsy identifies the damage at microscopic level. Diffuse panlobular [[steatosis]] and microvesicular fatty droplets with central nuclei are consistent with typical Reye's syndrome. A few patients may have multifocal spotty necrosis or centrilobular necrosis also. | |||
====Other Diagnostic Studies==== | |||
* [[Lumbar puncture]] and [[CSF]] analysis helps in differentiating from [[encephalitis]] and [[meningitis]]. | |||
==Treatment== | |||
* No specific therapy exists. | |||
* 10% glucose to correct [[hypoglycemia]]. | |||
* Maintenance of [[airway]] and [[circulation]] with adequate oxygen. | |||
* [[Steroids]] to decrease any brain [[edema]]. | |||
===Prevention=== | |||
* Avoid or reduce the use of [[aspirin]] in the children with the aforementioned infectious diseases. | |||
== References == | == References == | ||
{{reflist|2}} | {{reflist|2}} | ||
== External links == | == External links == | ||
* [http://www.reyessyndrome.org/ National Reye's Syndrome Foundation] | * [http://www.reyessyndrome.org/ National Reye's Syndrome Foundation] | ||
{{CNS diseases of the nervous system}} | {{CNS diseases of the nervous system}} | ||
| Line 128: | Line 187: | ||
[[Category:Syndromes]] | [[Category:Syndromes]] | ||
[[Category:Mature chapter]] | [[Category:Mature chapter]] | ||
[[Category:Disease]] | |||
[[de:Reye-Syndrom]] | [[de:Reye-Syndrom]] | ||
Latest revision as of 14:37, 4 October 2019
| https://https://www.youtube.com/watch?v=Esgq0C2xjdY%7C350}} |
For patient information click here
| Reye's syndrome | |
| ICD-10 | G93.7 |
|---|---|
| ICD-9 | 331.81 |
| DiseasesDB | 11463 |
| MedlinePlus | 001565 |
|
WikiDoc Resources for Reye's syndrome |
|
Articles |
|---|
|
Most recent articles on Reye's syndrome Most cited articles on Reye's syndrome |
|
Media |
|
Powerpoint slides on Reye's syndrome |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Reye's syndrome at Clinical Trials.gov Trial results on Reye's syndrome Clinical Trials on Reye's syndrome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Reye's syndrome NICE Guidance on Reye's syndrome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Reye's syndrome Discussion groups on Reye's syndrome Patient Handouts on Reye's syndrome Directions to Hospitals Treating Reye's syndrome Risk calculators and risk factors for Reye's syndrome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Reye's syndrome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] ; Associate Editor(s)-in-Chief: Aditya Govindavarjhulla, M.B.B.S. [2]
Overview
Reye's syndrome is a potentially fatal disease that causes numerous detrimental effects to many organs, especially the brain and liver. It is associated with aspirin consumption by children with viral diseases such as chicken pox.
Historical Perspective
The syndrome is named after Dr R. Douglas Reye, who, along with fellow Australians Dr. Graeme Morgan and Dr. Jim Baral, published the first study of the syndrome in 1963 in the British medical journal called The Lancet.[1] In retrospect, the occurrence of the syndrome may have first been reported in 1929. Also in 1963, Dr. George Johnson and colleagues published an investigation of an outbreak of influenza B that described 16 children who developed neurological problems, four of whom had a remarkably similar profile to Reye’s syndrome. Some investigators refer to this disorder as Reye-Johnson syndrome, although it is more commonly called Reye's syndrome. During the late 1970s and early 1980s, studies in Ohio, Michigan and Arizona[2]pointed to the use of aspirin during an upper respiratory tract or chicken pox infection as a possible trigger of the syndrome. Beginning in 1980, the CDC cautioned physicians and parents about the association between Reye’s syndrome and the use of salicylates in children and teenagers with chickenpox or virus-like illnesses. In 1982 the US Surgeon General issued an advisory and in 1986 the Food and Drug Administration required a Reye’s syndrome-related warning label for all aspirin-containing medications.
Pathophysiology
The disease causes fatty liver with minimal inflammation, and severe encephalopathy (with swelling of the brain). The liver may become slightly enlarged and firm, and there is a change in the appearance of the kidneys. Jaundice is not usually present.[3]
The precise mechanism by which Reye's syndrome occurs remains unknown. However, the major form of Reye’s syndrome reported in the United States is characteristically preceded by a viral-like flu illness or chickenpox. Many studies have demonstrated a strong association between aspirin taken for these viral illnesses and the development of Reye’s syndrome.
