Protirelin: Difference between revisions
No edit summary |
m (Protected "Protirelin": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (3 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{AV}} | ||
|genericName=protirelin | |||
|aOrAn=a | |||
|genericName= | |||
|aOrAn= | |||
a | |||
|drugClass=[[diagnostic agent]] | |drugClass=[[diagnostic agent]] | ||
|indicationType=diagnosis | |||
|indication=thyroid function | |||
|adverseReactions=[[hypertension]], [[hypotension]], [[lightheadedness]], [[flushing]], [[abdominal discomfort]], bad taste in mouth, [[nausea]], [[xerostomia]], [[headache]], Urgent desire to urinate<!--Black Box Warning--> | |||
| | |blackBoxWarningTitle=Title | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
| | |||
|adverseReactions= | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 42: | Line 15: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=*TRH is indicated as an adjunctive agent in the diagnostic assessment of thyroid function. As an adjunct to other diagnostic procedures, testing with TRH (protirelin) may yield useful information in patients with pituitary or [[hypothalamic]] dysfunction. | |||
|fdaLIADAdult= | *TRH is indicated as an adjunct to evaluate the effectiveness of [[thyrotropin]] suppression with a particular dose of [[T4]] in patients with nodular or diffuse [[goiter]]. A normal TSH baseline value and a minimal difference between the 30 minute and baseline response to TRH injection would indicate adequate suppression of the pituitary secretion of TSH. | ||
*TRH is indicated as an adjunctive agent in the diagnostic assessment of thyroid function. As an adjunct to other diagnostic procedures, testing with TRH (protirelin) may yield useful information in patients with pituitary or hypothalamic dysfunction. | *TRH may be used, adjunctively, for adjustment of thyroid hormone dosage given to patients with primary [[hypothyroidism]]. A normal or slightly blunted TSH response, thirty minutes following TRH injection, would indicate adequate replacement therapy. | ||
*TRH is indicated as an adjunct to evaluate the effectiveness of thyrotropin suppression with a particular dose of T4 in patients with nodular or diffuse goiter. A normal TSH baseline value and a minimal difference between the 30 minute and baseline response to TRH injection would indicate adequate suppression of the pituitary secretion of TSH. | |||
*TRH may be used, adjunctively, for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism. A normal or slightly blunted TSH response, thirty minutes following TRH injection, would indicate adequate replacement therapy. | |||
=====Dosing Information===== | =====Dosing Information===== | ||
*TRH is intended for intravenous administration with the patient in the supine position. The drug is administered as a bolus over a period of 15 to 30 seconds, with the patient remaining supine until all scheduled post injection blood samples have been taken. Blood pressure should be measured before TRH is administered and at frequent intervals during the first 15 minutes thereafter | *TRH is intended for intravenous administration with the patient in the [[supine]] position. The drug is administered as a bolus over a period of 15 to 30 seconds, with the patient remaining supine until all scheduled post injection blood samples have been taken. Blood pressure should be measured before TRH is administered and at frequent intervals during the first 15 minutes thereafter . Have the patient urinate before injecting TRH. | ||
*Adults: 500 μg. Doses between 200 and 500 μg have been used. 500 μg is considered the optimum dose to give the maximum response in the greatest number of patients. Doses greater than 500 μg are unlikely to elicit a greater TSH response. | *Adults: 500 μg. Doses between 200 and 500 μg have been used. 500 μg is considered the optimum dose to give the maximum response in the greatest number of patients. Doses greater than 500 μg are unlikely to elicit a greater TSH response. | ||
*Children age 6 to 16 years: 7 μg/kg body weight up to a dose of 500 μg. | *Children age 6 to 16 years: 7 μg/kg body weight up to a dose of 500 μg. | ||
| Line 55: | Line 26: | ||
*One blood sample for TSH assay should be drawn immediately prior to the injection of TRH, and a second sample should be obtained 30 minutes after injection. | *One blood sample for TSH assay should be drawn immediately prior to the injection of TRH, and a second sample should be obtained 30 minutes after injection. | ||
