Flavoxate: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EJ}} + & -{{EH}} + & -{{Editor Join}} + & -{{Editor Help}} +)) |
Rabin Bista (talk | contribs) No edit summary |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| IUPAC_name = 2-(1-piperidyl)ethyl 3-methyl-4-oxo-2- | |authorTag=<!--Overview-->{{RB}} | ||
| image = | |genericName=Flavoxate hydrochloride | ||
|aOrAn=a | |||
|drugClass=anticholinergic agent | |||
|indicationType=treatment | |||
|indication=[[dysuria]], [[urgency]], [[nocturia]], [[suprapubic pain]], frequency and incontinence as may occur in [[cystitis]], [[prostatitis]], [[urethritis]], [[urethrocystitis]]/urethrotrigonitis. | |||
|adverseReactions=[[Nausea]], [[vomiting]], [[headache]], [[blurred vision]], [[drowsiness]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult=====Indications==== | |||
* Flavoxate HCl tablets are indicated for symptomatic relief of [[dysuria]], [[urgency]], [[nocturia]], [[suprapubic pain]], [[frequency]] and [[incontinence]] as may occur in [[cystitis]], [[prostatitis]], [[urethritis]], [[urethrocystitis]]/[[urethrotrigonitis]]. Flavoxate HCl tablets are not indicated for definitive treatment, but are compatible with drugs used for the treatment of [[urinary tract infections]]. | |||
====Dosage==== | |||
=====Adults and children over 12 years of age===== | |||
* One or two 100 mg tablets 3 or 4 times a day. With improvement of symptoms, the dose may be reduced. This drug cannot be recommended for infants and children under 12 years of age because safety and efficacy have not been demonstrated in this age group. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed=* This drug cannot be recommended for infants and children under 12 years of age because safety and efficacy have not been demonstrated in this age group. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications=* Flavoxate HCl tablets are contraindicated in patients who have any of the following obstructive conditions: pyloric or [[duodenal obstruction]], obstructive intestinal lesions or [[ileus]], [[achalasia]], [[gastrointestinal hemorrhage]] and [[obstructive uropathies]] of the [[lower urinary tract]]. | |||
<!--Warnings--> | |||
|warnings=* Flavoxate HCl should be given cautiously in patients with suspected [[glaucoma]]. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials=* The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency. | |||
=====Gastrointestinal===== | |||
* [[Nausea]], [[vomiting]], [[dry mouth]]. | |||
=====CNS===== | |||
* [[Vertigo]], [[headache]], [[mental confusion]], especially in the elderly, [[drowsiness]], [[nervousness]]. | |||
=====Hematologic===== | |||
* [[Leukopenia]] (one case which was reversible upon discontinuation of the drug). | |||
=====Cardiovascular===== | |||
* [[Tachycardia]] and [[palpitation]]. | |||
=====Allergic===== | |||
* [[Urticaria]] and other [[dermatoses]], [[eosinophilia]] and [[hyperpyrexia]]. | |||
=====Ophthalmic===== | |||
* Increased ocular tension, [[blurred vision]], disturbance in eye [[accommodation]]. | |||
=====Renal===== | |||
* [[Dysuria]]. | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=* Reproduction studies have been performed in rats and rabbits at doses up to 34 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to flavoxate HCl. There are, however, no well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when flavoxate HCl is administered to a nursing woman. | |||
|useInPed=* Safety and effectiveness in children below the age of 12 years have not been established. | |||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Oral | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* The oral LD50 for flavoxate HCl in rats is 4273 mg/kg. The oral LD50 for flavoxate HCl in mice is 1837 mg/kg. | |||
* It is not known whether flavoxate HCl is dialyzable. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 459435750 | |||
| IUPAC_name = 2-(1-piperidyl)ethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate | |||
| image = Flavoxate Wiki Str.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|monograph|flavoxate-hydrochloride}} | |||
| MedlinePlus = a682706 | |||
| pregnancy_category = | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 15301-69-6 | | CAS_number = 15301-69-6 | ||
| ATC_prefix = G04 | | ATC_prefix = G04 | ||
| ATC_suffix = BD02 | | ATC_suffix = BD02 | ||
| ATC_supplemental = | | ATC_supplemental = | ||
| PubChem = 3354 | | PubChem = 3354 | ||
| DrugBank = | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| C=24 | H=25 | N=1 | O=4 | | DrugBank = DB01148 | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3237 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 3E74Y80MEY | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07961 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 5088 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1493 | |||
