Parkinson's disease pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| (59 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Parkinson's disease}} | {{Parkinson's disease}} | ||
{{CMG}} | {{CMG}} {{AE}} {{Fs}} | ||
==Overview== | ==Overview== | ||
The underlying [[pathophysiology]] of [[Parkinson's disease|Parkinson disease]] is [[dopamine]] depletion. Reduced number of [[dopaminergic]] [[neurons]] lead to increased inhibition of [[thalamus]] and as a result, decrease excitation of [[Cortex|brain cortex]], causing [[bradykinesia]]. pathologic [[hallmark]] of [[Parkinson's disease|PD]] is [[Lewy body|lewy bodies]] which are round [[cytoplasmic]] [[eosinophilic]] inclusions. This disease can have so many triggers ( Protein misfolding, Defective proteolysis, Mitochondrial dysfunction, Oxidative stress, Iron metabolism and Immunologic and inflammatory mechanisms) but the main etiology of neuronal degeneration is either apoptosis or necrosis. | |||
==Pathophysiology== | ==Pathophysiology== | ||
===Physiology=== | |||
The | *The [[substantia nigra]] ([[Substantia nigra|SN]]), [[striatum]] ([[Caudate nucleus|caudate]] and [[putamen]]), [[globus pallidus]] ([[Globus pallidus|GP]]), [[subthalamic nucleus]] ([[Subthalamic nucleus|STN]]) and [[thalamus]] contribute with each other to make the [[extrapyramidal system]] or [[basal ganglia]]. | ||
*The impulses from [[hippocampus]], [[amygdala]] and prefrontal supplementary motor area to the [[basal ganglia]] are [[Excitatory synapse|excitatory]] mediated by [[glutamate]]. | |||
*The major [[dopaminergic]] [[neurons]] are in [[substantia nigra]] and are responsible for [[dopaminergic]] input of [[striatum]]. The striatal output is [[Inhibitory synapses|inhibitory]] ([[GABA]]) despite the [[Excitatory synapse|excitatory]] ([[glutamate]]) output of [[Subthalamic nucleus|STN]] to the [[globus pallidus]] (medial and lateral). | |||
*There are 5 [[dopamine receptors]] (D1_D5) which are in [[basal ganglia]] and [[limbic system]]. [[D1 receptor|D1]] and [[D2 receptor|D2]] are mostly found in the dorsal [[striatum]] (motor) and are activated through [[dopaminergic]] pathway from [[Substantia nigra|SNc]], as a result, they are very important in the [[pathophysiology]] of Parkinson disease. D3 and D4 are located mostly in [[Mesolimbic system|mesolimbic]] or emotional part of the [[brain]] and D5 in [[hippocampus]]/[[hypothalamus]] area.<ref name="pmid11052222">{{cite journal |vauthors=Gerfen CR |title=Molecular effects of dopamine on striatal-projection pathways |journal=Trends Neurosci. |volume=23 |issue=10 Suppl |pages=S64–70 |date=October 2000 |pmid=11052222 |doi= |url=}}</ref> | |||
===Phatogenesis=== | |||
*The underlying [[pathophysiology]] of [[Parkinson's disease|Parkinson disease]] is [[dopamine]] depletion. In the course of the disease [[dopamine]] depletion of [[nigrostriatal pathway]] will lead to denervation hypersensitivity and increasing number of [[D2 receptor|D2]] receptors in dorsal [[putamen]].<ref name="pmid15509741">{{cite journal |vauthors=Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK |title=Dopamine modulates release from corticostriatal terminals |journal=J. Neurosci. |volume=24 |issue=43 |pages=9541–52 |date=October 2004 |pmid=15509741 |doi=10.1523/JNEUROSCI.2891-04.2004 |url=}}</ref> | |||
*There are two pathways in this system: Direct and indirect pathway. | |||
*Indirect pathway starts with [[inhibition]] of [[striatum]] via [[D2 receptor]] which in turn [[Inhibition|inhibits]] [[neurons]] of lateral [[Globus pallidus|GP]] by [[GABA]] which [[Inhibition|inhibits]] the inhibition of [[Subthalamic nucleus|STN]] by lateral [[Globus pallidus|GP]]. [[Subthalamic nucleus|STN]] provides [[Excitatory synapse|excitatory]] action on [[Globus pallidus|GP]] internal and [[Substantia nigra|SNr]] via [[glutamate]]. [[Globus pallidus|GPi]] inhibit [[thalamus]] by [[GABA]] but [[cortex]] input from [[thalamus]] is [[Excitatory synapse|excitatory]]. | |||
*Direct pathway starts with [[excitation]] of [[striatum]] by stimulation of [[D1 receptor|D1 receptors]], then [[striatum]] inhibits [[Globus pallidus|GP]] internal and [[Substantia nigra|SNr]] by [[GABA]] directly. Reduced number of [[dopaminergic]] [[neurons]] lead to increased inhibition of [[thalamus]] and as a result, decrease excitation of [[Cortex|brain cortex]], causing [[bradykinesia]].<ref name="pmid16830313">{{cite journal |vauthors=Gatev P, Darbin O, Wichmann T |title=Oscillations in the basal ganglia under normal conditions and in movement disorders |journal=Mov. Disord. |volume=21 |issue=10 |pages=1566–77 |date=October 2006 |pmid=16830313 |doi=10.1002/mds.21033 |url=}}</ref> | |||
*Our [[brain]] has some compensatory mechanism fighting [[dopamine]] depletion. It can increase the synthesis of [[dopamine]], [[gap junctions]] and the number of [[D2 receptor|D2 receptors]].<ref name="pmid11052221">{{cite journal |vauthors=Calabresi P, Centonze D, Bernardi G |title=Electrophysiology of dopamine in normal and denervated striatal neurons |journal=Trends Neurosci. |volume=23 |issue=10 Suppl |pages=S57–63 |date=October 2000 |pmid=11052221 |doi= |url=}}</ref><ref name="pmid12464455">{{cite journal |vauthors=Moore H, Grace AA |title=A role for electrotonic coupling in the striatum in the expression of dopamine receptor-mediated stereotypies |journal=Neuropsychopharmacology |volume=27 |issue=6 |pages=980–92 |date=December 2002 |pmid=12464455 |doi=10.1016/S0893-133X(02)00383-4 |url=}}</ref> It can also reduce the uptake of [[dopamine]] from synaptic space.<ref name="pmid16081470">{{cite journal |vauthors=Adams JR, van Netten H, Schulzer M, Mak E, Mckenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, Stoessl AJ |title=PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation |journal=Brain |volume=128 |issue=Pt 12 |pages=2777–85 |date=December 2005 |pmid=16081470 |doi=10.1093/brain/awh607 |url=}}</ref> | |||