*The TSH response to TRH is reduced by repetitive administration of the drug. Accordingly, if the TRH test is repeated, an interval of seven days before testing is recommended. | *The TSH response to TRH is reduced by repetitive administration of the drug. Accordingly, if the TRH test is repeated, an interval of seven days before testing is recommended. | ||
*Elevated serum lipids may interfere with the TSH assay. Thus, fasting (except in patients with hypopituitarism) or a low-fat meal is recommended prior to the test. | *Elevated serum lipids may interfere with the TSH assay. Thus, fasting (except in patients with [[hypopituitarism]]) or a low-fat meal is recommended prior to the test. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications= | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* Transient changes in blood pressure, either increases or decreases, frequently occur immediately following administration of TRH. Blood pressure should therefore be measured before TRH is administered and at frequent intervals during the first 15 minutes after its administration. | |||
*Increases in [[systolic pressure]] (usually less than 30 mm Hg) and/or increases in [[diastolic pressure]] (usually less than 20 mm Hg) have been observed more frequently than decreases in pressure. These changes have not ordinarily persisted for more than 15 minutes nor have they required therapy. More severe degrees of [[hypertension]] or [[hypotension]] with or without syncope have been reported in a few patients. To minimize the incidence and/or severity of [[hypotension]], the patient should be [[supine]] before, during, and after TRH administration. If a clinically important change in blood pressure occurs, monitoring of blood pressure should be continued until it returns to base-line levels. | |||
*Increases in systolic pressure (usually less than 30 mm Hg) and/or increases in diastolic pressure (usually less than 20 mm Hg) have been observed more frequently than decreases in pressure. These changes have not ordinarily persisted for more than 15 minutes nor have they required therapy. More severe degrees of hypertension or hypotension with or without syncope have been reported in a few patients. To minimize the incidence and/or severity of hypotension, the patient should be supine before, during, and after TRH administration. If a clinically important change in blood pressure occurs, monitoring of blood pressure should be continued until it returns to base-line levels. | |||
*TRH should not be administered to patients in whom marked, rapid changes in blood pressure would be dangerous unless the potential benefit clearly outweighs the potential risk | *TRH should not be administered to patients in whom marked, rapid changes in blood pressure would be dangerous unless the potential benefit clearly outweighs the potential risk | ||
| Line 120: | Line 62: | ||
=====General:===== | =====General:===== | ||
*Thyroid hormones reduce the TSH response to TRH. Accordingly, patients in whom TRH is to be used diagnostically should be taken off liothyronine (T3) approximately seven days prior to testing and should be taken off thyroid medications containing levothyroxine (T4), e.g., desiccated thyroid, thyroglobulin, or liotrix, at least 14 days before testing. Hormone therapy is NOT to be discontinued when the test is used to evaluate the effectiveness of thyroid suppression with a particular dose of T4 in patients with nodular or diffuse goiter, or for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism. | *Thyroid hormones reduce the TSH response to TRH. Accordingly, patients in whom TRH is to be used diagnostically should be taken off [[liothyronine]] (T3) approximately seven days prior to testing and should be taken off thyroid medications containing [[levothyroxine]] (T4), e.g., desiccated thyroid, [[thyroglobulin]], or liotrix, at least 14 days before testing. Hormone therapy is NOT to be discontinued when the test is used to evaluate the effectiveness of thyroid suppression with a particular dose of T4 in patients with nodular or diffuse goiter, or for adjustment of thyroid hormone dosage given to patients with primary [[hypothyroidism]]. | ||
*Chronic administration of levodopa has been reported to inhibit the TSH response to TRH. | *Chronic administration of [[levodopa]] has been reported to inhibit the TSH response to TRH. | ||
