Gout: Difference between revisions
| Line 9: | Line 9: | ||
== [[Gout historical perspective|Historical Perspective]] == | == [[Gout historical perspective|Historical Perspective]] == | ||
== [[Gout pathophysiology|Pathophysiology]]== | == [[Gout pathophysiology|Pathophysiology]]== | ||
Revision as of 13:38, 26 May 2020
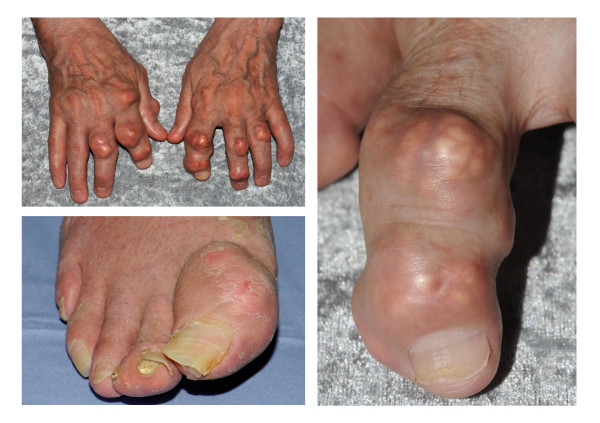
For patient information click here
|
Gout Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Gout On the Web |
|
American Roentgen Ray Society Images of Gout |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2] Synonyms and keywords: Urate crystal arthropathy; uric acid crystal deposition in joint; gouty arthritis; podagra
Overview
Historical Perspective
Pathophysiology
Differentiating Gout from other Diseases
Epidemiology and Demographics
Risk Factors
Screening
Natural History, Complications and Prognosis
Diagnosis
The diagnosis of gout is based upon the identification of intracellular monosodium urate (MSU) crystals in the synovial fluid aspirate of an affected joint, under polarizing light microscopy. But when this is not possible, a clinical diagnosis can be deduced with the help of classical clinical features, including the history and physical examination, laboratory findings, and various imaging studies.
Diagnosis of acute gout
- While the favored approach is to find MSU crystals in the synovial fluid aspirate of an affected joint, in clinical practice a crystal evaluation is routinely not done[2][3].
- When a patient is presenting with classic symptoms of rapid onset (within 24 hours), podagra, swelling, and erythema, supported by the presence of hyperuricemia, a clinical diagnosis of gout can easily be concluded. [4]
- When an arthrocentesis is done, synovial fluid should be examined readily under routine light and polarizing light microscopy and looked for negatively birefringent needle-shaped MSU crystals. [5]
- In addition, testing for cell counts with differential, gram staining and culture should also be done on the aspirate.
- The sensitivity of this technique in demonstrating negatively birefringent intra- and extracellular crystals in patients with gout flares is at least 85 percent, and the specificity for gout is 100 percent. [6] [7]. The sensitivity of can be further improved by examination of the sediment in a centrifuged specimen. [8]
| Criteria | Sensitivity | Specificity | |
|---|---|---|---|
| ARA (ACR) | 6 of 12 criteria | 70% | 79% |
| Rome | 2 of 4 criteria: • Painful joint swelling, abrupt onset, Clearing in 1-2 weeks initially • Serum uric acid: >7 in males; >6 in females • Presence of tophi • Urate crystals in synovial fluid or tissues |
70% | 83% |
| New York | 2 of 5 criteria: • 2 attacks of painful limb joint swelling • Abrupt onset and remission in 1—2 weeks initially • First MTP attack • Presence of a tophus • Response to colchicine-major reduction in inflammation within 48 h |
67% | 89% |
Several sets of diagnostic criteria exit (see table).[9]
| Sensitivity | Specificity | |
|---|---|---|
| > 5.88 mg/dl[10] | 95% | 53% |
| ≥ 6 mg/dl[11] | 86% | ? |
| ≥ 8 mg/dl[11] | 68% | ? |
A clinical prediction rule (online link) found that the following predicted urate crystals by aspiration:[10]
- Male
- Onset within one day
- Joint redness
- First metatarsaophalangeal joint
- Previous arthritis attack per patient
- History of hypertension or 1 or more cardiovascular diseases
- Serum uric acid level > 5.88 mg/dl
However, among patients with high scores, 20% did not have crystals. Only one of 381 patients had bacterial arthritis.
Diagnosis of intercritical and tophaceous gout
- In patients where a diagnosis of gout wasn’t ascertained during an acute flare, a synovial fluid analysis identifying urate crystals from the previously affected joints would allow a late establishment of the disease.
