D-dimer: Difference between revisions
Rim Halaby (talk | contribs) |
Rim Halaby (talk | contribs) |
||
| Line 245: | Line 245: | ||
===Cardiovascular Diseases=== | ===Cardiovascular Diseases=== | ||
====Ischemic Heart Disease==== | |||
Since D-dimers alterations reflect thrombus turnover, levels are expected to be increased in [[ischemic heart disease]] and [[coronary angina]]. D-dimer levels are significantly associated with a 1.5 fold increase in [[coronary artery disease]] (CAD) and in subclinical [[atherosclerosis]].<ref name="pmid7805229">{{cite journal| author=Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK| title=Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study. | journal=Circulation | year= 1995 | volume= 91 | issue= 2 | pages= 284-90 | pmid=7805229 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7805229 }} </ref> In patients with CAD, the median D-dimer value was 0.112 µg/mL vs. only 0.0028 µg/mL in patients without CAD. The positive correlation between D-dimer, [[fibrinogen]], plasma viscosity, and interleukin-6 seem to be of importance in understanding the association of D-dimer with inflammatory products.<ref name="pmid11597948">{{cite journal| author=Koenig W, Rothenbacher D, Hoffmeister A, Griesshammer M, Brenner H| title=Plasma fibrin D-dimer levels and risk of stable coronary artery disease: results of a large case-control study. | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 10 | pages= 1701-5 | pmid=11597948 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11597948 }} </ref> The most important value that D-dimer carries is its prediction of future cardiac events in various patient age groups, in both genders, and in patients with known peripheral arterial disease and myocardial infarction.<ref name="pmid9459337">{{cite journal| author=Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC| title=Fibrin D-dimer, tissue plasminogen activator, plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly Study. | journal=Thromb Haemost | year= 1998 | volume= 79 | issue= 1 | pages= 129-33 | pmid=9459337 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9459337 }} </ref><ref name="pmid11304479">{{cite journal| author=Lowe GD, Yarnell JW, Rumley A, Bainton D, Sweetnam PM| title=C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 4 | pages= 603-10 | pmid=11304479 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11304479 }} </ref><ref name="pmid11304480">{{cite journal| author=Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK| title=Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 4 | pages= 611-7 | pmid=11304480 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11304480 }} </ref><ref name="pmid10073948">{{cite journal| author=Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR et al.| title=Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. | journal=Arterioscler Thromb Vasc Biol | year= 1999 | volume= 19 | issue= 3 | pages= 493-8 | pmid=10073948 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10073948 }} </ref><ref name="pmid8100915">{{cite journal| author=Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA et al.| title=Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. | journal=Lancet | year= 1993 | volume= 342 | issue= 8863 | pages= 84-6 | pmid=8100915 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8100915 }} </ref><ref name="pmid10330382">{{cite journal| author=Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H et al.| title=Thrombogenic factors and recurrent coronary events. | journal=Circulation | year= 1999 | volume= 99 | issue= 19 | pages= 2517-22 | pmid=10330382 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10330382 }} </ref> Despite obvious associations, some still question the use of D-dimer in providing informative or additional clinical data in the diagnosis or management of myocardial ischemia.<ref name="pmid17541763">{{cite journal| author=Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Guidi GC| title=Diagnostic value of D-dimer measurement in patients referred to the emergency department with suspected myocardial ischemia. | journal=J Thromb Thrombolysis | year= 2008 | volume= 25 | issue= 3 | pages= 247-50 | pmid=17541763 | doi=10.1007/s11239-007-0060-6 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17541763 }} </ref> | Since D-dimers alterations reflect thrombus turnover, levels are expected to be increased in [[ischemic heart disease]] and [[coronary angina]]. D-dimer levels are significantly associated with a 1.5 fold increase in [[coronary artery disease]] (CAD) and in subclinical [[atherosclerosis]].<ref name="pmid7805229">{{cite journal| author=Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK| title=Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study. | journal=Circulation | year= 1995 | volume= 91 | issue= 2 | pages= 284-90 | pmid=7805229 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7805229 }} </ref> In patients with CAD, the median D-dimer value was 0.112 µg/mL vs. only 0.0028 µg/mL in patients without CAD. The positive correlation between D-dimer, [[fibrinogen]], plasma viscosity, and interleukin-6 seem to be of importance in understanding the association of D-dimer with inflammatory products.<ref name="pmid11597948">{{cite journal| author=Koenig W, Rothenbacher D, Hoffmeister A, Griesshammer M, Brenner H| title=Plasma fibrin D-dimer levels and risk of stable coronary artery disease: results of a large case-control study. | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 10 | pages= 1701-5 | pmid=11597948 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11597948 }} </ref> The most important value that D-dimer carries is its prediction of future cardiac events in various patient age groups, in both genders, and in patients with known peripheral arterial disease and myocardial infarction.<ref name="pmid9459337">{{cite journal| author=Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC| title=Fibrin D-dimer, tissue plasminogen activator, plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly Study. | journal=Thromb Haemost | year= 1998 | volume= 79 | issue= 1 | pages= 129-33 | pmid=9459337 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9459337 }} </ref><ref name="pmid11304479">{{cite journal| author=Lowe GD, Yarnell JW, Rumley A, Bainton D, Sweetnam PM| title=C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 4 | pages= 603-10 | pmid=11304479 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11304479 }} </ref><ref name="pmid11304480">{{cite journal| author=Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK| title=Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. | journal=Arterioscler Thromb Vasc Biol | year= 2001 | volume= 21 | issue= 4 | pages= 611-7 | pmid=11304480 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11304480 }} </ref><ref name="pmid10073948">{{cite journal| author=Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR et al.| title=Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. | journal=Arterioscler Thromb Vasc Biol | year= 1999 | volume= 19 | issue= 3 | pages= 493-8 | pmid=10073948 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10073948 }} </ref><ref name="pmid8100915">{{cite journal| author=Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA et al.| title=Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. | journal=Lancet | year= 1993 | volume= 342 | issue= 8863 | pages= 84-6 | pmid=8100915 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8100915 }} </ref><ref name="pmid10330382">{{cite journal| author=Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H et al.| title=Thrombogenic factors and recurrent coronary events. | journal=Circulation | year= 1999 | volume= 99 | issue= 19 | pages= 2517-22 | pmid=10330382 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10330382 }} </ref> Despite obvious associations, some still question the use of D-dimer in providing informative or additional clinical data in the diagnosis or management of myocardial ischemia.<ref name="pmid17541763">{{cite journal| author=Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Guidi GC| title=Diagnostic value of D-dimer measurement in patients referred to the emergency department with suspected myocardial ischemia. | journal=J Thromb Thrombolysis | year= 2008 | volume= 25 | issue= 3 | pages= 247-50 | pmid=17541763 | doi=10.1007/s11239-007-0060-6 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17541763 }} </ref> | ||