*It is not advisable to withdraw maintenance doses of adrenocortical drugs used in the therapy of known hypopituitarism. Several published reports have shown that prolonged treatment with glucocorticoids at physiologic doses has no significant effect on the TSH response to thyrotropin releasing hormone, but that the administration of pharmacologic doses of steroids reduces the TSH response. Therapeutic doses of acetylsalicylic acid (2 to 3.6 g/day) have been reported to inhibit the TSH response to protirelin. The ingestion of acetylsalicylic acid caused the peak level of TSH to decrease approximately 30% as compared to values obtained without acetylsalicylic acid administration. In both cases, the TSH peak occurred 30 minutes post-administration of protirelin. | *It is not advisable to withdraw maintenance doses of adrenocortical drugs used in the therapy of known [[hypopituitarism]]. Several published reports have shown that prolonged treatment with [[glucocorticoids]] at physiologic doses has no significant effect on the TSH response to [[thyrotropin releasing hormone]], but that the administration of pharmacologic doses of steroids reduces the TSH response. Therapeutic doses of [[acetylsalicylic]] acid (2 to 3.6 g/day) have been reported to inhibit the TSH response to protirelin. The ingestion of [[acetylsalicylic acid]] caused the peak level of TSH to decrease approximately 30% as compared to values obtained without [[acetylsalicylic acid]] administration. In both cases, the TSH peak occurred 30 minutes post-administration of protirelin. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*Side effects have been reported in about 50% of the patients tested with TRH. Generally, the side effects are moor, have occurred promptly, and have persisted for only a few minutes following injection. | |||
|clinicalTrials= | |||
*Side effects have been reported in about 50% of the patients tested with TRH. Generally, the side effects are moor, have occurred promptly, and have persisted for only a few minutes following injection. | |||
=====Cardiovascular reactions:===== | =====Cardiovascular reactions:===== | ||
*Marked changes in blood pressure, including both hypertension and hypotension with or without syncope, have been reported in a small number of patients. | *Marked changes in [[blood pressure]], including both [[hypertension]] and [[hypotension]] with or without [[syncope]], have been reported in a small number of patients. | ||
=====Endocrine reaction:===== | =====Endocrine reaction:===== | ||
*Breast enlargement and leakage in lactating women for up to two or three days. | *[[Breast enlargement]] and leakage in lactating women for up to two or three days. | ||
=====Other reactions:===== | =====Other reactions:===== | ||
*Headaches, sometimes severe, and transient amaurosis in patients with pituitary tumors. Rarely, convulsions may occur in patients with predisposing conditions, e.g., epilepsy, brain damage. Nausea; urge to urinate; flushed sensation; light-headedness; bad taste in mouth; abdominal discomfort; and dry mouth. | *[[Headaches]], sometimes severe, and transient [[amaurosis]] in patients with pituitary tumors. Rarely, convulsions may occur in patients with predisposing conditions, e.g., [[epilepsy]], brain damage. [[Nausea]]; urge to urinate; flushed sensation; [[light-headedness]]; bad taste in mouth; abdominal discomfort; and [[dry mouth]]. | ||
=====Less frequently reported were:===== | =====Less frequently reported were:===== | ||
*Anxiety; sweating; tightness in the throat; pressure in the chest; tingling sensation; drowsiness; and allergic reactions. | *[[Anxiety]]; [[sweating]]; tightness in the throat; pressure in the chest; [[tingling sensation]]; [[drowsiness]]; and allergic reactions. | ||
*Pituitary apoplexy requiring acute neurosurgical intervention has been reported infrequently for patients with pituitary macroadenomas following the acute administration of protirelin injection in the setting of combined anterior pituitary function testing in conjunction with LHRH and insulin. | *Pituitary apoplexy requiring acute neurosurgical intervention has been reported infrequently for patients with [[pituitary macroadenomas]] following the acute administration of protirelin injection in the setting of combined anterior pituitary function testing in conjunction with [[LHRH]] and [[insulin]]. | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=<!--Use in Specific Populations--> | |||
|drugInteractions= | |FDAPregCat=C | ||
|useInPregnancyAUS=There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
<!--Use in Specific Populations--> | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
