Propafenone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
MORTALITY:
|
Overview
Propafenone is an antiarrhythmic that is FDA approved for the {{{indicationType}}} of paroxysmal atrial fibrillation/flutter (PAF) associated with disabling symptoms in patients without structural heart disease, recurrent paroxysmal supraventricular tachycardia (PSVT) associated with disabling symptoms in patients without structural heart disease, and life-threatening ventricular arrhythmias, such as sustained ventricular tachycardia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include chest pain, edema, palpitations, constipation, nausea, taste alteration, vomiting, dizziness, anxiety, dyspnea, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
PAF, PSVT, or Ventricular Arrhythmia
- Dosing Information
- The dose of RYTHMOL must be individually titrated on the basis of response and tolerance.
- Initiate therapy with RYTHMOL 150 mg given every 8 hours (450 mg/day).
- Dosage may be increased at a minimum of 3 to 4 day intervals to 225 mg every 8 hours (675 mg/day).
- If additional therapeutic effect is needed, the dose of RYTHMOL may be increased to 300 mg every 8 hours (900 mg/day). The usefulness and safety of dosages exceeding 900 mg per day have not been established.
- In patients with hepatic impairment or those with significant widening of the QRS complex or second- or third-degree AV block, consider reducing the dose.
- As with other antiarrhythmic agents, in the elderly or in ventricular arrhythmia patients with marked previous myocardial damage, the dose of RYTHMOL should be increased more gradually during the initial phase of treatment.
- The combination of CYP3A4 inhibition and either CYP2D6 deficiency or CYP2D6 inhibition with the simultaneous administration of propafenone may significantly increase the concentration of propafenone and thereby increase the risk of proa
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Propafenone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of propafenone in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Propafenone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Propafenone in pediatric patients.
Contraindications
- Heart failure
- Cardiogenic shock
- Sinoatrial, atrioventricular, and intraventricular disorders of impulse generation or conduction (e.g., sick sinus syndrome, AV block) in the absence of an artificial pacemaker
- Brugada Syndrome
- Bradycardia
- Marked hypotension
- Bronchospastic disorders or severe obstructive pulmonary disease
- Marked electrolyte imbalance
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
MORTALITY:
|
- Proarrhythmic Effects
- Propafenone has caused new or worsened arrhythmias. Such proarrhythmic effects include sudden death and life-threatening ventricular arrhythmias such as ventricular fibrillation, ventricular tachycardia, asystole, and torsade de pointes. It may also worsen premature ventricular contractions or supraventricular arrhythmias, and it may prolong the QT interval. It is therefore essential that each patient given RYTHMOL be evaluated electrocardiographically prior to and during therapy to determine whether the response to RYTHMOL supports continued treatment. Because propafenone prolongs the QRS interval in the electrocardiogram, changes in the QT interval are difficult to interpret [see Clinical Pharmacology (12.2)].
- In a US uncontrolled, open-label, multicenter trial in subjects with symptomatic supraventricular tachycardia (SVT), 1.9% (9/474) of these subjects experienced ventricular tachycardia (VT) or ventricular fibrillation (VF) during the trial. However, in 4 of the 9 subjects, the ventricular tachycardia was of atrial origin. Six of the 9 subjects that developed ventricular arrhythmias did so within 14 days of onset of therapy. About 2.3% (11/474) of all subjects had a recurrence of SVT during the trial which could have been a change in the subjects’ arrhythmia behavior or could represent a proarrhythmic event. Case reports in patients treated with propafenone for atrial fibrillation/flutter have included increased premature ventricular contractions (PVCs), VT, VF, torsade de pointes, asystole, and death.
- Overall in clinical trials with RYTHMOL (which included subjects treated for ventricular arrhythmias, atrial fibrillation/flutter, and PSVT), 4.7% of all subjects had new or worsened ventricular arrhythmia possibly representing a proarrhythmic event (0.7% was an increase in PVCs; 4.0% a worsening, or new appearance, of VT or VF). Of the subjects who had worsening of VT (4%), 92% had a history of VT and/or VT/VF, 71% had coronary artery disease, and 68% had a prior myocardial infarction. The incidence of proarrhythmia in subjects with less serious or benign arrhythmias, which include subjects with an increase in frequency of PVCs, was 1.6%. Although most proarrhythmic events occurred during the first week of therapy, late events also were seen and the CAST trial [see Boxed Warning: Mortality] suggests that an increased risk of proarrythmia is present throughout treatment.