In a study by Tataru et al. in 2000 that recruited 1112 male and 299 female patients, the significant association between and previous MI was further emphasized.<ref name="pmid10901512">{{cite journal| author=Tataru MC, Heinrich J, Junker R, Schulte H, von Eckardstein A, Assmann G et al.| title=C-reactive protein and the severity of atherosclerosis in myocardial infarction patients with stable angina pectoris. | journal=Eur Heart J | year= 2000 | volume= 21 | issue= 12 | pages= 1000-8 | pmid=10901512 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10901512 }} </ref> | In a study by Tataru et al. in 2000 that recruited 1112 male and 299 female patients, the significant association between and previous MI was further emphasized.<ref name="pmid10901512">{{cite journal| author=Tataru MC, Heinrich J, Junker R, Schulte H, von Eckardstein A, Assmann G et al.| title=C-reactive protein and the severity of atherosclerosis in myocardial infarction patients with stable angina pectoris. | journal=Eur Heart J | year= 2000 | volume= 21 | issue= 12 | pages= 1000-8 | pmid=10901512 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10901512 }} </ref> | ||
====Atrial Fibrillation==== | |||
D-dimer levels are also elevated in patients with [[atrial fibrillation]] (AF), especially in those who are known to have established several embolism risk factors.<ref name="pmid17716992">{{cite journal| author=Cohen A, Ederhy S, Meuleman C, Di Angelantonio E, Dufaitre G, Boccara F| title=D-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism? | journal=Eur Heart J | year= 2007 | volume= 28 | issue= 18 | pages= 2179-80 | pmid=17716992 | doi=10.1093/eurheartj/ehm170 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17716992 }} </ref> In 2003, Somloi et al. measured D-dimer levels in 73 patients with AF prior to TEE-guided cardioversion; they concluded that although D-dimer is unspecific, D-dimer concentration < 0.06 µg/ml provides a negative predictive value of 98%.<ref name="pmid12842257">{{cite journal| author=Somlói M, Tomcsányi J, Nagy E, Bodó I, Bezzegh A| title=D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation. | journal=Am J Cardiol | year= 2003 | volume= 92 | issue= 1 | pages= 85-7 | pmid=12842257 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12842257 }} </ref> Habara et al. concluded that D-dimer concentration of 0.115 µg/mL was an optimal cut-off value to detect left atrial appendange (LAA) thrombus; the NPV was 97% irrespective of co-morbidities, such as [[congestive heart failure]], or [[stroke]].<ref name="pmid17716992">{{cite journal| author=Cohen A, Ederhy S, Meuleman C, Di Angelantonio E, Dufaitre G, Boccara F| title=D-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism? | journal=Eur Heart J | year= 2007 | volume= 28 | issue= 18 | pages= 2179-80 | pmid=17716992 | doi=10.1093/eurheartj/ehm170 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17716992 }} </ref> Even in AF patients receiving appropriate Warfarin anticoagulation therapy, D-dimer levels remained abnormally high.<ref name="pmid15992948">{{cite journal| author=Nozawa T, Inoue H, Hirai T, Iwasa A, Okumura K, Lee JD et al.| title=D-dimer level influences thromboembolic events in patients with atrial fibrillation. | journal=Int J Cardiol | year= 2006 | volume= 109 | issue= 1 | pages= 59-65 | pmid=15992948 | doi=10.1016/j.ijcard.2005.05.049 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15992948 }} </ref> | D-dimer levels are also elevated in patients with [[atrial fibrillation]] (AF), especially in those who are known to have established several embolism risk factors.<ref name="pmid17716992">{{cite journal| author=Cohen A, Ederhy S, Meuleman C, Di Angelantonio E, Dufaitre G, Boccara F| title=D-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism? | journal=Eur Heart J | year= 2007 | volume= 28 | issue= 18 | pages= 2179-80 | pmid=17716992 | doi=10.1093/eurheartj/ehm170 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17716992 }} </ref> In 2003, Somloi et al. measured D-dimer levels in 73 patients with AF prior to TEE-guided cardioversion; they concluded that although D-dimer is unspecific, D-dimer concentration < 0.06 µg/ml provides a negative predictive value of 98%.<ref name="pmid12842257">{{cite journal| author=Somlói M, Tomcsányi J, Nagy E, Bodó I, Bezzegh A| title=D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation. | journal=Am J Cardiol | year= 2003 | volume= 92 | issue= 1 | pages= 85-7 | pmid=12842257 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12842257 }} </ref> Habara et al. concluded that D-dimer concentration of 0.115 µg/mL was an optimal cut-off value to detect left atrial appendange (LAA) thrombus; the NPV was 97% irrespective of co-morbidities, such as [[congestive heart failure]], or [[stroke]].<ref name="pmid17716992">{{cite journal| author=Cohen A, Ederhy S, Meuleman C, Di Angelantonio E, Dufaitre G, Boccara F| title=D-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism? | journal=Eur Heart J | year= 2007 | volume= 28 | issue= 18 | pages= 2179-80 | pmid=17716992 | doi=10.1093/eurheartj/ehm170 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17716992 }} </ref> Even in AF patients receiving appropriate Warfarin anticoagulation therapy, D-dimer levels remained abnormally high.<ref name="pmid15992948">{{cite journal| author=Nozawa T, Inoue H, Hirai T, Iwasa A, Okumura K, Lee JD et al.| title=D-dimer level influences thromboembolic events in patients with atrial fibrillation. | journal=Int J Cardiol | year= 2006 | volume= 109 | issue= 1 | pages= 59-65 | pmid=15992948 | doi=10.1016/j.ijcard.2005.05.049 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15992948 }} </ref> | ||
| Line 255: | Line 257: | ||
According to Diego et al., elevated D-dimer level is significantly associated with increasing severity of atherosclerosis based on ultrasound detection of carotid plaques and overall cardiovascular decline.<ref name="pmid12871360">{{cite journal| author=de Maat MP, Bladbjerg EM, Drivsholm T, Borch-Johnsen K, Møller L, Jespersen J| title=Inflammation, thrombosis and atherosclerosis: results of the Glostrup study. | journal=J Thromb Haemost | year= 2003 | volume= 1 | issue= 5 | pages= 950-7 | pmid=12871360 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12871360 }} </ref><ref name="pmid12637131">{{cite journal| author=Cohen HJ, Harris T, Pieper CF| title=Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. | journal=Am J Med | year= 2003 | volume= 114 | issue= 3 | pages= 180-7 | pmid=12637131 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12637131 }} </ref> | According to Diego et al., elevated D-dimer level is significantly associated with increasing severity of atherosclerosis based on ultrasound detection of carotid plaques and overall cardiovascular decline.<ref name="pmid12871360">{{cite journal| author=de Maat MP, Bladbjerg EM, Drivsholm T, Borch-Johnsen K, Møller L, Jespersen J| title=Inflammation, thrombosis and atherosclerosis: results of the Glostrup study. | journal=J Thromb Haemost | year= 2003 | volume= 1 | issue= 5 | pages= 950-7 | pmid=12871360 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12871360 }} </ref><ref name="pmid12637131">{{cite journal| author=Cohen HJ, Harris T, Pieper CF| title=Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. | journal=Am J Med | year= 2003 | volume= 114 | issue= 3 | pages= 180-7 | pmid=12637131 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12637131 }} </ref> | ||
===Stroke=== | |||
Elevated D-dimer values can also be used to predict acute cerebrovascular events and subtypes. Levels increase remarkably during first 6 hours of [[stroke]] onset in patients with large occlusion and those being treated with intravenous [[thrombolytic]]s regardless of time of artery recanalization.<ref name="pmid19568692">{{cite journal| author=Skoloudík D, Bar M, Sanák D, Bardon P, Roubec M, Langová K et al.| title=D-dimers increase in acute ischemic stroke patients with the large artery occlusion, but do not depend on the time of artery recanalization. | journal=J Thromb Thrombolysis | year= 2010 | volume= 29 | issue= 4 | pages= 477-82 | pmid=19568692 | doi=10.1007/s11239-009-0372-9 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19568692 }} </ref> D-dimer seems to have shown association with infarction volume<ref name="pmid22053225">{{cite journal| author=Park YW, Koh EJ, Choi HY| title=Correlation between Serum D-Dimer Level and Volume in Acute Ischemic Stroke. | journal=J Korean Neurosurg Soc | year= 2011 | volume= 50 | issue= 2 | pages= 89-94 | pmid=22053225 | doi=10.3340/jkns.2011.50.2.89 | pmc=PMC3206284 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22053225 }} </ref> and with cardioembolic strokes but not atherothrombotic or lacunar strokes. According to Ageno et al. in 2002, the optimal D-dimer cut-off point for predicting cardioembolic [[stroke]] is 2 µg/mL with 93.2% specificity and 59.3% sensitivity. In contrast, the cut-off point for predicting lacunar stroke in the same study was 0.54 µg/mL with 96.2% specificity and 61.3% [[sensitivity]].