| | |useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInPregnancyAUS= | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with [[renal impairment]]. | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with [[hepatic impairment]]. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInLaborDelivery= | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are [[immunocompromised]]. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Intravenous | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
|overdose= | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Name = thyrotropin-releasing hormone | | Name = thyrotropin-releasing hormone | ||
| Line 240: | Line 132: | ||
| Band = 13.3 | | Band = 13.3 | ||
| LocusSupplementaryData = -q21 | | LocusSupplementaryData = -q21 | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| Line 290: | Line 176: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=<!--Structure--> | |||
|mechAction= | |structure=* Chemically, TRH (protirelin) is identified as 5-oxo-L-prolyl-L-histidyl-L-proline amide. It is a synthetic tripeptide that is believed to be structurally identical to the naturally-occurring [[thyrotropin-releasing hormone]] produced by the [[hypothalamus]]. The CAS Registry Number is 24305-27-9. The structural formula is: | ||
<!--Structure--> | |||
|structure= | |||
* Chemically, TRH (protirelin) is identified as 5-oxo-L-prolyl-L-histidyl-L-proline amide. It is a synthetic tripeptide that is believed to be structurally identical to the naturally-occurring thyrotropin-releasing hormone produced by the hypothalamus. The CAS Registry Number is 24305-27-9. The structural formula is: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
*TRH is supplied as a solution of 1 mL in a 5 mL vial. Each vial contains 500 mcg protirelin, 1.8 mg Methylparaben, 0.2 mg Propylparaben, and 9.0 mg Sodium Chloride. TRH is intended for intravenous administration following dilution with 1 mL sterile water for injection. | *TRH is supplied as a solution of 1 mL in a 5 mL vial. Each vial contains 500 mcg protirelin, 1.8 mg Methylparaben, 0.2 mg Propylparaben, and 9.0 mg Sodium Chloride. TRH is intended for intravenous administration following dilution with 1 mL sterile water for injection. | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Pharmacologically, TRH increases the release of the [[thyroid stimulating hormone]] (TSH) from the [[anterior pituitary]]. [[Prolactin]] release is also increased. It has recently been observed that approximately 65% of [[acromegalic]] patients tested respond with a rise in circulating growth hormone levels; the clinical significance is as yet not clear. Following intravenous administration, the mean plasma half-life of protirelin in normal subjects is approximately five minutes. TSH levels rise rapidly and reach a peak at 20 to 30 minutes. The decline in TSH levels takes place more slowly, approaching baseline levels after approximately three hours | |||
|PK= | |||
*Pharmacologically, TRH increases the release of the thyroid stimulating hormone (TSH) from the anterior pituitary. Prolactin release is also increased. It has recently been observed that approximately 65% of acromegalic patients tested respond with a rise in circulating growth hormone levels; the clinical significance is as yet not clear. Following intravenous administration, the mean plasma half-life of protirelin in normal subjects is approximately five minutes. TSH levels rise rapidly and reach a peak at 20 to 30 minutes. The decline in TSH levels takes place more slowly, approaching baseline levels after approximately three hours | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=<!--Patient Counseling Information--> | |||
|howSupplied= | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=*Thyrel TRH | |||
|brandNames= | |||
*Thyrel TRH | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=<!--Drug Shortage Status--> | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 17:01, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Protirelin is a diagnostic agent that is FDA approved for the diagnosis of thyroid function. Common adverse reactions include hypertension, hypotension, lightheadedness, flushing, abdominal discomfort, bad taste in mouth, nausea, xerostomia, headache, Urgent desire to urinate.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- TRH is indicated as an adjunctive agent in the diagnostic assessment of thyroid function. As an adjunct to other diagnostic procedures, testing with TRH (protirelin) may yield useful information in patients with pituitary or hypothalamic dysfunction.