- In a trial of sustained-release propafenone (RYTHMOL SR®), there were too few deaths to assess the long-term risk to patients. There were 5 deaths, 3 in the pooled group for RYTHMOL SR (0.8%) and 2 in the placebo group (1.6%). In the overall database of 8 trials of RYTHMOL SR and immediate-release RYTHMOL, the mortality rate was 2.5% per year on propafenone and 4.0% per year on placebo. Concurrent use of propafenone with other antiarrhythmic agents has not been well studied.
- Unmasking Brugada Syndrome
- Brugada Syndrome may be unmasked after exposure to RYTHMOL. Perform an ECG after initiation of RYTHMOL, and discontinue the drug if changes are suggestive of Brugada Syndrome [see Contraindications (4)].
- Use With Drugs That Prolong the QT Interval and Antiarrhythmic Agents
- The use of RYTHMOL in conjunction with other drugs that prolong the QT interval has not been extensively studied. Such drugs may include many antiarrhythmics, some phenothiazines, tricyclic antidepressants, and oral macrolides. Withhold Class IA and III antiarrhythmic agents for at least 5 half-lives prior to dosing with RYTHMOL. Avoid the use of propafenone with Class IA and III antiarrhythmic agents (including quinidine and amiodarone). There is only limited experience with the concomitant use of Class IB or IC antiarrhythmics.
- Drug Interactions: Simultaneous Use With Inhibitors of Cytochrome P450 Isoenzymes 2D6 and 3A4
- Propafenone is metabolized by CYP2D6, CYP3A4, and CYP1A2 isoenzymes. Approximately 6% of Caucasians in the US population are naturally deficient in CYP2D6 activity and to a somewhat lesser extent in other demographic groups. Drugs that inhibit these CYP pathways (such as desipramine, paroxetine, ritonavir, sertraline for CYP2D6; ketoconazole, erythromycin, saquinavir, and grapefruit juice for CYP3A4; and amiodarone and tobacco smoke for CYP1A2) can be expected to cause increased plasma levels of propafenone.
- Increased exposure to propafenone may lead to cardiac arrhythmias and exaggerated beta-adrenergic blocking activity. Because of its metabolism, the combination of CYP3A4 inhibition and either CYP2D6 deficiency or CYP2D6 inhibition in users of propafenone is potentially hazardous. Therefore, avoid simultaneous use of RYTHMOL with both a CYP2D6 inhibitor and a CYP3A4 inhibitor.
- Use in Patients With a History of Heart Failure
- Propafenone exerts a negative inotropic activity on the myocardium as well as beta-blockade effects and may provoke overt heart failure.
- In clinical trial experience with RYTHMOL, new or worsened congestive heart failure (CHF) has been reported in 3.7% of subjects with ventricular arrhythmia; of those 0.9% were considered probably or definitely related to propafenone HCl. Of the subjects with CHF probably related to propafenone, 80% had pre-existing heart failure and 85% had coronary artery disease. CHF attributable to propafenone HCl developed rarely (<0.2%) in ventricular arrhythmia subjects who had no previous history of CHF. CHF occurred in 1.9% of subjects studied with PAF or PSVT.
- In a US trial of RYTHMOL SR in subjects with symptomatic AF, heart failure was reported in 4 (1.0%) subjects receiving RYTHMOL SR (all doses), compared with 1 (0.8%) subject receiving placebo.
- Conduction Disturbances
- Propafenone slows atrioventricular conduction and may also cause dose-related first-degree AV block. Average PR interval prolongation and increases in QRS duration are also dose-related. Do not give propafenone to patients with atrioventricular and intraventricular conduction defects in the absence of a pacemaker [see Contraindications (4), Clinical Pharmacology (12.2)].
