Didanosine: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 77: | Line 77: | ||
=====Fat Redistribution===== | =====Fat Redistribution===== | ||
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. | Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
=====Adults===== | |||
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients. Selected clinical adverse reactions that occurred in combination with other antiretroviral agents are provided in TABLE 3. | |||
[[File:Didanosine Selected Clinical Adverse Reactions.png|thumb|none|600px]] | |||
In clinical trials using a buffered formulation of didanosine, pancreatitis resulting in death was observed in one patient who received didanosine plus stavudine plus nelfinavir, one patient who received didanosine plus stavudine plus indinavir, and 2 of 68 patients who received didanosine plus stavudine plus indinavir plus hydroxyurea. In an early access program, pancreatitis resulting in death was observed in one patient who received didanosine delayed-release capsules plus stavudine plus hydroxyurea plus ritonavir plus indinavir plus efavirenz [see Warnings and Precautions (5)]. | |||
The frequency of pancreatitis is dose related. In phase 3 studies with buffered formulations of didanosine, incidence ranged from 1% to 10% with doses higher than are currently recommended and 1% to 7% with recommended dose. | |||
Selected laboratory abnormalities that occurred in a study of didanosine delayed-release capsules in combination with other antiretroviral agents are shown in TABLE 4. | |||
[[File:Didanosine Selected Laboratory Abnormalities.png|thumb|none|600px]] | |||
=====Pediatric Patients===== | |||
In clinical trials, 743 pediatric patients between 2 weeks and 18 years of age have been treated with didanosine. Adverse reactions and laboratory abnormalities reported to occur in these patients were generally consistent with the safety profile of didanosine in adults. | |||
In pediatric phase 1 studies, pancreatitis occurred in 2 of 60 (3%) patients treated at entry doses below 300 mg/m2/day and in 5 of 38 (13%) patients treated at higher doses. In study ACTG 152, pancreatitis occurred in none of the 281 pediatric patients who received didanosine 120 mg/m2 every 12 hours and in less than 1% of the 274 pediatric patients who received didanosine 90 mg/m2 every 12 hours in combination with zidovudine. | |||
Retinal changes and optic neuritis have been reported in pediatric patients. | |||
|postmarketing=The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine, or a combination of these factors. | |||
* Blood and Lymphatic System Disorders - anemia, leukopenia, and thrombocytopenia. | |||
* Body as a Whole - abdominal pain, alopecia, anaphylactoid reaction, asthenia, chills/fever, pain, and redistribution/accumulation of body fat. | |||
* Digestive Disorders - anorexia, dyspepsia, and flatulence. | |||
* Exocrine Gland Disorders - pancreatitis (including fatal cases), sialoadenitis, parotid gland enlargement, dry mouth, and dry eyes. | |||
* Hepatobiliary Disorders - symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis; non-cirrhotic portal hypertension; hepatitis and liver failure. | |||
* Metabolic Disorders - diabetes mellitus, elevated serum alkaline phosphatase level, elevated serum amylase level, elevated serum gamma-glutamyltransferase level, elevated serum uric acid level, hypoglycemia, and hyperglycemia. | |||
* Musculoskeletal Disorders - myalgia (with or without increases in creatine kinase), rhabdomyolysis including acute renal failure and hemodialysis, arthralgia, and myopathy. | |||
* Ophthalmologic Disorders - retinal depigmentation and optic neuritis. | |||
=====Use with Stavudine- and Hydroxyurea-Based Regimens===== | |||
When didanosine is used in combination with other agents with similar toxicities, the incidence of these toxicities may be higher than when didanosine is used alone. Thus, patients treated with didanosine delayed-release capsules in combination with stavudine, with or without hydroxyurea, may be at increased risk for pancreatitis and hepatotoxicity, which may be fatal, and severe peripheral neuropathy. The combination of didanosine delayed-release capsules and hydroxyurea, with or without stavudine, should be avoided. | |||
|drugInteractions======Established Drug Interactions===== | |||
[[File:Didanosine Established Drug Interactions Based on Studies with Didanosine.png|thumb|none|600px]] | |||
Exposure to didanosine is increased when coadministered with tenofovir disoproxil fumarate. Increased exposure may cause or worsen didanosine-related clinical toxicities, including pancreatitis, symptomatic hyperlactatemia/lactic acidosis, and peripheral neuropathy. Coadministration of tenofovir disoproxil fumarate with didanosine delayed-release capsules should be undertaken with caution, and patients should be monitored closely for didanosine-related toxicities and clinical response. Didanosine delayed-release capsules should be suspended if signs or symptoms of pancreatitis, symptomatic hyperlactatemia, or lactic acidosis develop. Suppression of CD4 cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine at a dose of 400 mg daily. | |||
=====Predicted Drug Interactions===== | |||
[[File:Didanosine Predicted Drug Interactions with Didanosine.png|thumb|none|600px]] | |||
|FDAPregCat=B | |FDAPregCat=B | ||
|AUSPregCat=B2 | |AUSPregCat=B2 | ||
Revision as of 14:04, 4 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
See full prescribing information for complete Boxed Warning.