- TRH is indicated as an adjunct to evaluate the effectiveness of thyrotropin suppression with a particular dose of T4 in patients with nodular or diffuse goiter. A normal TSH baseline value and a minimal difference between the 30 minute and baseline response to TRH injection would indicate adequate suppression of the pituitary secretion of TSH.
- TRH may be used, adjunctively, for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism. A normal or slightly blunted TSH response, thirty minutes following TRH injection, would indicate adequate replacement therapy.
Dosing Information
- TRH is intended for intravenous administration with the patient in the supine position. The drug is administered as a bolus over a period of 15 to 30 seconds, with the patient remaining supine until all scheduled post injection blood samples have been taken. Blood pressure should be measured before TRH is administered and at frequent intervals during the first 15 minutes thereafter . Have the patient urinate before injecting TRH.
- Adults: 500 μg. Doses between 200 and 500 μg have been used. 500 μg is considered the optimum dose to give the maximum response in the greatest number of patients. Doses greater than 500 μg are unlikely to elicit a greater TSH response.
- Children age 6 to 16 years: 7 μg/kg body weight up to a dose of 500 μg.
- Infants and children up to 6 years: Experience is limited in this age group; doses of 7μg/kg have been administered.
- One blood sample for TSH assay should be drawn immediately prior to the injection of TRH, and a second sample should be obtained 30 minutes after injection.
- The TSH response to TRH is reduced by repetitive administration of the drug. Accordingly, if the TRH test is repeated, an interval of seven days before testing is recommended.
- Elevated serum lipids may interfere with the TSH assay. Thus, fasting (except in patients with hypopituitarism) or a low-fat meal is recommended prior to the test.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Protirelin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Protirelin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Protirelin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Protirelin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Protirelin in pediatric patients.
Contraindications
There is limited information regarding Protirelin Contraindications in the drug label.
Warnings
- Transient changes in blood pressure, either increases or decreases, frequently occur immediately following administration of TRH. Blood pressure should therefore be measured before TRH is administered and at frequent intervals during the first 15 minutes after its administration.
- Increases in systolic pressure (usually less than 30 mm Hg) and/or increases in diastolic pressure (usually less than 20 mm Hg) have been observed more frequently than decreases in pressure. These changes have not ordinarily persisted for more than 15 minutes nor have they required therapy. More severe degrees of hypertension or hypotension with or without syncope have been reported in a few patients. To minimize the incidence and/or severity of hypotension, the patient should be supine before, during, and after TRH administration. If a clinically important change in blood pressure occurs, monitoring of blood pressure should be continued until it returns to base-line levels.
- TRH should not be administered to patients in whom marked, rapid changes in blood pressure would be dangerous unless the potential benefit clearly outweighs the potential risk
Precautions
General:
- Thyroid hormones reduce the TSH response to TRH. Accordingly, patients in whom TRH is to be used diagnostically should be taken off liothyronine (T3) approximately seven days prior to testing and should be taken off thyroid medications containing levothyroxine (T4), e.g., desiccated thyroid, thyroglobulin, or liotrix, at least 14 days before testing. Hormone therapy is NOT to be discontinued when the test is used to evaluate the effectiveness of thyroid suppression with a particular dose of T4 in patients with nodular or diffuse goiter, or for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism.
- Chronic administration of levodopa has been reported to inhibit the TSH response to TRH.
- It is not advisable to withdraw maintenance doses of adrenocortical drugs used in the therapy of known hypopituitarism. Several published reports have shown that prolonged treatment with glucocorticoids at physiologic doses has no significant effect on the TSH response to thyrotropin releasing hormone, but that the administration of pharmacologic doses of steroids reduces the TSH response. Therapeutic doses of acetylsalicylic acid (2 to 3.6 g/day) have been reported to inhibit the TSH response to protirelin. The ingestion of acetylsalicylic acid caused the peak level of TSH to decrease approximately 30% as compared to values obtained without acetylsalicylic acid administration. In both cases, the TSH peak occurred 30 minutes post-administration of protirelin.