- Urate crystals are present in synovial fluid of all untreated gouty patients and in approximately 70 percent of those under urate-lowering therapy. 8624633 10577299 444319
- For tophaceous gout, demonstration of urate crystals in aspirates of tophi provides an easy way to confirm the diagnosis 10834006
Clinical diagnosis “rule” for acute gout
- In patients with acute gout where a diagnosis couldn’t be confirmed due to a negative synovial fluid analysis for MSU crystals, a clinical diagnostic approach can be implemented. 20625017
- This approach utilizes a set of clinical parameters with a scoring value. The parameters are derived from history, clinical presentation, and the laboratory findings. 25231179
- This approach has been shown to improve the accuracy of diagnosis without joint fluid analysis of a gout flare in primary care practice settings 20625017
- The model uses seven variables to calculate a total score to distinguish three levels of risk for gout. These are:
- Male sex (2 points)
- Previous patient-reported arthritis flare (2 points)
- Onset within one day (0.5 points)
- Joint redness (1 point)
- First metatarsal phalangeal joint involvement (2.5 points)
- Hypertension or at least one cardiovascular disease (1.5 points)
- Serum urate level greater than 5.88 mg/dL (3.5 points)
- Based upon the total score, patients can be identified as having low (≤4 points), intermediate (>4 to <8 points), or high (≥8 points) probability of having acute gout.
- In patients with an intermediate score (>4 but <8 points), a preliminary diagnosis of gout may be made for the purpose of clinical management based upon a prevalent clinical features favoring gout.
- This diagnostic approach is not recommended for patients presenting with oligoarticular and polyarticular arthritis, as it was developed studying patients with monoarthritis seen by family physicians.
Treatment
Clinical practice guidelines address treatment.[12][13][14] However, trials comparing glucocorticoids (steroids) and non-steroidal anti-inflammatory agents (NSAIDs) were not published till after the guidelines.
A nurse-led protocol with treatment goal of 6 mg/dL was beneficial[15].
Regarding medications, if there are no mitigating factors in choosing a drug, glucocorticoids, non-steroidal anti-inflammatory agents (NSAIDs), and colchicine all work; however, colchicine consistently causes drug toxicity.
A combination treatment is ice four times a day with oral prednisone 30 mg orally tapered over 6 days (30 mg for two days, 20 mg for two days, 10 mg for two days) and colchicine 0.6 mg/day.[16] An advantage of this regimen is the reduced toxicity from the low dose of colchicine and that the colchicine helps prevent flares if allopurinol is later started. Colchicine has been combined with NSAIDs[17] that are not metabolized by the CYP3A4 isoenzyme of cytochrome P-450 (naproxen is not metabolized by CYP3A4). Combining glucocorticoids with NSAIDs increased the risk for gastrointestinal drug toxicity[18]
Local ice
Ice packs, applied for 30 minutes 4 times per day, can help according to a randomized controlled trial without allocation concealment.[16] In this trial, ice reduced the visual pain analog score by an additional 33 mm beyond the reduction provided by a combination of glucocorticoids and colchicine.
Non-steroidal anti-inflammatory agents
Non-steroidal anti-inflammatory agents (NSAIDs) are better than placebo according to a randomized controlled trial of 30 total patients.[19] According to a summary of this trial, "the knee was affected in 14 cases and the great toe in only two cases. After 24 h, 67% of tenoxicam group had ≥50% reduction in pain compared with 26% of placebo group (P<0.05). However, at the end of the treatment (4 days), there was no significant difference between the groups."[20]
Glucocorticoids
| Patients | Interventions | Results | ||
|---|---|---|---|---|
| Steroid | NSAID | |||
| Janssens et al 2008[21] | 120 total patients with uric acid crystals on arthrocentesis | Prednisolone 35 mg once daily for 5 days | Naproxen 500 mg twice daily for 5 days | NSAID trended better (88% versus 80% response; p=0.3) No differences in rates of drug toxicity. |
| Man et al 2007[22] | 90 total patients with clinical diagnosis of gout† | Initially prednisolone 30 mg Followed by prednisolone 30 mg daily for 5 days and as needed acetaminophen |
Initially diclofenac 75 mg with indomethacin 50 mg Followed by indomethacin 50 mg every 8 hrs for 2 days then 25 mg every 8 hrs for 3 days and as needed acetaminophen. |
Steroids faster reduction in pain. Steroids used more acetaminophen. More adverse effects from indomethacin. Indomethacin trended to more relapses at 2 weeks (11% vs 17%). |
| Notes: † Clinical diagnosis of gout was "pain and warmth in a joint, and presented within 3 days of the onset of pain and also had 1 or more of the following: metatarsal-phalangeal joint involvement; knee or ankle joint involvement and aspirate containing crystals; or typical gouty arthritis, with either gouty tophi present or previous joint aspiration confirming the diagnosis of gout." Seven patients allowed arthrocentesis and all were positive for gout. | ||||
Randomized controlled trials find similar benefit from non-steroidal anti-inflammatory agents and oral glucocorticoids. In the first trial the reduction in visual analog scale after 5 days was 44.7 with prednisolone and 46.0 with naproxen.[22] Less adverse drug reactions occurred in the glucocorticoids group; however, the NSAID group received a high dose (50 mg every 8 hours for 2 days, followed by 25 mg every 8 hours for 3 days)[23].