<ref name="pmid12456231">{{cite journal| author=Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi D'Eril G et al.| title=Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. | journal=Arch Intern Med | year= 2002 | volume= 162 | issue= 22 | pages= 2589-93 | pmid=12456231 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12456231 }} </ref> Although it is suggested as a screening test for patients presenting with acute-onset [[headache]]s,<ref name="pmid16302160">{{cite journal| author=Squizzato A, Ageno W| title=D-dimer testing in ischemic stroke and cerebral sinus and venous thrombosis. | journal=Semin Vasc Med | year= 2005 | volume= 5 | issue= 4 | pages= 379-86 | pmid=16302160 | doi=10.1055/s-2005-922484 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16302160 }} </ref> triage for high-risk patients who require antithrombotic therapy, and evaluating progressing strokes, defined by clinical worsening of symptoms following admission, the prognostic role of D-dimer in cases of strokes is unclear and yet to be determined.<ref name="pmid16527998">{{cite journal| author=Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ| title=D-dimer predicts early clinical progression in ischemic stroke: confirmation using routine clinical assays. | journal=Stroke | year= 2006 | volume= 37 | issue= 4 | pages= 1113-5 | pmid=16527998 | doi=10.1161/01.STR.0000209240.63821.1a | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16527998 }} </ref> | Elevated D-dimer values can also be used to predict acute cerebrovascular events and subtypes. Levels increase remarkably during first 6 hours of [[stroke]] onset in patients with large occlusion and those being treated with intravenous [[thrombolytic]]s regardless of time of artery recanalization.<ref name="pmid19568692">{{cite journal| author=Skoloudík D, Bar M, Sanák D, Bardon P, Roubec M, Langová K et al.| title=D-dimers increase in acute ischemic stroke patients with the large artery occlusion, but do not depend on the time of artery recanalization. | journal=J Thromb Thrombolysis | year= 2010 | volume= 29 | issue= 4 | pages= 477-82 | pmid=19568692 | doi=10.1007/s11239-009-0372-9 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19568692 }} </ref> D-dimer seems to have shown association with infarction volume<ref name="pmid22053225">{{cite journal| author=Park YW, Koh EJ, Choi HY| title=Correlation between Serum D-Dimer Level and Volume in Acute Ischemic Stroke. | journal=J Korean Neurosurg Soc | year= 2011 | volume= 50 | issue= 2 | pages= 89-94 | pmid=22053225 | doi=10.3340/jkns.2011.50.2.89 | pmc=PMC3206284 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22053225 }} </ref> and with cardioembolic strokes but not atherothrombotic or lacunar strokes. According to Ageno et al. in 2002, the optimal D-dimer cut-off point for predicting cardioembolic [[stroke]] is 2 µg/mL with 93.2% specificity and 59.3% sensitivity. In contrast, the cut-off point for predicting lacunar stroke in the same study was 0.54 µg/mL with 96.2% specificity and 61.3% [[sensitivity]].<ref name="pmid12456231">{{cite journal| author=Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi D'Eril G et al.| title=Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes. | journal=Arch Intern Med | year= 2002 | volume= 162 | issue= 22 | pages= 2589-93 | pmid=12456231 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12456231 }} </ref> Although it is suggested as a screening test for patients presenting with acute-onset [[headache]]s,<ref name="pmid16302160">{{cite journal| author=Squizzato A, Ageno W| title=D-dimer testing in ischemic stroke and cerebral sinus and venous thrombosis. | journal=Semin Vasc Med | year= 2005 | volume= 5 | issue= 4 | pages= 379-86 | pmid=16302160 | doi=10.1055/s-2005-922484 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16302160 }} </ref> triage for high-risk patients who require antithrombotic therapy, and evaluating progressing strokes, defined by clinical worsening of symptoms following admission, the prognostic role of D-dimer in cases of strokes is unclear and yet to be determined.<ref name="pmid16527998">{{cite journal| author=Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ| title=D-dimer predicts early clinical progression in ischemic stroke: confirmation using routine clinical assays. | journal=Stroke | year= 2006 | volume= 37 | issue= 4 | pages= 1113-5 | pmid=16527998 | doi=10.1161/01.STR.0000209240.63821.1a | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16527998 }} </ref> | ||
Revision as of 14:29, 10 September 2013
|
WikiDoc Resources for D-dimer |
|
Articles |
|---|
|
Most recent articles on D-dimer |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on D-dimer at Clinical Trials.gov Clinical Trials on D-dimer at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on D-dimer
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating D-dimer Risk calculators and risk factors for D-dimer
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for D-dimer |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor(s)-In-Chief: C. Michael Gibson, M.S., M.D. [1], The APEX Trial Investigators; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
D-dimer is a fibrin degradation product. D-dimer levels are elevated in the plasma after the acute formation of a blood clot. The majority of patients with pulmonary embolism have some degree of endogenous fibrinolysis with an elevation in D-dimer levels, therefore there is a high negative predictive value in ruling out a pulmonary embolism when D-dimer levels are low. However a wide range of diseases are associated with mild degree of fibrinolysis which elevate D-dimer levels and contribute towards a reduced specificity and a poor positive predictive value of a high D-dimer level. This means that it is more likely that one can rule out a PE with a low D-dimer level, but cannot necessarily confirm the diagnosis of a PE based on a high D-dimer level. Other disease states that can also have a high d-dimer level include pneumonia, congestive heart failure (CHF), myocardial infarction (MI) and malignancy. False-negative values may occur in patients with prolonged symptoms of venous thromboembolism (≥14 days), patients on therapeutic heparin therapy, and patients with suspected deep venous thrombosis on oral anticoagulation, as these patients have will have low D-dimer levels in the presence of a PE.[1][2]
Historical Perspective
D-dimer testing was originally developed in the diagnosis of disseminated intravascular coagulation. In the 1990s, they turned out to be useful in diagnosing thromboembolic process.
Physiology
Fibrin degradation products (FDPs) are formed whenever fibrin is broken down by enzymes. In fact, FDP are formed as a result of the sequential actions of the following three different enzymes: thrombin, factor VIII and plasmin. Determining FDPs is not considered useful, as this does not indicate whether the fibrin is part of a blood clot (or being generated as part of inflammation).
D-dimers are unique in that they are the breakdown products of a fibrin mesh that has been stabilized by Factor XIII. This factor crosslinks the E-element to two D-elements. This is the final step in the generation of a thrombus.
Plasmin is a fibrinolytic enzyme that organizes clots and breaks down the fibrin mesh. It cannot, however, break down the bonds between one E and two D units. The protein fragment thus left over is a D-dimer.[3]
Shown below is an immage summarizing the formation of D-dimers and other fibrin degradation products as a result of the sequential action of the three enzymes: thrombin, factor VIII and plasmin.
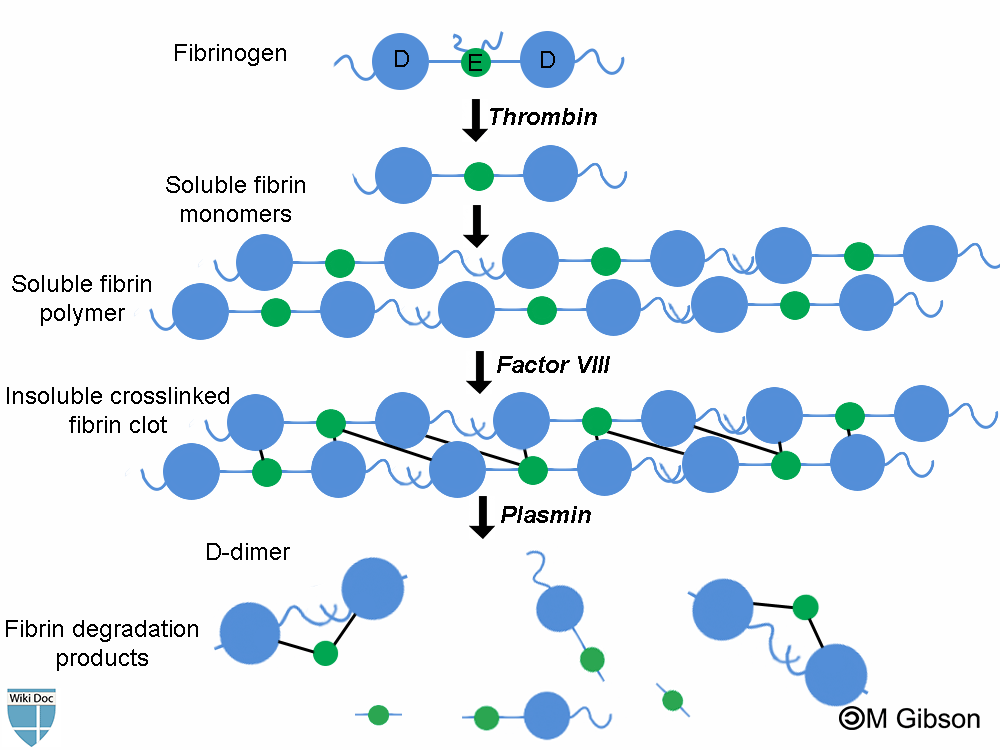
D-Dimer Test
D-dimer assays rely on monoclonal antibodies to bind to this specific protein fragment. The first patented MoAb of the kind was D Dimer-3B6/22, although others have been developed.
Indications
D-dimer testing is of clinical use when there is a suspicion of deep venous thrombosis (DVT) or pulmonary embolism (PE). In patients suspected of disseminated intravascular coagulation (DIC), D-dimers may aid in the diagnosis.
For DVT and PE, there are various scoring systems that are used to determine the a priori clinical probability of these diseases; the best-known were introduced by Wells et al (2003).
- For a very high score, or pretest probability, a D-dimer will make little difference and anticoagulant therapy will be initiated regardless of test results, and additional testing for DVT or pulmonary embolism may be performed.