The serious symptoms of Reye's syndrome appear to result from damage to cellular mitochondria, at least in the liver, and there are a number of ways that aspirin could cause or exacerbate mitochondrial damage. An increased risk of contracting Reye's syndrome is one of the main reasons that aspirin has not been recommended for use in children and teenagers, the age group for which the risk of lasting serious effects is highest.
Microscopic Pathology
Diffuse panlobular steatosis and microvesicular fatty droplets with central nuclei are consistent with typical Reye's syndrome. Few patients may have multifocal spotty necrosis or centrilobular necrosis.
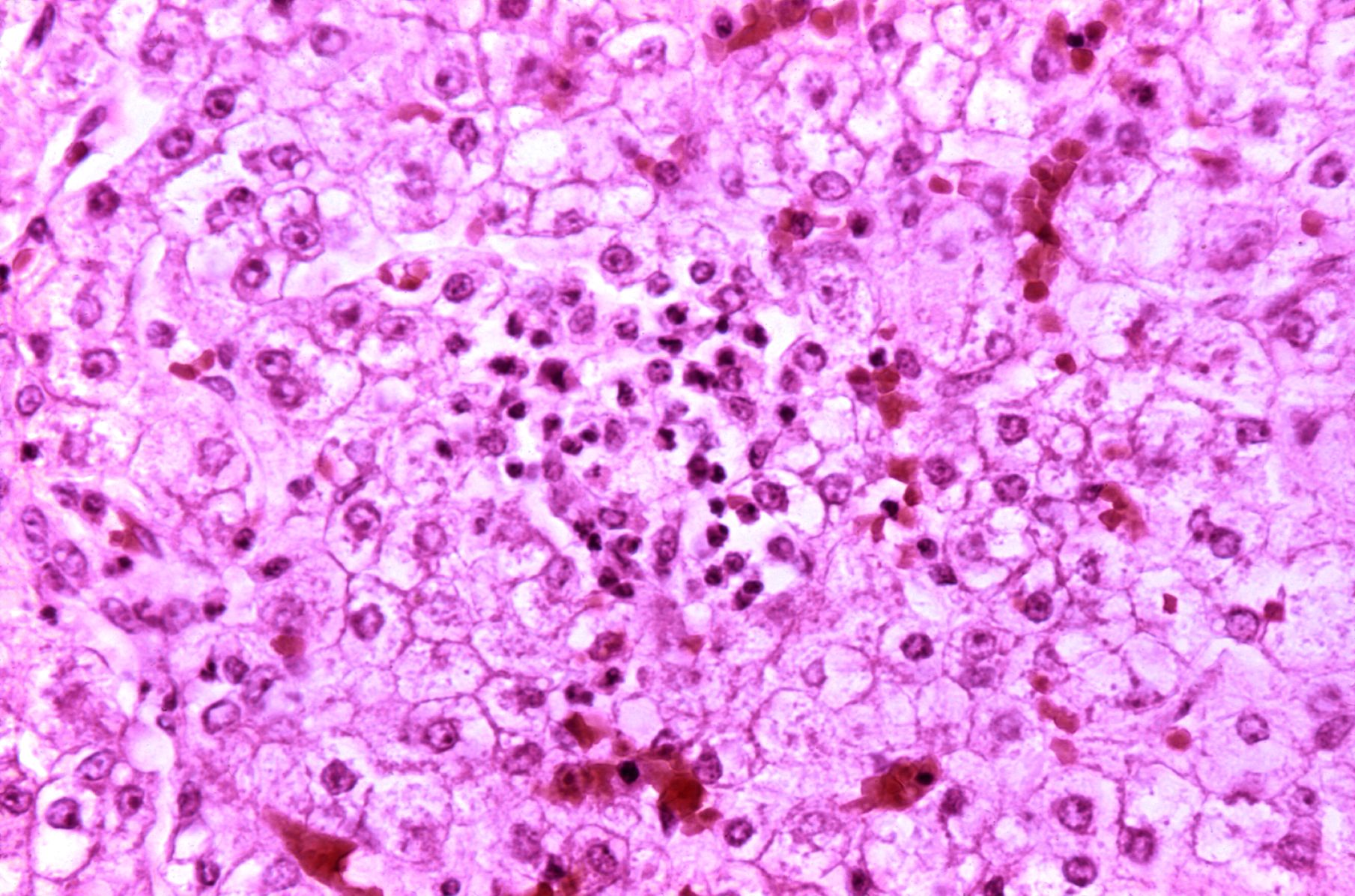
Causes
At least five epidemiologic studies published in US medical journals,[2] including one study that was supported by funds from the aspirin industry,[4] have confirmed an association between the development of Reye's syndrome and the use of aspirin (a salicylate compound) for treating the symptoms of influenza-like illnesses or chicken pox.[2]
Differentiating Reye syndrome from other Diseases
Reye's is often mistaken for diagnosis of other conditions due to the symptoms of encephalopathy.