*The main [[pathology]] seen in [[Parkinson disease|PD]] patients is neuronal loss, depigmentation and [[gliosis]] which are mostly seen in the [[locus ceruleus]] and [[substantia nigra]]. The normal number of pigmented neurons in [[Substantia nigra|SN]] in a normal individual is about 550,000, but in patient with [[Parkinson's disease|PD]] in can decrease as much as 66%.<ref name="pmid2010756">{{cite journal |vauthors=Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H |title=The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method |journal=J. Neurol. Neurosurg. Psychiatry |volume=54 |issue=1 |pages=30–3 |date=January 1991 |pmid=2010756 |pmc=1014294 |doi= |url=}}</ref> | |||
*In the normal aging process, neuronal loss occurs in the dorsal tier of [[Substantia nigra|SN]] pars compacta and the most of [[dopamine]] depletion is seen in [[caudate nucleus]]. But in Parkinson, loss of [[dopaminergic]] neurons occurs predominantly in ventrolateral portion of the [[Substantia nigra|SN]].<ref name="pmid15726582">{{cite journal |vauthors=Porritt M, Stanic D, Finkelstein D, Batchelor P, Lockhart S, Hughes A, Kalnins R, Howells D |title=Dopaminergic innervation of the human striatum in Parkinson's disease |journal=Mov. Disord. |volume=20 |issue=7 |pages=810–8 |date=July 2005 |pmid=15726582 |doi=10.1002/mds.20399 |url=}}</ref><ref name="pmid1933245">{{cite journal |vauthors=Fearnley JM, Lees AJ |title=Ageing and Parkinson's disease: substantia nigra regional selectivity |journal=Brain |volume=114 ( Pt 5) |issue= |pages=2283–301 |date=October 1991 |pmid=1933245 |doi= |url=}}</ref> | |||
*The other sites of the [[brain]] which are influenced by [[Parkinson's disease|PD]] are internal segment of the [[globus pallidus]], center median parafascicular complex, pedunculopontine tegmental nucleus, glutamatergic caudal intralaminar thalamic nuclei and [[hippocampus]].<ref name="pmid10716254">{{cite journal |vauthors=Henderson JM, Carpenter K, Cartwright H, Halliday GM |title=Degeneration of the centré median-parafascicular complex in Parkinson's disease |journal=Ann. Neurol. |volume=47 |issue=3 |pages=345–52 |date=March 2000 |pmid=10716254 |doi= |url=}}</ref><ref name="pmid12815657">{{cite journal |vauthors=Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA |title=Parkinson's disease is associated with hippocampal atrophy |journal=Mov. Disord. |volume=18 |issue=7 |pages=784–90 |date=July 2003 |pmid=12815657 |doi=10.1002/mds.10444 |url=}}</ref> | |||
*PD may have so many triggers but the main etiology of neuronal degeneration is either apoptosis or necrosis.<ref name="pmid16823471">{{cite journal |vauthors=Savitt JM, Dawson VL, Dawson TM |title=Diagnosis and treatment of Parkinson disease: molecules to medicine |journal=J. Clin. Invest. |volume=116 |issue=7 |pages=1744–54 |date=July 2006 |pmid=16823471 |pmc=1483178 |doi=10.1172/JCI29178 |url=}}</ref><ref name="pmid17372132">{{cite journal |vauthors=Lang AE |title=The progression of Parkinson disease: a hypothesis |journal=Neurology |volume=68 |issue=12 |pages=948–52 |date=March 2007 |pmid=17372132 |doi=10.1212/01.wnl.0000257110.91041.5d |url=}}</ref><ref name="pmid25071440">{{cite journal |vauthors=Atkin G, Paulson H |title=Ubiquitin pathways in neurodegenerative disease |journal=Front Mol Neurosci |volume=7 |issue= |pages=63 |date=2014 |pmid=25071440 |pmc=4085722 |doi=10.3389/fnmol.2014.00063 |url=}}</ref> | |||
====Protein misfolding==== | |||
*One of the main underlying cause of [[Parkinson's disease|PD]] is [[mutation]] in the gene of [[alpha-synuclein]] protein which is abundant in the [[CNS]]. | |||
*Its function is thought to be involved in [[synaptic]] function and [[plasticity]].<ref name="pmid12951565">{{cite journal |vauthors=Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K |title=The role of alpha-synuclein in Parkinson's disease: insights from animal models |journal=Nat. Rev. Neurosci. |volume=4 |issue=9 |pages=727–38 |date=September 2003 |pmid=12951565 |doi=10.1038/nrn1199 |url=}}</ref><ref name="pmid26790375">{{cite journal |vauthors=Calo L, Wegrzynowicz M, Santivañez-Perez J, Grazia Spillantini M |title=Synaptic failure and α-synuclein |journal=Mov. Disord. |volume=31 |issue=2 |pages=169–77 |date=February 2016 |pmid=26790375 |doi=10.1002/mds.26479 |url=}}</ref> | |||
*This [[Mutation|mutations]] lead to unfold [[alpha-synuclein]] and aggregation of insoluble [[protein]] and [[neuronal]] damage. | |||
*[[Lewy body|Lewy bodies]] which are characteristic of [[Parkinson's disease|PD]] are mostly build from [[alpha-synuclein]] [[protein]].<ref name="pmid9278044">{{cite journal |vauthors=Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M |title=Alpha-synuclein in Lewy bodies |journal=Nature |volume=388 |issue=6645 |pages=839–40 |date=August 1997 |pmid=9278044 |doi=10.1038/42166 |url=}}</ref> | |||
=== | ====Defective proteolysis==== | ||
*There are three pathways which control the [[protein]] [[homeostasis]] in cells: Molecular chaperons, the ubiquitin-proteasome system and autophagy-lysosomal pathway. | |||