- The incidence of first-degree, second-degree, and third-degree AV block observed in 2,127 subjects with ventricular arrhythmia was 2.5%, 0.6%, and 0.2%, respectively. Development of second- or third-degree AV block requires a reduction in dosage or discontinuation of propafenone HCl. Bundle branch block (1.2%) and intraventricular conduction delay (1.1%) have been reported in subjects receiving propafenone. Bradycardia has also been reported (1.5%). Experience in patients with sick sinus node syndrome is limited and these patients should not be treated with propafenone.
- In a US trial in 523 subjects with a history of symptomatic AF treated with RYTHMOL SR, sinus bradycardia (rate <50 beats/min) was reported with the same frequency with RYTHMOL SR and placebo.
- Effects on Pacemaker Threshold
- Propafenone may alter both pacing and sensing thresholds of implanted pacemakers and defibrillators. During and after therapy, monitor and re-program these devices accordingly.
- Agranulocytosis
- Agranulocytosis has been reported in patients receiving propafenone. Generally, the agranulocytosis occurred within the first 2 months of propafenone therapy and upon discontinuation of therapy; the white count usually normalized by 14 days. Unexplained fever or decrease in white cell count, particularly during the initial 3 months of therapy, warrant consideration of possible agranulocytosis or granulocytopenia. Instruct patients to report promptly any signs of infection such as fever, sore throat, or chills.
- Use in Patients With Hepatic Dysfunction
- Propafenone is highly metabolized by the liver. Severe liver dysfunction increases the bioavailability of propafenone to approximately 70% compared with 3% to 40% in patients with normal liver function. In 8 subjects with moderate to severe liver disease, the mean half-life was approximately 9 hours. Increased bioavailability of propafenone in these patients may result in excessive accumulation. Carefully monitor patients with impaired hepatic function for excessive pharmacological effects.
- Use in Patients With Renal Dysfunction
- Approximately 50% of propafenone metabolites are excreted in the urine following administration of RYTHMOL.
- In patients with impaired renal function, monitor for signs of overdosage [see Overdosage (10)].
- Use in Patients With Myasthenia Gravis
- Exacerbation of myasthenia gravis has been reported during propafenone therapy.
- Elevated ANA Titers
- Positive ANA titers have been reported in patients receiving propafenone. They have been reversible upon cessation of treatment and may disappear even in the face of continued propafenone therapy. These laboratory findings were usually not associated with clinical symptoms, but there is one published case of drug-induced lupus erythematosis (positive rechallenge); it resolved completely upon discontinuation of therapy. Carefully evaluate patients who develop an abnormal ANA test and, if persistent or worsening elevation of ANA titers is detected, consider discontinuing therapy.
- Impaired Spermatogenesis
- Reversible disorders of spermatogenesis have been demonstrated in monkeys, dogs, and rabbits after high-dose intravenous administration of propafenone. Evaluation of the effects of short-term administration of RYTHMOL on spermatogenesis in 11 normal subjects suggested that propafenone produced a reversible, short-term drop (within normal range) in sperm count.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Propafenone in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Propafenone in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Propafenone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Propafenone during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Propafenone with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Propafenone with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Propafenone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Propafenone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Propafenone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Propafenone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Propafenone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Propafenone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Propafenone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Propafenone in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Propafenone in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Propafenone in the drug label.