<!--Chemical data--> | |||
| C=24 | H=25 | N=1 | O=4 | |||
| molecular_weight = 391.46 g/mol | | molecular_weight = 391.46 g/mol | ||
| | | smiles = O=C(OCCN1CCCCC1)c4cccc2c4O/C(=C(\C2=O)C)c3ccccc3 | ||
| | | InChI = 1/C24H25NO4/c1-17-21(26)19-11-8-12-20(23(19)29-22(17)18-9-4-2-5-10-18)24(27)28-16-15-25-13-6-3-7-14-25/h2,4-5,8-12H,3,6-7,13-16H2,1H3 | ||
| | | InChIKey = SPIUTQOUKAMGCX-UHFFFAOYAW | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChI = 1S/C24H25NO4/c1-17-21(26)19-11-8-12-20(23(19)29-22(17)18-9-4-2-5-10-18)24(27)28-16-15-25-13-6-3-7-14-25/h2,4-5,8-12H,3,6-7,13-16H2,1H3 | ||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChIKey = SPIUTQOUKAMGCX-UHFFFAOYSA-N | ||
}} | }} | ||
= | <!--Mechanism of Action--> | ||
Flavoxate | |mechAction=* Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle. | ||
<!--Structure--> | |||
|structure=* Flavoxate HCl tablets contain flavoxate hydrochloride, a synthetic urinary tract spasmolytic. | |||
Flavoxate HCl is | * Chemically, flavoxate hydrochloride is 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate hydrochloride. The empirical formula of flavoxate hydrochloride is C24H25NO4•HCl. The molecular weight is 427.94. The structural formula appears below: | ||
: [[File:Flavoxate Str.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Flavoxate HCl is supplied in tablets for oral administration. Each round, white, film-coated Flavoxate HCl tablet is debossed "PAD" and "0115" on one side and plain on the other side and contains flavoxate hydrochloride, 100 mg. Inactive ingredients consist of colloidal [[silicon dioxide]], [[ethyl acrylate]], [[hypromellose]], [[lactose monohydrate]], [[magnesium stearate]], [[methyl methacrylate]], microcrystalline cellulose, nonoxynol 100 and sodium starch glycolate. Film coating is composed of hypromellose 2910 6cP and polyethylene glycol. | |||
<!--Pharmacodynamics--> | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK=* In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate HCl was excreted in the urine within 24 hours. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
* Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of flavoxate HCl have not been performed. | |||
<!--Clinical Studies--> | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied=* Flavoxate HCl Tablets, 100 mg, are supplied as round, white, film-coated tablets debossed "PAD" and "0115" on one side and plain on the other side, in bottles of 100. | |||
: 100 mg 100's: | |||
: NDC 0574-0115-01 | |||
|storage=* Store at 20° to 25°C (68° to 77°F) | |||
|packLabel=====PRINCIPAL DISPLAY PANEL - 100 MG TABLET BOTTLE==== | |||
Rx Only | |||
NDC 0574-0115-01 | |||
Flavoxate HCl Tablets | |||
100 mg | |||
100 Tablets | |||
: [[File:Flavoxate PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Flavoxate Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=* Patients should be informed that if drowsiness and blurred vision occur, they should not operate a motor vehicle or machinery or participate in activities where alertness is required. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* Urispas®<ref>{{Cite web | title = Flavoxate hydrochloride | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a9c44c4b-5b3c-45bf-9bc9-ce858e3d06c5}}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Latest revision as of 14:12, 3 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Flavoxate is a anticholinergic agent that is FDA approved for the treatment of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis.. Common adverse reactions include Nausea, vomiting, headache, blurred vision, drowsiness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Flavoxate HCl tablets are indicated for symptomatic relief of dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigonitis. Flavoxate HCl tablets are not indicated for definitive treatment, but are compatible with drugs used for the treatment of urinary tract infections.
Dosage
Adults and children over 12 years of age
- One or two 100 mg tablets 3 or 4 times a day. With improvement of symptoms, the dose may be reduced. This drug cannot be recommended for infants and children under 12 years of age because safety and efficacy have not been demonstrated in this age group.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flavoxate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flavoxate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- This drug cannot be recommended for infants and children under 12 years of age because safety and efficacy have not been demonstrated in this age group.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Flavoxate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Flavoxate in pediatric patients.