*[[Alpha-synuclein|Alpha synuclein]] processing is done by all of this three mechanisms and defect in any of them can cause aggregation of this [[protein]] and [[neuronal]] death.<ref name="pmid23580245">{{cite journal |vauthors=Lim KL, Zhang CW |title=Molecular events underlying Parkinson's disease - an interwoven tapestry |journal=Front Neurol |volume=4 |issue= |pages=33 |date=2013 |pmid=23580245 |pmc=3619247 |doi=10.3389/fneur.2013.00033 |url=}}</ref><ref name="pmid23580333">{{cite journal |vauthors=Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E |title=Lysosomal impairment in Parkinson's disease |journal=Mov. Disord. |volume=28 |issue=6 |pages=725–32 |date=June 2013 |pmid=23580333 |pmc=5131721 |doi=10.1002/mds.25462 |url=}}</ref><ref name="pmid24211851">{{cite journal |vauthors=Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ |title=Autophagy and apoptosis dysfunction in neurodegenerative disorders |journal=Prog. Neurobiol. |volume=112 |issue= |pages=24–49 |date=January 2014 |pmid=24211851 |doi=10.1016/j.pneurobio.2013.10.004 |url=}}</ref> | |||
====Mitochondrial dysfunction==== | |||
*The [[drug]] 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, an [[Analog (chemistry)|analog]] of mepridine is found to be associated with [[Parkinson's disease|PD]]. | |||
*The [[oxidation]] of this drug produces 1-methyl-4-phenylpyridium which inhibits complex one of mitochondria and result in [[cell]] damage. | |||
*Studies showed that the activity of this complex is decreased in [[Parkinson's disease|PD]] patients.<ref name="pmid15377875">{{cite journal |vauthors=Przedborski S, Tieu K, Perier C, Vila M |title=MPTP as a mitochondrial neurotoxic model of Parkinson's disease |journal=J. Bioenerg. Biomembr. |volume=36 |issue=4 |pages=375–9 |date=August 2004 |pmid=15377875 |doi=10.1023/B:JOBB.0000041771.66775.d5 |url=}}</ref><ref name="pmid22446186">{{cite journal |vauthors=Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB |title=Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling |journal=J. Clin. Invest. |volume=122 |issue=4 |pages=1354–67 |date=April 2012 |pmid=22446186 |pmc=3314472 |doi=10.1172/JCI61332 |url=}}</ref><ref name="pmid2566813">{{cite journal |vauthors=Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD |title=Mitochondrial complex I deficiency in Parkinson's disease |journal=Lancet |volume=1 |issue=8649 |pages=1269 |date=June 1989 |pmid=2566813 |doi= |url=}}</ref> | |||
</ref> | |||
=== | ====Oxidative stress==== | ||
*[[Reactive oxygen species]] including [[hydrogen peroxide]], superoxide anions and hydroxyradicals are [[toxic]] to [[neurons]] and cause [[neuronal]] damage. | |||
*They interact with [[membrane lipids]] and cause [[lipid peroxidation]] which can be seen in [[substantia nigra]] of [[Parkinson's disease|PD]] patients.<ref name="pmid15155938">{{cite journal |vauthors=Greenamyre JT, Hastings TG |title=Biomedicine. Parkinson's--divergent causes, convergent mechanisms |journal=Science |volume=304 |issue=5674 |pages=1120–2 |date=May 2004 |pmid=15155938 |doi=10.1126/science.1098966 |url=}}</ref><ref name="pmid14645467">{{cite journal |vauthors=Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT |title=Mechanism of toxicity in rotenone models of Parkinson's disease |journal=J. Neurosci. |volume=23 |issue=34 |pages=10756–64 |date=November 2003 |pmid=14645467 |doi= |url=}}</ref> | |||
*They can also cause protein misfolding by attacking disulfide isomerase through [[nitric oxide]]. Disulfide isomerase is a [[chaperone]] preventing the aggregation of [[proteins]].<ref name="pmid16724068">{{cite journal |vauthors=Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA |title=S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration |journal=Nature |volume=441 |issue=7092 |pages=513–7 |date=May 2006 |pmid=16724068 |doi=10.1038/nature04782 |url=}}</ref> | |||
====Iron metabolism==== | |||
*Studies showed that impaired [[iron metabolism]] leads to increase amount of [[iron]] in [[substantia nigra]] of [[Parkinson's disease|PD]] patients. | |||
*One of the underlying [[etiology]] of [[iron]] accommodation in [[Neuron|neuronal cells]] is the absence of [[tau protein]].<ref name="pmid17515544">{{cite journal |vauthors=Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM |title=Individual dopaminergic neurons show raised iron levels in Parkinson disease |journal=Neurology |volume=68 |issue=21 |pages=1820–5 |date=May 2007 |pmid=17515544 |doi=10.1212/01.wnl.0000262033.01945.9a |url=}}</ref><ref name="pmid22266337">{{cite journal |vauthors=Dusek P, Jankovic J, Le W |title=Iron dysregulation in movement disorders |journal=Neurobiol. Dis. |volume=46 |issue=1 |pages=1–18 |date=April 2012 |pmid=22266337 |doi=10.1016/j.nbd.2011.12.054 |url=}}</ref><ref name="pmid22286308">{{cite journal |vauthors=Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI |title=Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export |journal=Nat. Med. |volume=18 |issue=2 |pages=291–5 |date=January 2012 |pmid=22286308 |doi=10.1038/nm.2613 |url=}}</ref> | |||
}}</ref> | |||
}}</ref> | |||
}}</ref> | |||
=== | ====Immunologic and inflammatory mechanisms==== | ||
=== | *There are some studies supporting the idea of [[Immunology|immunologic]] mechanisms causing [[Parkinson's disease|PD]].<ref name="pmid19296921">{{cite journal |vauthors=Hirsch EC, Hunot S |title=Neuroinflammation in Parkinson's disease: a target for neuroprotection? |journal=Lancet Neurol |volume=8 |issue=4 |pages=382–97 |date=April 2009 |pmid=19296921 |doi=10.1016/S1474-4422(09)70062-6 |url=}}</ref> | ||