Pharmacology
| Template:Px | |
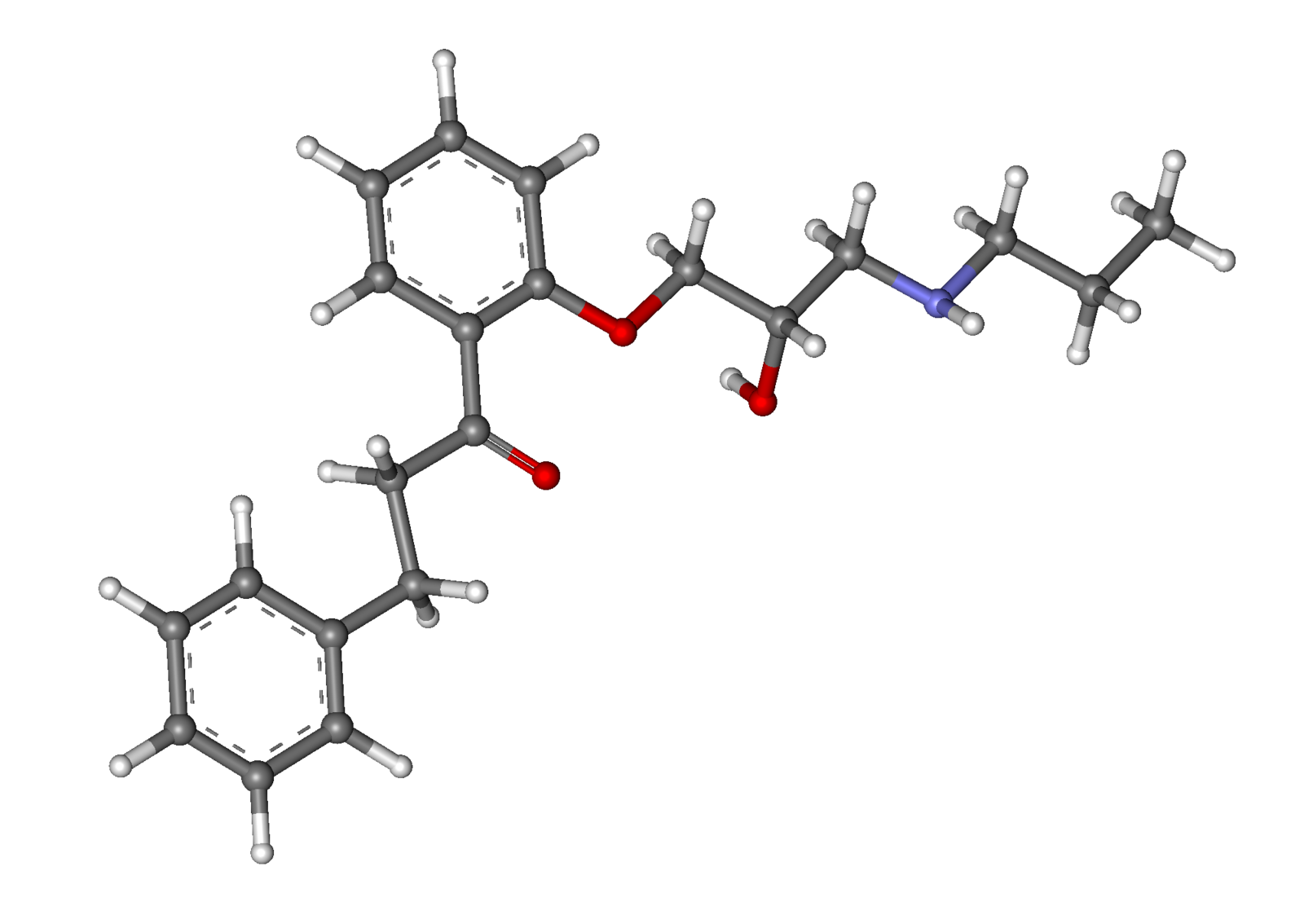
| |
Propafenone
| |
| Systematic (IUPAC) name | |
| 1-{2-[2-hydroxy-3-(propylamino)propoxy]phenyl}-3-phenylpropan-1-one | |
| Identifiers | |
| CAS number | |
| ATC code | C01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 341.444 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 97% |
| Metabolism | ? |
| Half life | 2-10 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
Prescription only |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Propafenone in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Propafenone in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Propafenone in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Propafenone in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Propafenone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Propafenone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Propafenone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Propafenone in the drug label.
Precautions with Alcohol
- Alcohol-Propafenone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Rythmol®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "RYTHMOL (propafenone hydrochloride) tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Propafenone |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Propafenone |Label Name=Propafenone11.png
}}
{{#subobject:
|Label Page=Propafenone |Label Name=Propafenone11.png
}}