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk.
|
Overview
Didanosine is a nucleoside reverse transcriptase inhibitor that is FDA approved for the treatment of human immunodeficiency virus (HIV)-1 infection.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include highlights: diarrhea, peripheral neurologic symptoms/neuropathy, nausea, headache, rash and vomiting
MICROMEDEX: Dermatologic: Pruritus (7% to 9% ), Rash (7% to 30% ) Gastrointestinal: Abdominal pain (7% to 13% ), Diarrhea (19% to 70% ), Nausea (24% to 53% ), Vomiting (12% to 30% ) Hepatic: Increased liver enzymes Neurologic: Headache (21% to 46%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Didanosine in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus (HIV)-1 infection.
- Should be administered on an empty stomach
- Total daily dose is based on body weight
- 20 kg to less than 25 kg: 200 mg once daily
- 25 kg to less than 60 kg: 250 mg once daily
- At least 60 kg: 400 mg once daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Didanosine in adult patients.
Non–Guideline-Supported Use
- Prophylaxis of occupational exposure to HIV
- Didanosine: 400 mg (if body weight is less than 60 kg, 125 mg twice daily or 250 mg once daily) daily, on an empty stomach, AND
- Lamivudine: 300 mg once daily or 150 mg twice daily for 4 weeks.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Didanosine in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus (HIV)-1 infection.
- Should be administered on an empty stomach
- Total daily dose is based on body weight
- 20 kg to less than 25 kg: 200 mg once daily
- 25 kg to less than 60 kg: 250 mg once daily
- At least 60 kg: 400 mg once daily
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Didanosine in pediatric patients.
Non–Guideline-Supported Use
- Prophylaxis of occupational exposure to HIV
- Didanosine: 400 mg (if body weight is less than 60 kg, 125 mg twice daily or 250 mg once daily) daily, on an empty stomach, AND
- Lamivudine: 300 mg once daily or 150 mg twice daily for 4 weeks.
Contraindications
These recommendations are based on either drug interaction studies or observed clinical toxicities.
Allopurinol
- Coadministration of didanosine and allopurinol is contraindicated because systemic exposures of didanosine are increased, which may increase didanosine-associated toxicity.
Ribavirin
- Coadministration of didanosine and ribavirin is contraindicated because exposures of the active metabolite of didanosine (dideoxyadenosine 5’-triphosphate) are increased. Fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in patients receiving both didanosine and ribavirin.
Warnings
|
WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
See full prescribing information for complete Boxed Warning.
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk.
|
Pancreatitis
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with signs or symptoms of pancreatitis and discontinued in patients with confirmed pancreatitis. Patients treated with didanosine delayed-release capsules in combination with stavudine may be at increased risk for pancreatitis.
When treatment with life-sustaining drugs known to cause pancreatic toxicity is required, suspension of didanosine delayed-release capsules therapy is recommended. In patients with risk factors for pancreatitis, didanosine delayed-release capsules should be used with extreme caution and only if clearly indicated. Patients with advanced HIV-1 infection, especially the elderly, are at increased risk of pancreatitis and should be followed closely. Patients with renal impairment may be at greater risk for pancreatitis if treated without dose adjustment. The frequency of pancreatitis is dose related.
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk. Particular caution should be exercised when administering didanosine delayed-release capsules to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with didanosine delayed-release capsules should be suspended in any patient who develops clinical signs or symptoms with or without laboratory findings consistent with symptomatic hyperlactatemia, lactic acidosis, or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Hepatic Toxicity
The safety and efficacy of didanosine delayed-release capsules have not been established in HIV-infected patients with significant underlying liver disease. During combination antiretroviral therapy, patients with preexisting liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities, including severe and potentially fatal hepatic adverse events, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Hepatotoxicity and hepatic failure resulting in death were reported during postmarketing surveillance in HIV-infected patients treated with hydroxyurea and other antiretroviral agents. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. This combination should be avoided.
Non-cirrhotic Portal Hypertension
Postmarketing cases of non-cirrhotic portal hypertension have been reported, including cases leading to liver transplantation or death. Cases of didanosine-associated non-cirrhotic portal hypertension were confirmed by liver biopsy in patients with no evidence of viral hepatitis. Onset of signs and symptoms ranged from months to years after start of didanosine therapy. Common presenting features included elevated liver enzymes, esophageal varices, hematemesis, ascites, and splenomegaly.