*In [[Parkinson's disease|PD]] patients there is elevated amounts of [[cyclooxygenase-2]] which is the rate limiting enzyme in [[Prostaglandin E|prostaglandin E2]] synthesis.<ref name="pmid12702778">{{cite journal |vauthors=Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S |title=Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=100 |issue=9 |pages=5473–8 |date=April 2003 |pmid=12702778 |pmc=154369 |doi=10.1073/pnas.0837397100 |url=}}</ref> | |||
*[[Neuron|Neuronal cell]] death can also occur due to infiltration of [[CD4+ T cells]].<ref name="pmid19104149">{{cite journal |vauthors=Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S |title=Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease |journal=J. Clin. Invest. |volume=119 |issue=1 |pages=182–92 |date=January 2009 |pmid=19104149 |pmc=2613467 |doi=10.1172/JCI36470 |url=}}</ref> | |||
==Genetics== | |||
== | *There are some evidence showing that there is an association between [[Parkinson's disease|PD]] and [[genetic]]. | ||
*This role is higher when Parkinson disease occurs in the individual younger than 50 years old.<ref name="pmid23389780">{{cite journal |vauthors=Singleton AB, Farrer MJ, Bonifati V |title=The genetics of Parkinson's disease: progress and therapeutic implications |journal=Mov. Disord. |volume=28 |issue=1 |pages=14–23 |date=January 2013 |pmid=23389780 |pmc=3578399 |doi=10.1002/mds.25249 |url=}}</ref> | |||
*These studies also demonstrate that if a person has a first degree with [[Parkinson's disease|PD]], the risk of developing [[Parkinson's disease|PD]] is 2 to 3 times higher than normal population. Conversely, in 25 to 50 % of PD patients we can find at least one first degree having [[Parkinson's disease|PD]].<ref name="pmid8710070">{{cite journal |vauthors=Marder K, Tang MX, Mejia H, Alfaro B, Côté L, Louis E, Groves J, Mayeux R |title=Risk of Parkinson's disease among first-degree relatives: A community-based study |journal=Neurology |volume=47 |issue=1 |pages=155–60 |date=July 1996 |pmid=8710070 |doi= |url=}}</ref> | |||
Some of specific genes involving in [[Parkinson's disease|PD]] are: | |||
*[[Glucocerebrosidase|Glucocerebrosidase gene]] <ref name="pmid19846850">{{cite journal |vauthors=Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG |title=Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease |journal=N. Engl. J. Med. |volume=361 |issue=17 |pages=1651–61 |date=October 2009 |pmid=19846850 |pmc=2856322 |doi=10.1056/NEJMoa0901281 |url=}}</ref> | |||
*[[SNCA]]-associated PD <ref name="pmid17761553">{{cite journal |vauthors=Klein C, Schlossmacher MG |title=Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder |journal=Neurology |volume=69 |issue=22 |pages=2093–104 |date=November 2007 |pmid=17761553 |doi=10.1212/01.wnl.0000271880.27321.a7 |url=}}</ref> | |||
*[[LRRK2]]-associated PD <ref name="pmid11891824">{{cite journal |vauthors=Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F |title=A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1 |journal=Ann. Neurol. |volume=51 |issue=3 |pages=296–301 |date=March 2002 |pmid=11891824 |doi= |url=}}</ref> | |||
*[[Parkin]]-associated PD <ref name="pmid10824074">{{cite journal |vauthors=Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denèfle P, Wood NW, Agid Y, Brice A |title=Association between early-onset Parkinson's disease and mutations in the parkin gene |journal=N. Engl. J. Med. |volume=342 |issue=21 |pages=1560–7 |date=May 2000 |pmid=10824074 |doi=10.1056/NEJM200005253422103 |url=}}</ref> | |||
*[[PINK1]]-associated PD <ref name="pmid15087508">{{cite journal |vauthors=Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW |title=Hereditary early-onset Parkinson's disease caused by mutations in PINK1 |journal=Science |volume=304 |issue=5674 |pages=1158–60 |date=May 2004 |pmid=15087508 |doi=10.1126/science.1096284 |url=}}</ref> | |||
*[[DJ-1]]-associated PD <ref name="pmid12446870">{{cite journal |vauthors=Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P |title=Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism |journal=Science |volume=299 |issue=5604 |pages=256–9 |date=January 2003 |pmid=12446870 |doi=10.1126/science.1077209 |url=}}</ref> | |||
==Microscopic Pathology== | |||
[[ | *The pathologic [[hallmark]] of [[Parkinson's disease|PD]] is the presence of [[Lewy body|lewy bodies]], which are round [[cytoplasmic]] [[eosinophilic]] inclusions. The content of these bodies are mostly [[Alpha-synuclein|alpha synuclein]] and [[ubiquitin]], but we can also find [[Complement|complement proteins]], microflament subunits, and parkin substrate protein.<ref name="pmid14991825">{{cite journal |vauthors=Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, Harigaya Y, Sasaki A, Takahashi R, Abe K |title=Pael-R is accumulated in Lewy bodies of Parkinson's disease |journal=Ann. Neurol. |volume=55 |issue=3 |pages=439–42 |date=March 2004 |pmid=14991825 |doi=10.1002/ana.20064 |url=}}</ref> | ||