- Various inborn metabolic disorders - specific metabolic tests are done and any co-morbidity will help in differentiating from Reye's syndrome.
- Viral encephalitis - presence of meningeal signs and symptoms may exclude the diagnosis.
- Drug overdose or poisoning - careful and detailed history helps in identifying the drug or poison.
- Head trauma - history of trauma and abrasion will provide clue to diagnosis.
- Hepatic failure due to other causes - associated co-morbid conditions.
- Meningitis - presence of meningeal signs will exclude the diagnosis
- Renal failure - symptoms like increase in blood pressure, electrolyte abnormalities, pedal edema help in the diagnosis.
Epidemiology and Demographics
In 1980, after CDC began cautioning physicians and parents about the association between Reye’s syndrome and the use of salicylates in children with chickenpox or virus-like illnesses, the incidence of Reye's syndrome in the United States began to decline. In the United States between 1980 and 1997, the number of reported cases of Reye’s syndrome decreased from 555 cases in 1980 to about 2 cases per year since 1994. During this time period 93% of reported cases for which racial data were available occurred in whites and the median age was six years. A viral illness occurred in 93% of cases in the preceding three week period. For the period 1991-1994, the annual rate of hospitalizations due to Reye’s syndrome in the US was estimated to be between 0.2 and 1.1 per million population less than 18 years of age.
During the 1980s, a case-control study carried out in the United Kingdom also demonstrated an associate between Reye’s syndrome and aspirin exposure.[5] In June 1986, the United Kingdom Committee on Safety of Medicines issued warnings against the use of aspirin in children under 12 years of age and warning labels on aspirin-containing medications were introduced. UK surveillance for Reye’s syndrome documented a decline in the incidence of Reye’s syndrome following 1986. The reported incidence rate of Reye’s syndrome decreased from a high of 0.63 per 100,000 population less than 12 years of age in 1983/84 to 0.11 in 1990/91.
From November 1995 to November 1996 in France, a national survey of pediatric departments for children under 15 years of age with unexplained encephalopathy and a three fold (or greater) increase in serum aminotransferase and/or ammonia led to the identification of nine definite cases of Reye’s syndrome (0.79 cases per million children). Eight of the nine children with Reye’s syndrome were found to have been exposed to aspirin. In part because of this survey result, the French Medicines Agency reinforced the international attention to the relationship between aspirin and Reye’s syndrome by issuing its own public and professional warnings about this relationship.[6]
Some studies indicate that a significant percentage of cases, particularly in very young children, are later re-categorized as other disorders or conditions -- as high as 25% in the UK and 50% in Australia. These re-categorized disorders, unlike the characteristic Reye’s syndrome, are not strongly linked to exposure to aspirin.
Age
- Reye’s syndrome occurs almost exclusively in children although it has been reported to occur in adults.
- However, adults do not appear to be as vulnerable to permanent neural or liver damage.
- The surveillance for Reye’s syndrome in the US is focused on patients under 18 years of age.
Risk Factors
- Reye's syndrome is most often seen in children ages 4 - 12.
- A few infections like chicken pox, influenza and other upper respiratory tract infections.