- For a moderate or low score, or pretest probability:[4]
- A negative D-dimer test will virtually rule out thromboembolism: the degree to which the d-dimer reduces the probability of thrombotic disease is dependent on the test properties of the specific test used in your clinical setting: most available d-dimer tests with a negative result will reduce the probability of thromboembolic disease to less than 1% if the pretest probability is less than 15-20%
- If the D-dimer reads high, then further testing (ultrasound of the leg veins or lung scintigraphy or CT scanning) is required to confirm the presence of thrombus. Anticoagulant therapy may be started at this point or withheld until further tests confirm the diagnosis, depending on the clinical situation.
In some hospitals, they are measured by laboratories after a form is completed showing the probability score and only if the probability score is low or intermediate. This would reduce the need for unnecessary tests in those who are high-probability.[5]
Reference Range
Most sampling kits have 0-300 ng/ml as normal range. Values exceeding 250, 300 or 500 ng/ml (different for various kits) are considered positive.
For patients over age 50 a value of ageX10 may be abnormal.[6][7][8]
Types of Assays
- ELISA (e.g. Vidas)
- Latex turbidimetric assay (automated immunoassay, e.g. Roche Tina-quant, MDA D-dimer)
- Enhanced microlatex
- Latex-enhanced photometric
- Whole Blood Agglutination (e.g. SimpliRED)
- Rapid Lateral Flow (e.g. Clearview Simplify)
Test Properties
Various kits have a 93-95% sensitivity and about 50% specificity in the diagnosis of thrombotic disease.[9]
- False positive readings can be due to various causes: liver disease, high rheumatoid factor, inflammation, malignancy, trauma, pregnancy, recent surgery as well as advanced age
- False negative readings can occur if the sample is taken either too early after thrombus formation or if testing is delayed for several days. Additionally, the presence of anti-coagulation can render the test negative because it prevents thrombus extension.
- Likelihood ratios are derived from sensitivity and specificity to adjust pretest probability.
D-dimer and Thromboembolism
Abnormal Levels
Plasma D-dimer levels > 500 ng/mL are abnormal.[10]
Sensitivity and Specificity
Sensitivity[10]
ELISA (p=0.020), quantitative rapid ELISA (p=0.016) and semi-quantitative ELISA (p=0.047) are shown to be statistically superior to whole-blood agglutination.
Specificity[10]
Qualitative rapid ELISA has shown to be statistically superior to ELISA (p=0.004), quantitative rapid ELISA (p=0.002), semi-quantitative rapid ELISA (p=0.001), quantitative (p=0.005) and semi-quantitative latex agglutination assays (p=0.019).
| Method | Sensitivity (95% CI) | Specificity (95% CI) | Positive Likelihood Ratio (95% CI) | Negative Likelihood Ratio (95% CI) | Time to obtain Results |
|---|---|---|---|---|---|
| Enzyme-linked immunosorbent assay (ELISA) | 0.95 (0.85 to 1.00) | NS | NS | 0.13 (0.03 to 0.58) | ≥ 8 hours |
| Quantitative rapid ELISA | 0.95 (0.83 to 1.00) | NS | NS | 0.13 (0.02 to 0.84) | 30 mins |
| Semi-Quantitative rapid ELISA | 0.93 (0.79 to 1.00) | NS | NS | 0.20 (0.04 to 0.96) | 10 mins |
| Qualitative rapid ELISA | NS | 0.68 (0.50 to 0.87) | NS | 0.11 (0.01 to 0.93) | 10 mins |
| Quantitative Latex Agglutination | NS | NS | NS | NS | 10-15 mins |
| Semi-quantitative Latex Agglutination | NS | NS | NS | 0.17 (0.04 to 0.78) | 5 mins |
| Whole-Blood Agglutination | NS | 0.74 (0.60 to 0.88) | NS | NS | 2 mins |
Causes of Elevated D-Dimer
- Age
- Aortic dissection
- Atherosclerosis
- Atrial fibrillation
- Heart failure
- Ischemic heart disease
- Liver disease
- Malignancy
- Pregnancy
- Primary pulmonary hypertension
- Renal disease
- Sickle cell disease
- Stroke
- Surgery
- Thromboembolism
- Trauma
Hemodynamically Stable Patients
Incidence of Thromboembolic Events in Hemodynamically Stable Patients
| Condition | Incidence of thromboembolic event (%) |
|---|---|
| Patients not receiving anticoagulation with negative CT findings. | 1.5%[11][12] |
| Patients with a high d-dimer level | 1.5% |
| Patients with a normal d-dimer level | 0.5%[11] |
- Multidetector CT is indicated in hemodynamically stable patients with a high clinical probability of PE and/or patients with elevated plasma d-dimer levels secondary to the lack of specificity.[12][13]
- In patients with low-to-moderate suspicion of PE, a normal D-dimer level is considered sufficient to exclude the possibility of pulmonary embolism.[14]
Flowchart Summarizing the Role of D-dimer in the Diagnosis of PE
| Patients with suspection of Pulmonary embolism | |||||||||||||||||||||||
| Clinically Low or Moderate | Clinically High | ||||||||||||||||||||||
| D-Dimer Positive | |||||||||||||||||||||||
| D-Dimer Negative | |||||||||||||||||||||||
| No treatment | Further Tests | Further Tests | |||||||||||||||||||||
A new D-Dimer (DDMR) analyzer has shown to be more accurate in excluding patients with a low clinical pre-test probability.[15]
Prognostic Role of D-dimer
Mortality and Thromboembolism
- Several studies have investigated the role of D-dimer as a prognostic marker for patients diagnosed with pulmonary embolism. In fact, according to several studies D-dimer level is suggested to have a prognostic role as higher levels of D-dimer are associated with a higher mortality risk.[16]
- Measurement of the level of D-dimer was done on 366 patients presenting to the emergency department. Follow up on these patients revealed a higher mortality risk among patients having a D-dimer level higher than 5500 mg/L. In fact, the overall mortality increased from 1.1% to 9% among patients with D-dimer levels less than 1500mg/L and greater than 5500 mg/L respectively. The sensitivity and specificity of D-dimer in predicting mortality were 95% and 26% respectively, while the PPV and NPV were 7 % and 99% respectively.[17]
- Another study supported the same association of high D-dimer levels and increased mortality risk and suggested that the best cut-off level of D-dimer to predict mortality is more than 3000 ng/mL (OR= 7.29, CI=95%). In addition to their association with higher mortality risk, elevated levels of D-dimer are associated with centrally located pulmonary embolism.[18]
- Data results from RIETE registry also supports the association between high levels of D-dimer and fatality from pulmonary embolism (OR=1.8, CI=95%) as well as higher risk of major bleeding.[19]
- The prognostic role of D-dimer in hemodynamically stable patients does not have a solid ground. In fact, mixed results are present regarding the association between D-dimer and mortality. According to a study conducted on 292 stable patients with PE, high levels of D-dimer more than 5000 ng/mL were not associated with a higher mortality.[20]
Recurrence of Thromboembolism
- D-dimer seems to have a good prognostic role in predicting recurrence of thromboembolism. According to PROLONG study, normal levels of D-dimer 1 month following discontinuation of anticoagulation were associated with a decreased level of thromboembolism recurrences.[21]
- PROLONG II study investigated the association between the levels of D-dimers more than one month after anticoagulation suspension for unprovoked venous thromboembolism. The results of this study showed that patients who have high levels of D-dimer at 3 months after anticoagulation suspension have higher thromboembolism recurrences risks than patients who have normal levels of D-dimer. Hence, these studies suggest that the measurement of D-dimer levels at several months intervals following anticoagulation suspension in patient suffering from a first episode of VTE might be beneficial in triaging patients and targeting their therapies.[20]
Sepsis and Mortality
- Increase level of D-dimer is correlated with worsening severity and death. For instance, according to one study higher D-dimer levels were correlated with high risk of 28 day mortality such as the odds ratio are 2.07 (CI=95%) and 3.03 (CI=95%) in patients having a D-dimer level >1180 and >2409 respectively.[22]
- On the other hand, its decrease was associated with resolution of sepsis.[23][24][25]
- Higher D-dimer levels were correlated with high risk of 28 day mortality such as the odds ratio are 2.07 (CI=95%) and 3.03 (CI=95%) in patients having a D-dimer level >1180 and >2409 respectively.[22]
Primary Pulmonary Hypertension
Elevated D-dimer levels are shown to be associated with idiopathic primary pulmonary hypertension (PPH) and correlates with severity of disease, New York Heart Association (NYHA) functional class, and survival when evaluated in a small study that included 14 patients.[26][27]
Liver Disease
D-dimer is suggested to have a prognostic role in liver disease because it was found to be a significant predictor of death.[28]
Malignancy
Not only is D-dimer associated with the presence of malignancy, but also it correlates with tumor bulk, metastasis, and overall patient prognosis and mortality.[29]
D-Dimer and Non Thromboembolism Conditions