[[ | *The pathologic manifestations of [[apoptosis]] include condensation of [[chromatin]] and [[cytoplasm]], fragmentation of cell and lysosome-mediated phagocytosis.<ref name="pmid18187492">{{cite journal |vauthors=Pan T, Kondo S, Le W, Jankovic J |title=The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease |journal=Brain |volume=131 |issue=Pt 8 |pages=1969–78 |date=August 2008 |pmid=18187492 |doi=10.1093/brain/awm318 |url=}}</ref> Neuronal [[apoptosis]] occurs in normal individuals (0.5 percent of [[substantia nigra]] [[neurons]]) but in [[Parkinson's disease|PD]] patients this can be as high as 2 percent.<ref name="pmid10809400">{{cite journal |vauthors=Jellinger KA |title=Cell death mechanisms in Parkinson's disease |journal=J Neural Transm (Vienna) |volume=107 |issue=1 |pages=1–29 |date=2000 |pmid=10809400 |doi=10.1007/s007020050001 |url=}}</ref><ref name="pmid12666099">{{cite journal |vauthors=Tatton WG, Chalmers-Redman R, Brown D, Tatton N |title=Apoptosis in Parkinson's disease: signals for neuronal degradation |journal=Ann. Neurol. |volume=53 Suppl 3 |issue= |pages=S61–70; discussion S70–2 |date=2003 |pmid=12666099 |doi=10.1002/ana.10489 |url=}}</ref>. | ||
[[File:Histological_sample_of_Substantia_nigra_in_Parkinson's_disease.jpg|500px|none|thumb|https://librepathology.org/wiki/File:Histological_sample_of_Substantia_nigra_in_Parkinson%27s_disease.jpg]] | |||
[[File:Lewy_bodies_(alpha_synuclein_inclusions).jpg|500px|none|thumb|https://librepathology.org/wiki/File:Lewy_bodies_(alpha_synuclein_inclusions).jpg]] | |||
[[File:Lewy Body alphaSynuclein.jpg|500px|none|thumb|https://librepathology.org/wiki/File:Lewy_Body_alphaSynuclein.jpg]] | |||
[[File:628px-Journal.pone.0008247.g001.png|500px|none|thumb|https://librepathology.org/wiki/File:Journal.pone.0008247.g001.png]] | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 19:09, 17 October 2021
|
Parkinson's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Parkinson's disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Parkinson's disease pathophysiology |
|
Risk calculators and risk factors for Parkinson's disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Fahimeh Shojaei, M.D.
Overview
The underlying pathophysiology of Parkinson disease is dopamine depletion. Reduced number of dopaminergic neurons lead to increased inhibition of thalamus and as a result, decrease excitation of brain cortex, causing bradykinesia. pathologic hallmark of PD is lewy bodies which are round cytoplasmic eosinophilic inclusions. This disease can have so many triggers ( Protein misfolding, Defective proteolysis, Mitochondrial dysfunction, Oxidative stress, Iron metabolism and Immunologic and inflammatory mechanisms) but the main etiology of neuronal degeneration is either apoptosis or necrosis.
Pathophysiology
Physiology
- The substantia nigra (SN), striatum (caudate and putamen), globus pallidus (GP), subthalamic nucleus (STN) and thalamus contribute with each other to make the extrapyramidal system or basal ganglia.
- The impulses from hippocampus, amygdala and prefrontal supplementary motor area to the basal ganglia are excitatory mediated by glutamate.
- The major dopaminergic neurons are in substantia nigra and are responsible for dopaminergic input of striatum. The striatal output is inhibitory (GABA) despite the excitatory (glutamate) output of STN to the globus pallidus (medial and lateral).
- There are 5 dopamine receptors (D1_D5) which are in basal ganglia and limbic system. D1 and D2 are mostly found in the dorsal striatum (motor) and are activated through dopaminergic pathway from SNc, as a result, they are very important in the pathophysiology of Parkinson disease. D3 and D4 are located mostly in mesolimbic or emotional part of the brain and D5 in hippocampus/hypothalamus area.[1]
Phatogenesis
- The underlying pathophysiology of Parkinson disease is dopamine depletion. In the course of the disease dopamine depletion of nigrostriatal pathway will lead to denervation hypersensitivity and increasing number of D2 receptors in dorsal putamen.[2]
- There are two pathways in this system: Direct and indirect pathway.
- Indirect pathway starts with inhibition of striatum via D2 receptor which in turn inhibits neurons of lateral GP by GABA which inhibits the inhibition of STN by lateral GP. STN provides excitatory action on GP internal and SNr via glutamate. GPi inhibit thalamus by GABA but cortex input from thalamus is excitatory.
- Direct pathway starts with excitation of striatum by stimulation of D1 receptors, then striatum inhibits GP internal and SNr by GABA directly. Reduced number of dopaminergic neurons lead to increased inhibition of thalamus and as a result, decrease excitation of brain cortex, causing bradykinesia.[3]
- Our brain has some compensatory mechanism fighting dopamine depletion. It can increase the synthesis of dopamine, gap junctions and the number of D2 receptors.[4][5] It can also reduce the uptake of dopamine from synaptic space.[6]
- The main pathology seen in PD patients is neuronal loss, depigmentation and gliosis which are mostly seen in the locus ceruleus and substantia nigra. The normal number of pigmented neurons in SN in a normal individual is about 550,000, but in patient with PD in can decrease as much as 66%.[7]
- In the normal aging process, neuronal loss occurs in the dorsal tier of SN pars compacta and the most of dopamine depletion is seen in caudate nucleus. But in Parkinson, loss of dopaminergic neurons occurs predominantly in ventrolateral portion of the SN.[8][9]
- The other sites of the brain which are influenced by PD are internal segment of the globus pallidus, center median parafascicular complex, pedunculopontine tegmental nucleus, glutamatergic caudal intralaminar thalamic nuclei and hippocampus.[10][11]
- PD may have so many triggers but the main etiology of neuronal degeneration is either apoptosis or necrosis.[12][13][14]
Protein misfolding
- One of the main underlying cause of PD is mutation in the gene of alpha-synuclein protein which is abundant in the CNS.