Patients receiving didanosine delayed-release capsules should be monitored for early signs of portal hypertension (e.g., thrombocytopenia and splenomegaly) during routine medical visits. Appropriate laboratory testing including liver enzymes, serum bilirubin, albumin, complete blood count, and international normalized ratio (INR) and ultrasonography should be considered. Didanosine delayed-release capsules should be discontinued in patients with evidence of non-cirrhotic portal hypertension.
Peripheral Neuropathy
Peripheral neuropathy, manifested by numbness, tingling, or pain in the hands or feet, has been reported in patients receiving didanosine therapy. Peripheral neuropathy has occurred more frequently in patients with advanced HIV disease, in patients with a history of neuropathy, or in patients being treated with neurotoxic drug therapy, including stavudine. Discontinuation of didanosine delayed-release capsules should be considered in patients who develop peripheral neuropathy.
Retinal Changes and Optic Neuritis
Retinal changes and optic neuritis have been reported in patients taking didanosine. Periodic retinal examinations should be considered for patients receiving didanosine delayed-release capsules.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including didanosine delayed-release capsules. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients. Selected clinical adverse reactions that occurred in combination with other antiretroviral agents are provided in TABLE 3.

In clinical trials using a buffered formulation of didanosine, pancreatitis resulting in death was observed in one patient who received didanosine plus stavudine plus nelfinavir, one patient who received didanosine plus stavudine plus indinavir, and 2 of 68 patients who received didanosine plus stavudine plus indinavir plus hydroxyurea. In an early access program, pancreatitis resulting in death was observed in one patient who received didanosine delayed-release capsules plus stavudine plus hydroxyurea plus ritonavir plus indinavir plus efavirenz [see Warnings and Precautions (5)].
The frequency of pancreatitis is dose related. In phase 3 studies with buffered formulations of didanosine, incidence ranged from 1% to 10% with doses higher than are currently recommended and 1% to 7% with recommended dose.
Selected laboratory abnormalities that occurred in a study of didanosine delayed-release capsules in combination with other antiretroviral agents are shown in TABLE 4.
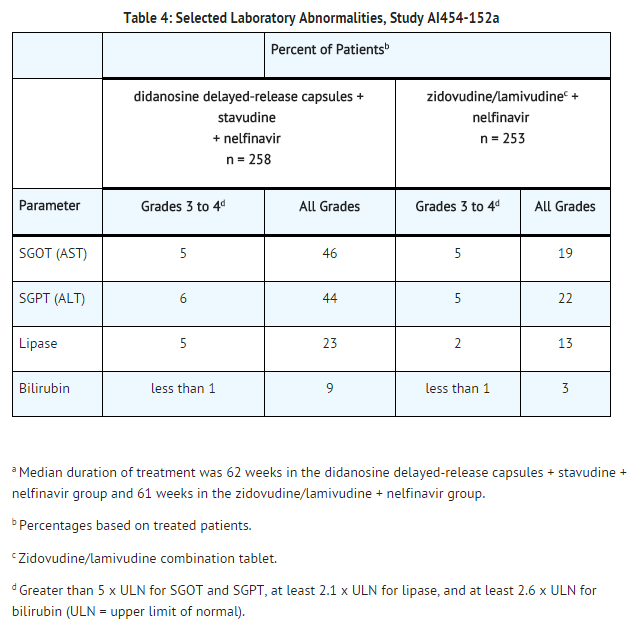
Pediatric Patients
In clinical trials, 743 pediatric patients between 2 weeks and 18 years of age have been treated with didanosine. Adverse reactions and laboratory abnormalities reported to occur in these patients were generally consistent with the safety profile of didanosine in adults.
In pediatric phase 1 studies, pancreatitis occurred in 2 of 60 (3%) patients treated at entry doses below 300 mg/m2/day and in 5 of 38 (13%) patients treated at higher doses. In study ACTG 152, pancreatitis occurred in none of the 281 pediatric patients who received didanosine 120 mg/m2 every 12 hours and in less than 1% of the 274 pediatric patients who received didanosine 90 mg/m2 every 12 hours in combination with zidovudine.
Retinal changes and optic neuritis have been reported in pediatric patients.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine, or a combination of these factors.
- Blood and Lymphatic System Disorders - anemia, leukopenia, and thrombocytopenia.
- Body as a Whole - abdominal pain, alopecia, anaphylactoid reaction, asthenia, chills/fever, pain, and redistribution/accumulation of body fat.