Contraindications
- Flavoxate HCl tablets are contraindicated in patients who have any of the following obstructive conditions: pyloric or duodenal obstruction, obstructive intestinal lesions or ileus, achalasia, gastrointestinal hemorrhage and obstructive uropathies of the lower urinary tract.
Warnings
- Flavoxate HCl should be given cautiously in patients with suspected glaucoma.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions have been observed, but there are not enough data to support an estimate of their frequency.
Gastrointestinal
CNS
- Vertigo, headache, mental confusion, especially in the elderly, drowsiness, nervousness.
Hematologic
- Leukopenia (one case which was reversible upon discontinuation of the drug).
Cardiovascular
- Tachycardia and palpitation.
Allergic
- Urticaria and other dermatoses, eosinophilia and hyperpyrexia.
Ophthalmic
- Increased ocular tension, blurred vision, disturbance in eye accommodation.
Renal
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Flavoxate in the drug label.
Drug Interactions
There is limited information regarding Flavoxate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at doses up to 34 times the human dose and revealed no evidence of impaired fertility or harm to the fetus due to flavoxate HCl. There are, however, no well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Flavoxate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Flavoxate during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when flavoxate HCl is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in children below the age of 12 years have not been established.
Geriatic Use
There is no FDA guidance on the use of Flavoxate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Flavoxate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Flavoxate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Flavoxate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Flavoxate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Flavoxate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Flavoxate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Flavoxate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Flavoxate in the drug label.
Overdosage
- The oral LD50 for flavoxate HCl in rats is 4273 mg/kg. The oral LD50 for flavoxate HCl in mice is 1837 mg/kg.
- It is not known whether flavoxate HCl is dialyzable.
Pharmacology
| |
Flavoxate
| |
| Systematic (IUPAC) name | |
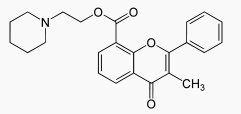
| 2-(1-piperidyl)ethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate | |
| Identifiers | |
| CAS number | |
| ATC code | G04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 391.46 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Flavoxate hydrochloride counteracts smooth muscle spasm of the urinary tract and exerts its effect directly on the muscle.
Structure
- Flavoxate HCl tablets contain flavoxate hydrochloride, a synthetic urinary tract spasmolytic.
- Chemically, flavoxate hydrochloride is 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4H-1-benzopyran-8-carboxylate hydrochloride. The empirical formula of flavoxate hydrochloride is C24H25NO4•HCl. The molecular weight is 427.94. The structural formula appears below:
- Flavoxate HCl is supplied in tablets for oral administration. Each round, white, film-coated Flavoxate HCl tablet is debossed "PAD" and "0115" on one side and plain on the other side and contains flavoxate hydrochloride, 100 mg. Inactive ingredients consist of colloidal silicon dioxide, ethyl acrylate, hypromellose, lactose monohydrate, magnesium stearate, methyl methacrylate, microcrystalline cellulose, nonoxynol 100 and sodium starch glycolate. Film coating is composed of hypromellose 2910 6cP and polyethylene glycol.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Flavoxate in the drug label.
Pharmacokinetics
- In a single study of 11 normal male subjects, the time to onset of action was 55 minutes. The peak effect was observed at 112 minutes. 57% of the flavoxate HCl was excreted in the urine within 24 hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Mutagenicity studies and long-term studies in animals to determine the carcinogenic potential of flavoxate HCl have not been performed.
Clinical Studies
There is limited information regarding Clinical Studies of Flavoxate in the drug label.
How Supplied
- Flavoxate HCl Tablets, 100 mg, are supplied as round, white, film-coated tablets debossed "PAD" and "0115" on one side and plain on the other side, in bottles of 100.
- 100 mg 100's:
- NDC 0574-0115-01
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Flavoxate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 100 MG TABLET BOTTLE
Rx Only
NDC 0574-0115-01
Flavoxate HCl Tablets
100 mg
100 Tablets
Ingredients and Appearance
{{#ask: Label Page::Flavoxate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed that if drowsiness and blurred vision occur, they should not operate a motor vehicle or machinery or participate in activities where alertness is required.
Precautions with Alcohol
- Alcohol-Flavoxate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Urispas®[1]
Look-Alike Drug Names
There is limited information regarding Flavoxate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.