D-dimer lab test can be elevated in conditions other than venous thromboembolism. Because D-dimer is a sensitive test that lacks specificity, it is considered only useful in ruling out DVT and/or PE. Since D-dimer elevation is a physiologic process related to fibrinolytic activity that counteracts the extrinsic coagulation pathway activation, it is understandably not exclusive to venous thromboembolism (VTE) and can be present in other physiologic and pathological processes.[30] D-dimer elevation is not diagnostic of PE and can sometimes be a misleading lab value that is cost-inefficient, predisposes patients to high doses of unnecessary computed tomography (CT) radiation exposure, and delays appropriate diagnostic and therapeutic work-up.[31][32]
Age
D-dimer levels physiologically increase with age, making the usefulness of D-dimer among the elderly less significant. The exact mechanism of D-dimer increase with age is poorly understood. It is thought to be related to the expected increase in patient co-morbidities and thrombotic events that occur with age, and that also happen to elevate D-dimer levels. The use of D-dimer in elderly nonetheless remains helpful in diagnosing VTE in low and intermediate risk patients. Age-adjusted D-dimer levels are thought to be useful, especially for the elderly. However, specific age-adjusted values have not been released yet.[33]
Aortic Dissection
Elevated levels of D-dimer lab test has been used to rapidly rule out emergencies such as acute aortic dissection (AAD). More than 15 studies that enrolled more than 400 patients have evaluated the use of D-dimer in AAD. With the absence of specific biomarkers, the clinical diagnosis of AAD remains a challenge for clinicians based on clinical suspicion alone. A meta analysis for D-dimer testing in AAD revealed that D-dimer has 97% sensitivity and 59% specificity in diagnosis of AAD. The diagnostic cut-off D-dimer value for patients with AAD ranges between 0.1 and 0.9 µg/mL., with sensitivities ranging between 100% and 86% respectively. Using D-dimer cut-off value similar to that for PE at a level of 0.5 µg/mL is considered an appropriate level that has a negative predictive value that approximately reaches 100%.[30]
Renal Disease
D-dimer levels is correlated with nephrotic syndrome and other renal diseases. While some postulate that D-dimer elevation is associated with renal clearance,[34] data is conflicting as to whether D-dimer elevation may be less likely correlated with renal clearance as much as it is associated with proteinuria.[35] Nevertheless, the increase of hemostatic markers, such as D-dimer in renal disease, are considered risk factors for VTE in patients with renal disease.[36]
Nephrotic syndrome is considered a hypercoagulable state that is notoriously associated with DVT and PE. Among 100 patients with proteinuria, 53% had elevated D-dimer levels. When proteinuria was more than 1g/24 hours, elevation of D-dimer levels was seen in 69% of patients with proteinuria. D-dimer is believed to be related to the heavy proteinuria in nephrotic syndrome and subsequent hepatic synthesis of fibrinogen, where strong association between D-dimer elevation and hypoalbuminemia is found. It is also suggested that elevated serum fibrinopeptide A, thrombin-antithrombin III complex, along with products of thrombin and prothrombin, and the state of activated hemostasis in nephrotic syndrome causes the elevation of D-dimer with no evidence of clinical thrombosis.[37][38][35]
Sepsis and Septic Shock
D-dimer levels are almost always increased in patients with sepsis, septic shock, and disseminated intravascular coagulation (DIC). According to the Recombinant Human Activated Protein C Woldwide Evaluation in Severe Sepsis (PROWESS) trial that included 1,690 septic patients, D-dimer was elevated in approximately 100% of patients.[23][24][25]
Surgery
D-dimer levels may be elevated after surgery and trauma independent of VTE and PE. The diagnosis of post-operative VTE, a common complication following surgery, becomes even a more challenging diagnosis for this specific subset of patients given the unpredictable and heterogeneous variation of post-operative D-dimer levels. The dynamics behind D-dimer elevation following surgery and trauma are poorly understood.[39]
In a study of 154 patients categorized according to different types of abdominal surgeries, surgeries that did not include entering the abdominal cavity did not reveal elevation in D-dimer. In contrast, approximately 44% of open and laparoscopic intra-abdominal and retroperitoneal (and liver) surgeries were associated with elevated D-dimer levels that normalized after 25 and 38 days post-operatively respectively. D-dimer was found to generally peak around day 7 post-operation. The right time post-operatively to use D-dimer without the effect of the surgery itself is yet to be determined, but believed to be more than 5 weeks following intra-abdominal and retroperitoneal. It is thought that following peak, D-dimer levels decline at a rate of 6% every day.( PMID: 19474701 - Dindo et al. 200). The length of the surgery was associated with the elevation of D-dimer. However, no cut-off surgery length is determined.[40]
Orthopedic surgeries are also associated with an increase in D-dimer levels. In a study that recruited 78 patients with cemented or hybrid total hip replacement and uncemented total knee replacement. During the first 7 days post-op, D-dimers were significantly elevated particularly on day 1 and 7 post-operatively, showing a double-peak distribution.[41][42]
Sickle Cell Disease
The pathogenesis and clinical manifestations of sickle cell disease are mostly related to its hypercoagulable sickle-shaped red blood cells that contain phosphatydil serine moieties that contribute to their thrombogenic nature. In addition, endothelial dysfunction, sluggish blood flow, and increased transit time, all of which are associated with generation of subclinical or clinically relevant thrombin, are all factors generally augmented in patients with sickle cell disease. Elevated D-dimer levels is commonly found in up to 68% of homozygous sickle cell disease patients experiencing sickling crises and frequently associated with abnormal chest X-ray (CXR) findings.[43][44]
Primary Pulmonary Hypertension
The sensitivity of D-dimer in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH) is low in comparison to its sensitivity in other utilities. In a study that included 34 patients with CTEPH, the sensitivity of D-dimer in diagnosing CTEPH was only 37%, whereas the specificity was 46%. Hence, it cannot be used to rule in or rule out CTEPH.[26][27]
Liver Disease
Cirrhosis is considered a hypercoagulable state due to altered physiology of hemostasis secondary to the disease due to the physiological role the liver plays in the synthesis of thrombopoietin and coagulation factors,[45] decrease in fibrinolytic inhibitors, and reduced clearance of tissue plasminogen activator.[46] Platelet dysfunction and thrombocytopenia are frequent in liver cirrhosis, along with prolonged prothrombin time (PT) and activated partial thromboplastin time (APTT).[45] Elevated D-dimer level is seen in more than 75% of patients with advanced liver disease. Significant elevation correlates with worse liver outcomes, as demonstrated by Child-Pugh classification. It demonstrates features of fibrinolysis in these patients when levels are just above 0.2 µg/mL.[47][48] In a study that included 188 patients, D-dimer was considered of high specificity in patients with Child-Pugh class A or B, of cut-off values > 0.56 µg/mL and > 1.18 µg/mL respectively; whereas it was highly sensitive in patients with class C with cut-off value > 0.77 µg/mL with lower specificity in this particular class probably due to patients’ advanced state of liver dysfunction. [49]
D-dimer elevation is notably seen in patients with portal vein thrombosis (PVT) regardless of Child-Pugh class, a complication of portal hypertension that affects approximately 0.6-26% of patients with liver cirrhosis in general and approximately 35% of patients with cirrhosis from hepatocellular carcinoma (HCC).[46] In patients with worse outcomes of chronic liver disease Child-Pugh class C, D-dimer level of ≥ 0.55 µg/mL was 100% sensitive to diagnose PVT when measured.[50]
Normal Pregnancy
Malignancy
D-dimer is significantly associated with increased incidence of malignancy. The younger patient population, especially under 60 years, seem to be of particular concern for overt or occult cancer forms when D-dimer values are > 4 µg/mL .[51] Data regarding the correlation of malignancy with D-dimer shows that increasing D-dimer values are significantly more associated with malignancy than lower, yet abnormal, values. In patients with D-dimer > 8 µg/mL, the rate of malignancy following an episode of DVT was approximately 50%.[52] Similarly, patients with thrombosis who have low D-dimer values < 1 µg/mL are less likely to have an underlying malignancy.[53]