- Digestive Disorders - anorexia, dyspepsia, and flatulence.
- Exocrine Gland Disorders - pancreatitis (including fatal cases), sialoadenitis, parotid gland enlargement, dry mouth, and dry eyes.
- Hepatobiliary Disorders - symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis; non-cirrhotic portal hypertension; hepatitis and liver failure.
- Metabolic Disorders - diabetes mellitus, elevated serum alkaline phosphatase level, elevated serum amylase level, elevated serum gamma-glutamyltransferase level, elevated serum uric acid level, hypoglycemia, and hyperglycemia.
- Musculoskeletal Disorders - myalgia (with or without increases in creatine kinase), rhabdomyolysis including acute renal failure and hemodialysis, arthralgia, and myopathy.
- Ophthalmologic Disorders - retinal depigmentation and optic neuritis.
Use with Stavudine- and Hydroxyurea-Based Regimens
When didanosine is used in combination with other agents with similar toxicities, the incidence of these toxicities may be higher than when didanosine is used alone. Thus, patients treated with didanosine delayed-release capsules in combination with stavudine, with or without hydroxyurea, may be at increased risk for pancreatitis and hepatotoxicity, which may be fatal, and severe peripheral neuropathy. The combination of didanosine delayed-release capsules and hydroxyurea, with or without stavudine, should be avoided.
Drug Interactions
Established Drug Interactions

Exposure to didanosine is increased when coadministered with tenofovir disoproxil fumarate. Increased exposure may cause or worsen didanosine-related clinical toxicities, including pancreatitis, symptomatic hyperlactatemia/lactic acidosis, and peripheral neuropathy. Coadministration of tenofovir disoproxil fumarate with didanosine delayed-release capsules should be undertaken with caution, and patients should be monitored closely for didanosine-related toxicities and clinical response. Didanosine delayed-release capsules should be suspended if signs or symptoms of pancreatitis, symptomatic hyperlactatemia, or lactic acidosis develop. Suppression of CD4 cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine at a dose of 400 mg daily.
Predicted Drug Interactions
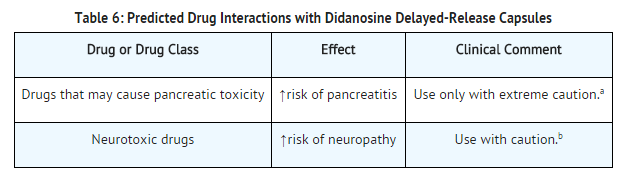
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
There is no FDA guidance on usage of Didanosine in women who are pregnant.
Pregnancy Category (AUS): B2
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Didanosine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Didanosine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Didanosine in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Didanosine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Didanosine in geriatric settings.
Gender
There is no FDA guidance on the use of Didanosine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Didanosine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Didanosine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Didanosine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Didanosine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Didanosine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Didanosine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Didanosine and IV administrations.
Overdosage
There is no known antidote for didanosine overdosage. In phase 1 studies, in which buffered formulations of didanosine were initially administered at doses ten times the currently recommended dose, toxicities included: pancreatitis, peripheral neuropathy, diarrhea, hyperuricemia, and hepatic dysfunction. Didanosine is not dialyzable by peritoneal dialysis, although there is some clearance by hemodialysis.
Pharmacology
There is limited information regarding Didanosine Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Didanosine Mechanism of Action in the drug label.
Structure
There is limited information regarding Didanosine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Didanosine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Didanosine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Didanosine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Didanosine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Didanosine How Supplied in the drug label.
Storage
There is limited information regarding Didanosine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Didanosine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Didanosine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Didanosine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Didanosine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Videx
- Videx EC
- Videx Pediatric
Look-Alike Drug Names
There is limited information regarding Didanosine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [4]
Overview
Didanosine (2′,3′-dideoxyinosine,DDI) is sold under the trade names Videx and Videx EC. It is a reverse transcriptase inhibitor, effective against HIV and used in combination with other antiretroviral drug therapy as part of highly active antiretroviral therapy (HAART).
Category
Antiretroviral
US Brand Names
VIDEX®, VIDEX EC®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Didanosine (ddI) is a nucleoside structural analogue of guanosine. It differs from other nucleoside analogues, because it does not have any of the regular bases, instead it has hypoxanthine attached to the sugar ring. Within the cell, ddI is phosphorylated to the active metabolite of dideoxyadenosine triphosphate, ddATP, by cellular enzymes. Like other anti-HIV nucleoside analogs, it acts as a chain terminator by incorporation and inhibits viral reverse transcriptase by competing with natural deoxyadenosine triphosphate (dATP).