In the second randomized controlled trial statistically equal effect resulted from prednisolone 35 mg orally per day or naproxen 500 mg orally twice per day; however there was an insignificant 8% improvement in the NSAID group.[21] There were no significant differences in drug toxicity.
Colchicine
Colchicine is better than placebo according to a systematic review by the Cochrane Collaboration[24] that found a single randomized controlled trial of 43 patients[25]. In this study, colchicine 1 mg orally, followed by 0.5 mg every two hours led to a 50% reduction in pain in about 70% of patients compared to about 35% of patients who received placebo. However, all patients had drug toxicity from colchicine and in 90% of the patients toxicity occurred before 50% reduction in pain.
Regarding the best dose, 1.2 mg followed by 0.6 mg in 1 hour may be as effective as higher dose.[26]
To avoid drug toxicity, lower doses of colchicine (0.6 per day) have been used in combination with glucocorticoids.[16] The UK National Library for Health recommends 0.5 mg two to four times a day.[27]
Anti-cytokines
The monoclonal antibody against interleukin-1 beta, canakinumab, may help according to a randomized controlled trial.[28]
Prognosis
Acute flares
Without treatment, one third of flares improve within 2 days.[25]
Case Studies
Related Chapter
External Links
- "Answers and Questions on Gout". U.S. National Institutes of Health—National Institute of Arthritis and Musculoskeletal and Skin Diseases. September 28th, 2007. Retrieved 2007-08-28. Check date values in:
|date=(help) - "Coffee Consumption and Reduced Gout Risk". Drinking coffee reduces risk of gout in middle age men. U.S. National Institutes of Health. Retrieved 2007-05-25.
References
- ↑ Roddy, Edward (2011). "Revisiting the pathogenesis of podagra: why does gout target the foot?". Journal of Foot and Ankle Research. 4 (1). doi:10.1186/1757-1146-4-13. ISSN 1757-1146.
- ↑ Neogi T (2011). "Clinical practice. Gout". N Engl J Med. 364 (5): 443–52. doi:10.1056/NEJMcp1001124. PMID 21288096.
- ↑ Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G (2004). "Purine-rich foods, dairy and protein intake, and the risk of gout in men". N Engl J Med. 350 (11): 1093–103. doi:10.1056/NEJMoa035700. PMID 15014182.
- ↑ http://pubmed.gov/16707533
- ↑ http://pubmed.gov/13773775
- ↑ http://pubmed.gov/856219
- ↑ http://pubmed.gov/16462524
- ↑ http://pubmed.gov/10803751
- ↑ 9.0 9.1 Malik A, Schumacher HR, Dinnella JE, Clayburne GM (2009). "Clinical diagnostic criteria for gout: comparison with the gold standard of synovial fluid crystal analysis". J Clin Rheumatol. 15 (1): 22–4. doi:10.1097/RHU.0b013e3181945b79. PMID 19125136.
- ↑ 10.0 10.1 10.2 Janssens HJ, Fransen J, van de Lisdonk EH, van Riel PL, van Weel C, Janssen M (2010). "A diagnostic rule for acute gouty arthritis in primary care without joint fluid analysis". Arch Intern Med. 170 (13): 1120–6. doi:10.1001/archinternmed.2010.196. PMID 20625017.
- ↑ 11.0 11.1 11.2 Schlesinger N, Norquist JM, Watson DJ (2009). "Serum urate during acute gout". J. Rheumatol. 36 (6): 1287–9. doi:10.3899/jrheum.080938. PMID 19369457. Unknown parameter
|month=ignored (help) - ↑ Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T; et al. (2012). "2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis". Arthritis Care Res (Hoboken). 64 (10): 1447–61. doi:10.1002/acr.21773. PMID 23024029.