According to the Vienna Cancer and Thrombosis Study (CATS) that evaluated 1178 cancer patients, D-dimer was highest at a median of approximately 1.2 µg/mL in pancreatic cancer, followed by 1.08 µg/mL in gastric cancer, then 0.84 µg/mL in lung cancer, 0.81 µg/mL in colorectal cancers.[54] Significant association was also seen with lower D-dimer values and other malignancies, such as brain cancer (0.66 µg/mL), lymphomas (0.61 µg/mL), prostate cancer (0.46 µg/mL) and finally breast cancers (0.46 µg/mL).[54]
Cardiovascular Diseases
Ischemic Heart Disease
Since D-dimers alterations reflect thrombus turnover, levels are expected to be increased in ischemic heart disease and coronary angina. D-dimer levels are significantly associated with a 1.5 fold increase in coronary artery disease (CAD) and in subclinical atherosclerosis.[55] In patients with CAD, the median D-dimer value was 0.112 µg/mL vs. only 0.0028 µg/mL in patients without CAD. The positive correlation between D-dimer, fibrinogen, plasma viscosity, and interleukin-6 seem to be of importance in understanding the association of D-dimer with inflammatory products.[56] The most important value that D-dimer carries is its prediction of future cardiac events in various patient age groups, in both genders, and in patients with known peripheral arterial disease and myocardial infarction.[57][58][59][60][61][62] Despite obvious associations, some still question the use of D-dimer in providing informative or additional clinical data in the diagnosis or management of myocardial ischemia.[63]
In a study by Tataru et al. in 2000 that recruited 1112 male and 299 female patients, the significant association between and previous MI was further emphasized.[64]
Atrial Fibrillation
D-dimer levels are also elevated in patients with atrial fibrillation (AF), especially in those who are known to have established several embolism risk factors.[65] In 2003, Somloi et al. measured D-dimer levels in 73 patients with AF prior to TEE-guided cardioversion; they concluded that although D-dimer is unspecific, D-dimer concentration < 0.06 µg/ml provides a negative predictive value of 98%.[66] Habara et al. concluded that D-dimer concentration of 0.115 µg/mL was an optimal cut-off value to detect left atrial appendange (LAA) thrombus; the NPV was 97% irrespective of co-morbidities, such as congestive heart failure, or stroke.[65] Even in AF patients receiving appropriate Warfarin anticoagulation therapy, D-dimer levels remained abnormally high.[67]
It was further found that AF patients with D-dimer levels > 0.15 µg/mL were at a significantly greater risk of thrombo-embolic events than other patient subgroups. As such, the level 0.15 µg/mL is currently considered an accepted threshold value for increased rate of thrombo-embolic events in patients with AF. Nonetheless, increased number of risk factors bypasses the importance of D-dimer in AF patients and thus, patients with multiple risk factors are at high risk of thrombo-embolic events regardless of D-dimer levels and anticoagulation therapy. As such, D-dimer alone in patients with AF must be coupled to clinical risk factors for adequate assessment of thrombo-embolic risk.[67]
According to Diego et al., elevated D-dimer level is significantly associated with increasing severity of atherosclerosis based on ultrasound detection of carotid plaques and overall cardiovascular decline.[68][69]
Stroke
Elevated D-dimer values can also be used to predict acute cerebrovascular events and subtypes. Levels increase remarkably during first 6 hours of stroke onset in patients with large occlusion and those being treated with intravenous thrombolytics regardless of time of artery recanalization.[70] D-dimer seems to have shown association with infarction volume[71] and with cardioembolic strokes but not atherothrombotic or lacunar strokes. According to Ageno et al. in 2002, the optimal D-dimer cut-off point for predicting cardioembolic stroke is 2 µg/mL with 93.2% specificity and 59.3% sensitivity. In contrast, the cut-off point for predicting lacunar stroke in the same study was 0.54 µg/mL with 96.2% specificity and 61.3% sensitivity.[72] Although it is suggested as a screening test for patients presenting with acute-onset headaches,[73] triage for high-risk patients who require antithrombotic therapy, and evaluating progressing strokes, defined by clinical worsening of symptoms following admission, the prognostic role of D-dimer in cases of strokes is unclear and yet to be determined.[74]
ESC 2008 Guideline Recommendations [75]
Suspected Non High-risk PE Patients (DO NOT EDIT)[75]
| Class I |
| "1. Plasma D-dimer measurement is recommended in emergency department patients to reduce the need for unnecessary imaging and irradiation, preferably with the use of a highly sensitive assay. (Level of Evidence: A) " |
Low Clinical Probability (DO NOT EDIT)[75]
| Class I |
| "1. Normal D-dimer level using either a highly or moderately sensitive assay excludes pulmonary embolism. (Level of Evidence: A) " |
Intermediate Clinical Probability (DO NOT EDIT)[75]
| Class I |
| "1. Normal D-dimer level using a highly sensitive assay excludes pulmonary embolism. (Level of Evidence: A) " |
| Class IIa |
| "1. Further testing should be considered if D-dimer level is normal when using a less sensitive assay. (Level of Evidence: B) " |
High Clinical Probability (DO NOT EDIT)[75]
| Class III |
| "1. D-dimer measurement is not recommended in high clinical probability patients as a normal result does not safely exclude pulmonary embolism even when using a highly sensitive assay. (Level of Evidence: C) " |
References
- ↑ Bruinstroop E, van de Ree MA, Huisman MV (2009). "The use of D-dimer in specific clinical conditions: a narrative review". Eur J Intern Med. 20 (5): 441–6. doi:10.1016/j.ejim.2008.12.004. PMID 19712840.
- ↑ Agnelli G, Becattini C (2010). "Acute pulmonary embolism". N Engl J Med. 363 (3): 266–74. doi:10.1056/NEJMra0907731. PMID 20592294.
- ↑ Adam SS, Key NS, Greenberg CS (2009). "D-dimer antigen: current concepts and future prospects". Blood. 113 (13): 2878–87. doi:10.1182/blood-2008-06-165845. PMID 19008457 Check
|pmid=value (help). - ↑ Wells PS, Anderson DR, Rodger M; et al. (2003). "Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis". N. Engl. J. Med. 349 (13): 1227–35. doi:10.1056/NEJMoa023153. PMID 14507948.
- ↑ Rathbun, SW (2004). "Clinical utility of D-dimer in patients with suspected pulmonary embolism and nondiagnostic lung scans or negative CT findings". Chest (125): 851. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA; et al. (2013). "Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis". BMJ. 346: f2492. doi:10.1136/bmj.f2492. PMC 3643284. PMID 23645857.
- ↑ van Es J, Mos I, Douma R, Erkens P, Durian M, Nizet T; et al. (2012). "The combination of four different clinical decision rules and an age-adjusted D-dimer cut-off increases the number of patients in whom acute pulmonary embolism can safely be excluded". Thromb Haemost. 107 (1): 167–71. doi:10.1160/TH11-08-0587. PMID 22072293.
- ↑ Douma RA, le Gal G, Söhne M, Righini M, Kamphuisen PW, Perrier A; et al. (2010). "Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts". BMJ. 340: c1475. doi:10.1136/bmj.c1475. PMID 20354012.
- ↑ Schrecengost JE, LeGallo RD, Boyd JC, Moons KG, Gonias SL, Rose CE Jr, Bruns DE. Comparison of diagnostic accuracies in outpatients and hospitalized patients of D-dimer testing for the evaluation of suspected pulmonary embolism. Clin Chem 2003;49:1483-90. PMID 12928229.
- ↑ 10.0 10.1 10.2 Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, Biel RK, Bharadia V, Kalra NK (2004). "D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review". Annals of Internal Medicine. 140 (8): 589–602. PMID 15096330. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ 11.0 11.1 Perrier A, Roy PM, Sanchez O, Le Gal G, Meyer G, Gourdier AL; et al. (2005). "Multidetector-row computed tomography in suspected pulmonary embolism". N Engl J Med. 352 (17): 1760–8. doi:10.1056/NEJMoa042905. PMID 15858185. in: J Fam Pract. 2005 Aug;54(8):653, 657
- ↑ 12.0 12.1 van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW; et al. (2006). "Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography". JAMA. 295 (2): 172–9. doi:10.1001/jama.295.2.172. PMID 16403929.
- ↑ Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF (2009). "D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism". AJR Am J Roentgenol. 193 (2): 425–30. doi:10.2214/AJR.08.2186. PMID 19620439.
- ↑ Bounameaux H, de Moerloose P, Perrier A, Reber G (1994). "Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview". Thromb. Haemost. 71 (1): 1–6. PMID 8165626.
- ↑ Gosselin RC, Wu JR, Kottke-Marchant K, Peetz D, Christie DJ, Muth H; et al. (2012). "Evaluation of the Stratus® CS Acute Care™ D-dimer assay (DDMR) using the Stratus® CS STAT Fluorometric Analyzer: A prospective multisite study for exclusion of pulmonary embolism and deep vein thrombosis". Thromb Res. doi:10.1016/j.thromres.2011.12.015. PMID 22245223.