- Its function is thought to be involved in synaptic function and plasticity.[15][16]
- This mutations lead to unfold alpha-synuclein and aggregation of insoluble protein and neuronal damage.
- Lewy bodies which are characteristic of PD are mostly build from alpha-synuclein protein.[17]
Defective proteolysis
- There are three pathways which control the protein homeostasis in cells: Molecular chaperons, the ubiquitin-proteasome system and autophagy-lysosomal pathway.
- Alpha synuclein processing is done by all of this three mechanisms and defect in any of them can cause aggregation of this protein and neuronal death.[18][19][20]
Mitochondrial dysfunction
- The drug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, an analog of mepridine is found to be associated with PD.
- The oxidation of this drug produces 1-methyl-4-phenylpyridium which inhibits complex one of mitochondria and result in cell damage.
- Studies showed that the activity of this complex is decreased in PD patients.[21][22][23]
Oxidative stress
- Reactive oxygen species including hydrogen peroxide, superoxide anions and hydroxyradicals are toxic to neurons and cause neuronal damage.
- They interact with membrane lipids and cause lipid peroxidation which can be seen in substantia nigra of PD patients.[24][25]
- They can also cause protein misfolding by attacking disulfide isomerase through nitric oxide. Disulfide isomerase is a chaperone preventing the aggregation of proteins.[26]
Iron metabolism
- Studies showed that impaired iron metabolism leads to increase amount of iron in substantia nigra of PD patients.
- One of the underlying etiology of iron accommodation in neuronal cells is the absence of tau protein.[27][28][29]
Immunologic and inflammatory mechanisms
- There are some studies supporting the idea of immunologic mechanisms causing PD.[30]
- In PD patients there is elevated amounts of cyclooxygenase-2 which is the rate limiting enzyme in prostaglandin E2 synthesis.[31]
- Neuronal cell death can also occur due to infiltration of CD4+ T cells.[32]
Genetics
- There are some evidence showing that there is an association between PD and genetic.
- This role is higher when Parkinson disease occurs in the individual younger than 50 years old.[33]
- These studies also demonstrate that if a person has a first degree with PD, the risk of developing PD is 2 to 3 times higher than normal population. Conversely, in 25 to 50 % of PD patients we can find at least one first degree having PD.[34]
Some of specific genes involving in PD are:
- Glucocerebrosidase gene [35]
- SNCA-associated PD [36]
- LRRK2-associated PD [37]
- Parkin-associated PD [38]
- PINK1-associated PD [39]
- DJ-1-associated PD [40]
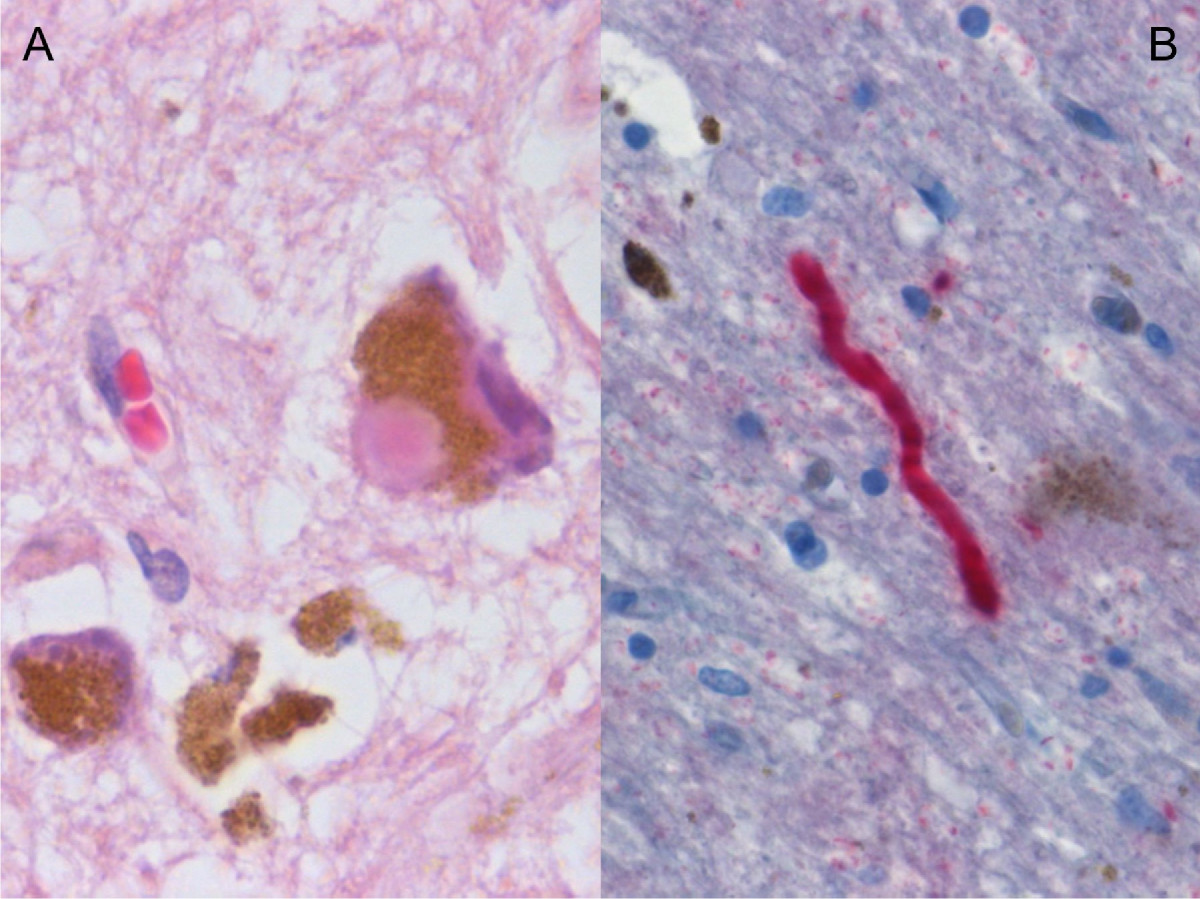
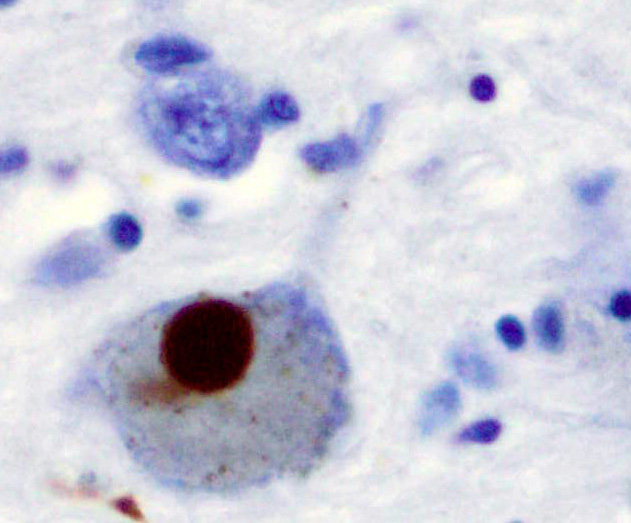
Microscopic Pathology
- The pathologic hallmark of PD is the presence of lewy bodies, which are round cytoplasmic eosinophilic inclusions. The content of these bodies are mostly alpha synuclein and ubiquitin, but we can also find complement proteins, microflament subunits, and parkin substrate protein.[41]
- The pathologic manifestations of apoptosis include condensation of chromatin and cytoplasm, fragmentation of cell and lysosome-mediated phagocytosis.[42] Neuronal apoptosis occurs in normal individuals (0.5 percent of substantia nigra neurons) but in PD patients this can be as high as 2 percent.[43][44].

References