Natural History, Complications and Prognosis
Reye's syndrome progresses through five stages, explained below:
- Stage I
- Persistent, heavy vomiting that is not relieved by eating
- Generalized lethargy
- General mental symptoms, e.g. confusion
- Nightmares
- Stage II
- Stupor caused by minor brain inflammation
- Hyperventilation
- Fatty liver (found by biopsy)
- Hyperactive reflexes
- Stage III
- Continuation of Stage I and II symptoms
- Possible coma
- Possible cerebral edema
- Rarely, respiratory arrest
- Stage IV
- Deepening coma
- Large pupils with minimal response to light
- Minimal but still present hepatic dysfunction
- Stage V
- Very rapid onset following stage IV
- Deep coma
- Seizures
- Respiratory failure
- Flaccidity
- Extremely high blood ammonia (above 300mg per 100mL of blood
- Death
Prognosis
Documented cases of Reye’s syndrome in adults have only been very rarely reported. The recovery of adults with the syndrome is generally complete, with liver and brain function returning to normal within two weeks of the illness. In children however, mild to severe permanent brain damage is possible, especially in infants. Over thirty percent of the cases reported in the United States from 1981 through 1997 died. Early diagnosis is vital, otherwise death or severe brain damage may follow.
Diagnosis
History
A directed history should be obtained to ascertain the duration of infection, use of aspirin and any other associated co-morbid conditions (diarrhea or vomiting).
Symptoms
Physical Examination
Appearance of the patient
- The child may be lethargic or in a unusual posture(Decerebrate rigidity)
Vital Signs
- Temperature - may be increased due to infection
- Blood pressure - may be low due to volume loss from diarrhea or vomiting
- Pulse - may be in high rate and low in volume
Skin
- Loss of skin turgor may be seen in dehydration
Eyes
- Staring gaze may be seen
- Double vision may be present
Lungs
- Hyperventilation may be seen
Extremities
- Extensor spasms may be seen
Neurologic
All these findings may not be seen in the patient but if present they are significant
- Stupor
- Delirium
- Decerebrate rigidity
- Hearing loss
- Speech difficulties
- Flaccidity / weakness of arms or legs
- Seizures
Laboratory Findings
Blood Studies
- Complete blood count and differential count to establish infectious cause.
- ESR is elevated as a result of infection.
- Arterial blood gas analysis to monitor acid base status.
Electrolyte and Biomarker Studies
- Liver enzymes are elevated due to damage to liver
- Blood ammonia level is elevated.
- Hypoglycemia may be noticed.
CT and MRI Scans
- To differentiate from other causes of encephalopathy.
Biopsy
- Liver biopsy identifies the damage at microscopic level. Diffuse panlobular steatosis and microvesicular fatty droplets with central nuclei are consistent with typical Reye's syndrome. A few patients may have multifocal spotty necrosis or centrilobular necrosis also.
Other Diagnostic Studies
- Lumbar puncture and CSF analysis helps in differentiating from encephalitis and meningitis.
Treatment
- No specific therapy exists.
- 10% glucose to correct hypoglycemia.
- Maintenance of airway and circulation with adequate oxygen.
- Steroids to decrease any brain edema.
Prevention
- Avoid or reduce the use of aspirin in the children with the aforementioned infectious diseases.
References
- ↑ Reye RD, Morgan G, Baral J (1963). "Encephalopathy and fatty degeneration of the viscera. A Disease entity in childhood". Lancet. 2: 749–52. PMID 14055046.
- ↑ 2.0 2.1 2.2 Mortimer EA (1987). "Reye's syndrome, salicylates, epidemiology, and public health policy". JAMA. 257 (14): 941. PMID 3820516.
- ↑ Suchy, FJ, el al.; Sokol, RJ; Balistreri, WF (2007). Liver Disease in Children. Cambridge: Cambridge University Press. ISBN 0-5218-5657-4.
- ↑ Forsyth BW, Horwitz RI, Acampora D; et al. (1989). "New epidemiologic evidence confirming that bias does not explain the aspirin/Reye's syndrome association". JAMA. 261 (17): 2517–24. PMID 2704111.
- ↑ Hall SM, Plaster PA, Glasgow JF, Hancock P (1988). "Preadmission antipyretics in Reye's syndrome". Arch. Dis. Child. 63 (7): 857–66. PMID 3415311.
- ↑ Autret-Leca E, Jonville-Béra AP, Llau ME; et al. (2001). "Incidence of Reye's syndrome in France: a hospital-based survey". Journal of clinical epidemiology. 54 (8): 857–62. PMID 11470397.
External links
Template:CNS diseases of the nervous system