- ↑ Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T; et al. (2012). "2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia". Arthritis Care Res (Hoboken). 64 (10): 1431–46. doi:10.1002/acr.21772. PMID 23024028.
- ↑ Zhang W, Doherty M, Bardin T; et al. (2006). "EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT)". Ann. Rheum. Dis. 65 (10): 1312–24. doi:10.1136/ard.2006.055269. PMID 16707532. Unknown parameter
|month=ignored (help) - ↑ Doherty M, Jenkins W, Richardson H, Sarmanova A, Abhishek A, Ashton D; et al. (2018). "Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial". Lancet. 392 (10156): 1403–1412. doi:10.1016/S0140-6736(18)32158-5. PMC 6196879. PMID 30343856.
- ↑ 16.0 16.1 16.2 Schlesinger N, Detry MA, Holland BK; et al. (2002). "Local ice therapy during bouts of acute gouty arthritis". J. Rheumatol. 29 (2): 331–4. PMID 11838852.
- ↑ Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA (2004). "Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis". J. Rheumatol. 31 (12): 2429–32. PMID 15570646. Unknown parameter
|month=ignored (help) - ↑ Fries JF, Williams CA, Bloch DA, Michel BA (1991). "Nonsteroidal anti-inflammatory drug-associated gastropathy: incidence and risk factor models". Am. J. Med. 91 (3): 213–22. PMID 1892140. Unknown parameter
|month=ignored (help) - ↑ García de la Torre, Ignacio. (1987) Estudio doble-ciego paralelo, comparativo con tenoxicam vs placebo en artritis gotosa aguda (A comparative, double-blind, parallel study with tenoxicam vs placebo in acute gouty arthritis). Invet Med Int '14:'92–7 [Abstract in Spanish]
- ↑ Sutaria S, Katbamna R, Underwood M (2006). "Effectiveness of interventions for the treatment of acute and prevention of recurrent gout--a systematic review". Rheumatology (Oxford). 45 (11): 1422–31. doi:10.1093/rheumatology/kel071. PMID 16632483. Unknown parameter
|month=ignored (help) - ↑ 21.0 21.1 Janssens HJ, Janssen M, van de Lisdonk EH, van Riel PL, van Weel C (2008). "Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial". Lancet. 371 (9627): 1854–60. doi:10.1016/S0140-6736(08)60799-0. PMID 18514729. Unknown parameter
|month=ignored (help) - ↑ 22.0 22.1 Man CY, Cheung IT, Cameron PA, Rainer TH (2007). "Comparison of oral prednisolone/paracetamol and oral indomethacin/paracetamol combination therapy in the treatment of acute goutlike arthritis: a double-blind, randomized, controlled trial". Annals of emergency medicine. 49 (5): 670–7. doi:10.1016/j.annemergmed.2006.11.014. PMID 17276548.
- ↑ Henry D, Lim LL, Garcia Rodriguez LA; et al. (1996). "Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis". BMJ. 312 (7046): 1563–6. PMC 2351326. PMID 8664664. Unknown parameter
|month=ignored (help) - ↑ Schlesinger N, Schumacher R, Catton M, Maxwell L (2006). "Colchicine for acute gout". Cochrane Database Syst Rev (4): CD006190. doi:10.1002/14651858.CD006190. PMID 17054279.
- ↑ 25.0 25.1 Ahern MJ, Reid C, Gordon TP, McCredie M, Brooks PM, Jones M (1987). "Does colchicine work? The results of the first controlled study in acute gout". Aust N Z J Med. 17 (3): 301–4. doi:10.1111/j.1445-5994.1987.tb01232.x. PMID 3314832. Unknown parameter
|month=ignored (help) Summary at Bandolier - ↑ Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW (2010). "High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study". Arthritis Rheum. 62 (4): 1060–8. doi:10.1002/art.27327. PMID 20131255.
- ↑ CKS (2007) Gout - Management (Topic Review). Clinical Knowledge Summaries. http://cks.library.nhs.uk/gout/management [Accessed: Date]
- ↑ So A, De Meulemeester M, Pikhlak A, Yücel AE, Richard D, Murphy V; et al. (2010). "Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study". Arthritis Rheum. 62 (10): 3064–76. doi:10.1002/art.27600. PMID 20533546.
Template:Diseases of the musculoskeletal system and connective tissue
ar:نقرس bg:Подагра cs:Dna da:Gigt de:Gicht eo:Podagro fa:نقرس io:Kiragro id:Gout it:Gotta he:שיגדון lb:Giicht ms:Gout nl:Jicht no:Urinsyregikt sk:Dna sr:Гихт fi:Kihti sv:Gikt te:గౌటు