- ↑ Gerfen CR (October 2000). "Molecular effects of dopamine on striatal-projection pathways". Trends Neurosci. 23 (10 Suppl): S64–70. PMID 11052222.
- ↑ Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK (October 2004). "Dopamine modulates release from corticostriatal terminals". J. Neurosci. 24 (43): 9541–52. doi:10.1523/JNEUROSCI.2891-04.2004. PMID 15509741.
- ↑ Gatev P, Darbin O, Wichmann T (October 2006). "Oscillations in the basal ganglia under normal conditions and in movement disorders". Mov. Disord. 21 (10): 1566–77. doi:10.1002/mds.21033. PMID 16830313.
- ↑ Calabresi P, Centonze D, Bernardi G (October 2000). "Electrophysiology of dopamine in normal and denervated striatal neurons". Trends Neurosci. 23 (10 Suppl): S57–63. PMID 11052221.
- ↑ Moore H, Grace AA (December 2002). "A role for electrotonic coupling in the striatum in the expression of dopamine receptor-mediated stereotypies". Neuropsychopharmacology. 27 (6): 980–92. doi:10.1016/S0893-133X(02)00383-4. PMID 12464455.
- ↑ Adams JR, van Netten H, Schulzer M, Mak E, Mckenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, Stoessl AJ (December 2005). "PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation". Brain. 128 (Pt 12): 2777–85. doi:10.1093/brain/awh607. PMID 16081470.
- ↑ Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H (January 1991). "The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method". J. Neurol. Neurosurg. Psychiatry. 54 (1): 30–3. PMC 1014294. PMID 2010756.
- ↑ Porritt M, Stanic D, Finkelstein D, Batchelor P, Lockhart S, Hughes A, Kalnins R, Howells D (July 2005). "Dopaminergic innervation of the human striatum in Parkinson's disease". Mov. Disord. 20 (7): 810–8. doi:10.1002/mds.20399. PMID 15726582.
- ↑ Fearnley JM, Lees AJ (October 1991). "Ageing and Parkinson's disease: substantia nigra regional selectivity". Brain. 114 ( Pt 5): 2283–301. PMID 1933245.
- ↑ Henderson JM, Carpenter K, Cartwright H, Halliday GM (March 2000). "Degeneration of the centré median-parafascicular complex in Parkinson's disease". Ann. Neurol. 47 (3): 345–52. PMID 10716254.
- ↑ Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA (July 2003). "Parkinson's disease is associated with hippocampal atrophy". Mov. Disord. 18 (7): 784–90. doi:10.1002/mds.10444. PMID 12815657.
- ↑ Savitt JM, Dawson VL, Dawson TM (July 2006). "Diagnosis and treatment of Parkinson disease: molecules to medicine". J. Clin. Invest. 116 (7): 1744–54. doi:10.1172/JCI29178. PMC 1483178. PMID 16823471.
- ↑ Lang AE (March 2007). "The progression of Parkinson disease: a hypothesis". Neurology. 68 (12): 948–52. doi:10.1212/01.wnl.0000257110.91041.5d. PMID 17372132.
- ↑ Atkin G, Paulson H (2014). "Ubiquitin pathways in neurodegenerative disease". Front Mol Neurosci. 7: 63. doi:10.3389/fnmol.2014.00063. PMC 4085722. PMID 25071440.
- ↑ Maries E, Dass B, Collier TJ, Kordower JH, Steece-Collier K (September 2003). "The role of alpha-synuclein in Parkinson's disease: insights from animal models". Nat. Rev. Neurosci. 4 (9): 727–38. doi:10.1038/nrn1199. PMID 12951565.
- ↑ Calo L, Wegrzynowicz M, Santivañez-Perez J, Grazia Spillantini M (February 2016). "Synaptic failure and α-synuclein". Mov. Disord. 31 (2): 169–77. doi:10.1002/mds.26479. PMID 26790375.
- ↑ Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (August 1997). "Alpha-synuclein in Lewy bodies". Nature. 388 (6645): 839–40. doi:10.1038/42166. PMID 9278044.