Adverse Reactions
Clinical Trials Experience
- Side effects have been reported in about 50% of the patients tested with TRH. Generally, the side effects are moor, have occurred promptly, and have persisted for only a few minutes following injection.
Cardiovascular reactions:
- Marked changes in blood pressure, including both hypertension and hypotension with or without syncope, have been reported in a small number of patients.
Endocrine reaction:
- Breast enlargement and leakage in lactating women for up to two or three days.
Other reactions:
- Headaches, sometimes severe, and transient amaurosis in patients with pituitary tumors. Rarely, convulsions may occur in patients with predisposing conditions, e.g., epilepsy, brain damage. Nausea; urge to urinate; flushed sensation; light-headedness; bad taste in mouth; abdominal discomfort; and dry mouth.
Less frequently reported were:
- Anxiety; sweating; tightness in the throat; pressure in the chest; tingling sensation; drowsiness; and allergic reactions.
- Pituitary apoplexy requiring acute neurosurgical intervention has been reported infrequently for patients with pituitary macroadenomas following the acute administration of protirelin injection in the setting of combined anterior pituitary function testing in conjunction with LHRH and insulin.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Protirelin in the drug label.
Drug Interactions
There is limited information regarding Protirelin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
There is no FDA guidance on usage of Protirelin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Protirelin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Protirelin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Protirelin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Protirelin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Protirelin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Protirelin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Protirelin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Protirelin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Protirelin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Protirelin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Protirelin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Protirelin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Protirelin in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Protirelin in the drug label.
Pharmacology
| |
Protirelin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | V04 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 362.38367 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Protirelin Mechanism of Action in the drug label.
Structure
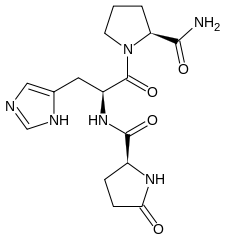
- Chemically, TRH (protirelin) is identified as 5-oxo-L-prolyl-L-histidyl-L-proline amide. It is a synthetic tripeptide that is believed to be structurally identical to the naturally-occurring thyrotropin-releasing hormone produced by the hypothalamus. The CAS Registry Number is 24305-27-9. The structural formula is:
- TRH is supplied as a solution of 1 mL in a 5 mL vial. Each vial contains 500 mcg protirelin, 1.8 mg Methylparaben, 0.2 mg Propylparaben, and 9.0 mg Sodium Chloride. TRH is intended for intravenous administration following dilution with 1 mL sterile water for injection.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Protirelin in the drug label.
Pharmacokinetics
- Pharmacologically, TRH increases the release of the thyroid stimulating hormone (TSH) from the anterior pituitary. Prolactin release is also increased. It has recently been observed that approximately 65% of acromegalic patients tested respond with a rise in circulating growth hormone levels; the clinical significance is as yet not clear. Following intravenous administration, the mean plasma half-life of protirelin in normal subjects is approximately five minutes. TSH levels rise rapidly and reach a peak at 20 to 30 minutes. The decline in TSH levels takes place more slowly, approaching baseline levels after approximately three hours
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Protirelin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Protirelin in the drug label.
How Supplied
There is limited information regarding Protirelin How Supplied in the drug label.
Storage
There is limited information regarding Protirelin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Protirelin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Protirelin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Protirelin in the drug label.
Precautions with Alcohol
- Alcohol-Protirelin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Thyrel TRH
Look-Alike Drug Names
There is limited information regarding Protirelin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Protirelin |Label Name=Protirelin06.png
}}
{{#subobject:
|Label Page=Protirelin |Label Name=Protirelin07.png
}}