- ↑ Sanchez O, Planquette B, Roux A, Gosset-Woimant M, Meyer G (2012). "Triaging in pulmonary embolism". Semin Respir Crit Care Med. 33 (2): 156–62. doi:10.1055/s-0032-1311794. PMID 22648488.
- ↑ Aujesky D, Roy PM, Guy M, Cornuz J, Sanchez O, Perrier A (2006). "Prognostic value of D-dimer in patients with pulmonary embolism". Thromb Haemost. 96 (4): 478–82. PMID 17003925.
- ↑ Klok FA, Djurabi RK, Nijkeuter M, Eikenboom HC, Leebeek FW, Kramer MH; et al. (2008). "High D-dimer level is associated with increased 15-d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity". Br J Haematol. 140 (2): 218–22. doi:10.1111/j.1365-2141.2007.06888.x. PMID 18028485.
- ↑ Lobo JL, Zorrilla V, Aizpuru F, Grau E, Jiménez D, Palareti G; et al. (2009). "D-dimer levels and 15-day outcome in acute pulmonary embolism. Findings from the RIETE Registry". J Thromb Haemost. 7 (11): 1795–801. doi:10.1111/j.1538-7836.2009.03576.x. PMID 19691481.
- ↑ 20.0 20.1 Stein PD, Janjua M, Matta F, Alrifai A, Jaweesh F, Chughtai HL (2011). "Prognostic value of D-dimer in stable patients with pulmonary embolism". Clin Appl Thromb Hemost. 17 (6): E183–5. doi:10.1177/1076029610395129. PMID 21288930.
- ↑ Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A; et al. (2006). "D-dimer testing to determine the duration of anticoagulation therapy". N Engl J Med. 355 (17): 1780–9. doi:10.1056/NEJMoa054444. PMID 17065639. Review in: ACP J Club. 2007 Mar-Apr;146(2):29 Review in: Evid Based Med. 2007 Apr;12(2):45
- ↑ 22.0 22.1 Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gómez CI; et al. (2012). "D-dimer is a significant prognostic factor in patients with suspected infection and sepsis". Am J Emerg Med. 30 (9): 1991–9. doi:10.1016/j.ajem.2012.04.033. PMID 22795996.
- ↑ 23.0 23.1 Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A; et al. (2001). "Efficacy and safety of recombinant human activated protein C for severe sepsis". N Engl J Med. 344 (10): 699–709. doi:10.1056/NEJM200103083441001. PMID 11236773.
- ↑ 24.0 24.1 Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A; et al. (2004). "Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism [ISRCTN74215569]". Crit Care. 8 (2): R82–90. doi:10.1186/cc2459. PMC 420030. PMID 15025782.
- ↑ 25.0 25.1 Nguyen HB, Rivers EP, Abrahamian FM, Moran GJ, Abraham E, Trzeciak S; et al. (2006). "Severe sepsis and septic shock: review of the literature and emergency department management guidelines". Ann Emerg Med. 48 (1): 28–54. doi:10.1016/j.annemergmed.2006.02.015. PMID 16781920.
- ↑ 26.0 26.1 Shitrit D, Bendayan D, Bar-Gil-Shitrit A, Huerta M, Rudensky B, Fink G; et al. (2002). "Significance of a plasma D-dimer test in patients with primary pulmonary hypertension". Chest. 122 (5): 1674–8. PMID 12426270.
- ↑ 27.0 27.1 Arunthari V, Burger CD (2009). "Utility of d-dimer in the diagnosis of patients with chronic thromboembolic pulmonary hypertension". Open Respir Med J. 3: 85–9. doi:10.2174/1874306400903010085. PMC 2703202. PMID 19572023.
- ↑ Primignani M, Dell'Era A, Bucciarelli P, Bottasso B, Bajetta MT, de Franchis R; et al. (2008). "High-D-dimer plasma levels predict poor outcome in esophageal variceal bleeding". Dig Liver Dis. 40 (11): 874–81. doi:10.1016/j.dld.2008.01.010. PMID 18329968.
- ↑ Dirix LY, Salgado R, Weytjens R, Colpaert C, Benoy I, Huget P; et al. (2002). "Plasma fibrin D-dimer levels correlate with tumour volume, progression rate and survival in patients with metastatic breast cancer". Br J Cancer. 86 (3): 389–95. doi:10.1038/sj.bjc.6600069. PMC 2375200. PMID 11875705.
- ↑ 30.0 30.1 Sodeck G, Domanovits H, Schillinger M, Ehrlich MP, Endler G, Herkner H; et al. (2007). "D-dimer in ruling out acute aortic dissection: a systematic review and prospective cohort study". Eur Heart J. 28 (24): 3067–75. doi:10.1093/eurheartj/ehm484. PMID 17986466.
- ↑ Dunn KL, Wolf JP, Dorfman DM, Fitzpatrick P, Baker JL, Goldhaber SZ (2002). "Normal D-dimer levels in emergency department patients suspected of acute pulmonary embolism". J Am Coll Cardiol. 40 (8): 1475–8. PMID 12392839. Review in: J Fam Pract. 2003 Feb;52(2):99, 103
- ↑ Chopra N, Doddamreddy P, Grewal H, Kumar PC (2012). "An elevated D-dimer value: a burden on our patients and hospitals". Int J Gen Med. 5: 87–92. doi:10.2147/IJGM.S25027. PMC 3273370. PMID 22319245.
- ↑ Der Sahakian G, Claessens YE, Allo JC, Kansao J, Kierzek G, Pourriat JL (2010). "Accuracy of D-Dimers to Rule Out Venous Thromboembolism Events across Age Categories". Emerg Med Int. 2010: 185453. doi:10.1155/2010/185453. PMC 3195346. PMID 22046531.
- ↑ Shlipak MG, Fried LF, Stehman-Breen C, Siscovick D, Newman AB (2004). "Chronic renal insufficiency and cardiovascular events in the elderly: findings from the Cardiovascular Health Study". Am J Geriatr Cardiol. 13 (2): 81–90. PMID 15010654.
- ↑ 35.0 35.1 Sexton DJ, Clarkson MR, Mazur MJ, Plant WD, Eustace JA (2012). "Serum D-dimer concentrations in nephrotic syndrome track with albuminuria, not estimated glomerular filtration rate". Am J Nephrol. 36 (6): 554–60. doi:10.1159/000345475. PMID 23221061.
- ↑ Dubin R, Cushman M, Folsom AR, Fried LF, Palmas W, Peralta CA; et al. (2011). "Kidney function and multiple hemostatic markers: cross sectional associations in the multi-ethnic study of atherosclerosis". BMC Nephrol. 12: 3. doi:10.1186/1471-2369-12-3. PMC 3037849. PMID 21269477.
- ↑ Chen TY, Huang CC, Tsao CJ (1993). "Hemostatic molecular markers in nephrotic syndrome". Am J Hematol. 44 (4): 276–9. PMID 8238000.
- ↑ Singhal R, Brimble KS (2006). "Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management". Thromb Res. 118 (3): 397–407. doi:10.1016/j.thromres.2005.03.030. PMID 15990160.
- ↑ Lippi G, Veraldi GF, Fraccaroli M, Manzato F, Cordiano C, Guidi G (2001). "Variation of plasma D-dimer following surgery: implications for prediction of postoperative venous thromboembolism". Clin Exp Med. 1 (3): 161–4. PMID 11833854.
- ↑ Dindo D, Breitenstein S, Hahnloser D, Seifert B, Yakarisik S, Asmis LM; et al. (2009). "Kinetics of D-dimer after general surgery". Blood Coagul Fibrinolysis. 20 (5): 347–52. doi:10.1097/MBC.0b013e32832a5fe6. PMID 19474701.
- ↑ Shiota N, Sato T, Nishida K, Matsuo M, Takahara Y, Mitani S; et al. (2002). "Changes in LPIA D-dimer levels after total hip or knee arthroplasty relevant to deep-vein thrombosis diagnosed by bilateral ascending venography". J Orthop Sci. 7 (4): 444–50. doi:10.1007/s007760200077. PMID 12181657.
- ↑ Rafee A, Herlikar D, Gilbert R, Stockwell RC, McLauchlan GJ (2008). "D-Dimer in the diagnosis of deep vein thrombosis following total hip and knee replacement: a prospective study". Ann R Coll Surg Engl. 90 (2): 123–6. doi:10.1308/003588408X261627. PMC 2443306. PMID 18325211.
- ↑ Dar J, Mughal I, Hassan H, Al Mekki TE, Chapunduka Z, Hassan IS (2010). "Raised D-dimer levels in acute sickle cell crisis and their correlation with chest X-ray abnormalities". Ger Med Sci. 8: Doc25. doi:10.3205/000114. PMC 2975260. PMID 21063468.