- ↑ Lim KL, Zhang CW (2013). "Molecular events underlying Parkinson's disease - an interwoven tapestry". Front Neurol. 4: 33. doi:10.3389/fneur.2013.00033. PMC 3619247. PMID 23580245.
- ↑ Dehay B, Martinez-Vicente M, Caldwell GA, Caldwell KA, Yue Z, Cookson MR, Klein C, Vila M, Bezard E (June 2013). "Lysosomal impairment in Parkinson's disease". Mov. Disord. 28 (6): 725–32. doi:10.1002/mds.25462. PMC 5131721. PMID 23580333.
- ↑ Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ (January 2014). "Autophagy and apoptosis dysfunction in neurodegenerative disorders". Prog. Neurobiol. 112: 24–49. doi:10.1016/j.pneurobio.2013.10.004. PMID 24211851.
- ↑ Przedborski S, Tieu K, Perier C, Vila M (August 2004). "MPTP as a mitochondrial neurotoxic model of Parkinson's disease". J. Bioenerg. Biomembr. 36 (4): 375–9. doi:10.1023/B:JOBB.0000041771.66775.d5. PMID 15377875.
- ↑ Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB (April 2012). "Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling". J. Clin. Invest. 122 (4): 1354–67. doi:10.1172/JCI61332. PMC 3314472. PMID 22446186.
- ↑ Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (June 1989). "Mitochondrial complex I deficiency in Parkinson's disease". Lancet. 1 (8649): 1269. PMID 2566813.
- ↑ Greenamyre JT, Hastings TG (May 2004). "Biomedicine. Parkinson's--divergent causes, convergent mechanisms". Science. 304 (5674): 1120–2. doi:10.1126/science.1098966. PMID 15155938.
- ↑ Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT (November 2003). "Mechanism of toxicity in rotenone models of Parkinson's disease". J. Neurosci. 23 (34): 10756–64. PMID 14645467.
- ↑ Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA (May 2006). "S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration". Nature. 441 (7092): 513–7. doi:10.1038/nature04782. PMID 16724068.
- ↑ Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM (May 2007). "Individual dopaminergic neurons show raised iron levels in Parkinson disease". Neurology. 68 (21): 1820–5. doi:10.1212/01.wnl.0000262033.01945.9a. PMID 17515544.
- ↑ Dusek P, Jankovic J, Le W (April 2012). "Iron dysregulation in movement disorders". Neurobiol. Dis. 46 (1): 1–18. doi:10.1016/j.nbd.2011.12.054. PMID 22266337.
- ↑ Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI (January 2012). "Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export". Nat. Med. 18 (2): 291–5. doi:10.1038/nm.2613. PMID 22286308.
- ↑ Hirsch EC, Hunot S (April 2009). "Neuroinflammation in Parkinson's disease: a target for neuroprotection?". Lancet Neurol. 8 (4): 382–97. doi:10.1016/S1474-4422(09)70062-6. PMID 19296921.
- ↑ Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S (April 2003). "Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5473–8. doi:10.1073/pnas.0837397100. PMC 154369. PMID 12702778.
- ↑ Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (January 2009). "Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease". J. Clin. Invest. 119 (1): 182–92. doi:10.1172/JCI36470. PMC 2613467. PMID 19104149.
- ↑ Singleton AB, Farrer MJ, Bonifati V (January 2013). "The genetics of Parkinson's disease: progress and therapeutic implications". Mov. Disord. 28 (1): 14–23. doi:10.1002/mds.25249. PMC 3578399. PMID 23389780.
- ↑ Marder K, Tang MX, Mejia H, Alfaro B, Côté L, Louis E, Groves J, Mayeux R (July 1996). "Risk of Parkinson's disease among first-degree relatives: A community-based study". Neurology. 47 (1): 155–60. PMID 8710070.
- ↑ Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Dürr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG (October 2009). "Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease". N. Engl. J. Med. 361 (17): 1651–61. doi:10.1056/NEJMoa0901281. PMC 2856322. PMID 19846850.
- ↑ Klein C, Schlossmacher MG (November 2007). "Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder". Neurology. 69 (22): 2093–104. doi:10.1212/01.wnl.0000271880.27321.a7. PMID 17761553.
- ↑ Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F (March 2002). "A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1". Ann. Neurol. 51 (3): 296–301. PMID 11891824.
- ↑ Lücking CB, Dürr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denèfle P, Wood NW, Agid Y, Brice A (May 2000). "Association between early-onset Parkinson's disease and mutations in the parkin gene". N. Engl. J. Med. 342 (21): 1560–7. doi:10.1056/NEJM200005253422103. PMID 10824074.
- ↑ Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW (May 2004). "Hereditary early-onset Parkinson's disease caused by mutations in PINK1". Science. 304 (5674): 1158–60. doi:10.1126/science.1096284. PMID 15087508.
- ↑ Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (January 2003). "Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism". Science. 299 (5604): 256–9. doi:10.1126/science.1077209. PMID 12446870.
- ↑ Murakami T, Shoji M, Imai Y, Inoue H, Kawarabayashi T, Matsubara E, Harigaya Y, Sasaki A, Takahashi R, Abe K (March 2004). "Pael-R is accumulated in Lewy bodies of Parkinson's disease". Ann. Neurol. 55 (3): 439–42. doi:10.1002/ana.20064. PMID 14991825.
- ↑ Pan T, Kondo S, Le W, Jankovic J (August 2008). "The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson's disease". Brain. 131 (Pt 8): 1969–78. doi:10.1093/brain/awm318. PMID 18187492.
- ↑ Jellinger KA (2000). "Cell death mechanisms in Parkinson's disease". J Neural Transm (Vienna). 107 (1): 1–29. doi:10.1007/s007020050001. PMID 10809400.
- ↑ Tatton WG, Chalmers-Redman R, Brown D, Tatton N (2003). "Apoptosis in Parkinson's disease: signals for neuronal degradation". Ann. Neurol. 53 Suppl 3: S61–70, discussion S70–2. doi:10.1002/ana.10489. PMID 12666099.