- ↑ Setty BN, Rao AK, Stuart MJ (2001). "Thrombophilia in sickle cell disease: the red cell connection". Blood. 98 (12): 3228–33. PMID 11719358.
- ↑ 45.0 45.1 Lisman T, Porte RJ (2010). "Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences". Blood. 116 (6): 878–85. doi:10.1182/blood-2010-02-261891. PMID 20400681.
- ↑ 46.0 46.1 Sacerdoti D, Serianni G, Gaiani S, Bolognesi M, Bombonato G, Gatta A (2007). "Thrombosis of the portal venous system". J Ultrasound. 10 (1): 12–21. doi:10.1016/j.jus.2007.02.007. PMC 3478708. PMID 23396402.
- ↑ Violi F, Ferro D, Basili S, Saliola M, Quintarelli C, Alessandri C; et al. (1995). "Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis". Gastroenterology. 109 (2): 531–9. PMID 7615203.
- ↑ Violi F, Ferro D, Basili S, Quintarelli C, Musca A, Cordova C; et al. (1993). "Hyperfibrinolysis resulting from clotting activation in patients with different degrees of cirrhosis. The CALC Group. Coagulation Abnormalities in Liver Cirrhosis". Hepatology. 17 (1): 78–83. PMID 8423044.
- ↑ Zhang DL, Hao JY, Yang N (2013). "Value of D-dimer and protein S for diagnosis of portal vein thrombosis in patients with liver cirrhosis". J Int Med Res. 41 (3): 664–72. doi:10.1177/0300060513483413. PMID 23637275.
- ↑ Fimognari FL, De Santis A, Piccheri C, Moscatelli R, Gigliotti F, Vestri A; et al. (2005). "Evaluation of D-dimer and factor VIII in cirrhotic patients with asymptomatic portal venous thrombosis". J Lab Clin Med. 146 (4): 238–43. doi:10.1016/j.lab.2005.06.003. PMID 16194685.
- ↑ Schutgens RE, Beckers MM, Haas FJ, Biesma DH (2005). "The predictive value of D-dimer measurement for cancer in patients with deep vein thrombosis". Haematologica. 90 (2): 214–9. PMID 15710574.
- ↑ Paneesha S, Cheyne E, French K, Delgado J, Borg A, Rose P (2005). "High D-dimer level at presentation in patients with venous thrombosis is a marker for malignancy". Haematologica. 90 (12 Suppl): ELT08. PMID 16464765.
- ↑ Rege KP, Jones S, Day J, Hoggarth CE (2004). "In proven deep vein thrombosis, a low positive D-Dimer score is a strong negative predictor for associated malignancy". Thromb Haemost. 91 (6): 1219–22. doi:10.1267/THRO04061219. PMID 15175810.
- ↑ 54.0 54.1 Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O; et al. (2012). "High D-dimer levels are associated with poor prognosis in cancer patients". Haematologica. 97 (8): 1158–64. doi:10.3324/haematol.2011.054718. PMC 3409812. PMID 22371182.
- ↑ Salomaa V, Stinson V, Kark JD, Folsom AR, Davis CE, Wu KK (1995). "Association of fibrinolytic parameters with early atherosclerosis. The ARIC Study. Atherosclerosis Risk in Communities Study". Circulation. 91 (2): 284–90. PMID 7805229.
- ↑ Koenig W, Rothenbacher D, Hoffmeister A, Griesshammer M, Brenner H (2001). "Plasma fibrin D-dimer levels and risk of stable coronary artery disease: results of a large case-control study". Arterioscler Thromb Vasc Biol. 21 (10): 1701–5. PMID 11597948.
- ↑ Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC (1998). "Fibrin D-dimer, tissue plasminogen activator, plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly Study". Thromb Haemost. 79 (1): 129–33. PMID 9459337.
- ↑ Lowe GD, Yarnell JW, Rumley A, Bainton D, Sweetnam PM (2001). "C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis?". Arterioscler Thromb Vasc Biol. 21 (4): 603–10. PMID 11304479.
- ↑ Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK (2001). "Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study". Arterioscler Thromb Vasc Biol. 21 (4): 611–7. PMID 11304480.
- ↑ Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR; et al. (1999). "Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study". Arterioscler Thromb Vasc Biol. 19 (3): 493–8. PMID 10073948.
- ↑ Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA; et al. (1993). "Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease". Lancet. 342 (8863): 84–6. PMID 8100915.
- ↑ Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H; et al. (1999). "Thrombogenic factors and recurrent coronary events". Circulation. 99 (19): 2517–22. PMID 10330382.
- ↑ Lippi G, Filippozzi L, Montagnana M, Salvagno GL, Guidi GC (2008). "Diagnostic value of D-dimer measurement in patients referred to the emergency department with suspected myocardial ischemia". J Thromb Thrombolysis. 25 (3): 247–50. doi:10.1007/s11239-007-0060-6. PMID 17541763.
- ↑ Tataru MC, Heinrich J, Junker R, Schulte H, von Eckardstein A, Assmann G; et al. (2000). "C-reactive protein and the severity of atherosclerosis in myocardial infarction patients with stable angina pectoris". Eur Heart J. 21 (12): 1000–8. PMID 10901512.
- ↑ 65.0 65.1 Cohen A, Ederhy S, Meuleman C, Di Angelantonio E, Dufaitre G, Boccara F (2007). "D-dimers in atrial fibrillation: a further step in risk stratification of thrombo-embolism?". Eur Heart J. 28 (18): 2179–80. doi:10.1093/eurheartj/ehm170. PMID 17716992.
- ↑ Somlói M, Tomcsányi J, Nagy E, Bodó I, Bezzegh A (2003). "D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation". Am J Cardiol. 92 (1): 85–7. PMID 12842257.
- ↑ 67.0 67.1 Nozawa T, Inoue H, Hirai T, Iwasa A, Okumura K, Lee JD; et al. (2006). "D-dimer level influences thromboembolic events in patients with atrial fibrillation". Int J Cardiol. 109 (1): 59–65. doi:10.1016/j.ijcard.2005.05.049. PMID 15992948.
- ↑ de Maat MP, Bladbjerg EM, Drivsholm T, Borch-Johnsen K, Møller L, Jespersen J (2003). "Inflammation, thrombosis and atherosclerosis: results of the Glostrup study". J Thromb Haemost. 1 (5): 950–7. PMID 12871360.
- ↑ Cohen HJ, Harris T, Pieper CF (2003). "Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly". Am J Med. 114 (3): 180–7. PMID 12637131.
- ↑ Skoloudík D, Bar M, Sanák D, Bardon P, Roubec M, Langová K; et al. (2010). "D-dimers increase in acute ischemic stroke patients with the large artery occlusion, but do not depend on the time of artery recanalization". J Thromb Thrombolysis. 29 (4): 477–82. doi:10.1007/s11239-009-0372-9. PMID 19568692.
- ↑ Park YW, Koh EJ, Choi HY (2011). "Correlation between Serum D-Dimer Level and Volume in Acute Ischemic Stroke". J Korean Neurosurg Soc. 50 (2): 89–94. doi:10.3340/jkns.2011.50.2.89. PMC 3206284. PMID 22053225.
- ↑ Ageno W, Finazzi S, Steidl L, Biotti MG, Mera V, Melzi D'Eril G; et al. (2002). "Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes". Arch Intern Med. 162 (22): 2589–93. PMID 12456231.
- ↑ Squizzato A, Ageno W (2005). "D-dimer testing in ischemic stroke and cerebral sinus and venous thrombosis". Semin Vasc Med. 5 (4): 379–86. doi:10.1055/s-2005-922484. PMID 16302160.
- ↑ Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ (2006). "D-dimer predicts early clinical progression in ischemic stroke: confirmation using routine clinical assays". Stroke. 37 (4): 1113–5. doi:10.1161/01.STR.0000209240.63821.1a. PMID 16527998.
- ↑ 75.0 75.1 75.2 75.3 75.4 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U, Klepetko W, Mayer E, Remy-Jardin M, Bassand JP (2008). "Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)". Eur. Heart J. 29 (18): 2276–315. doi:10.1093/eurheartj/ehn310. PMID 18757870. Retrieved 2011-12-07. Unknown parameter
|month=ignored (help)
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- CS1 errors: PMID
- CS1 maint: Explicit use of et al.
- Pages with citations using unsupported parameters
- Pages using citations with accessdate and no URL
- CS1 maint: PMC format
- Laboratory Test
- Chemical pathology
- Hematology
- Fibrinolytic system
- Cardiology
- Pulmonology